Abstract
This study investigated the crystallographic plane dependence of the reaction of AlN and AlGaN using heated-pressurized water under saturated vapor pressure. The results show that the reaction strongly depends on the crystallographic orientation plane, with no reaction in the +c-plane, the formation of an AlOOH-altered layer in the −c-plane, and etching in the a- and m-planes. These results suggest that the exfoliation mechanism of AlGaN grown on periodically formed AlN nanopillars on sapphire substrates using heated-pressurized water involves etching of a- and m-plane crystals, demonstrating that the proposed method is highly reproducible and versatile for large-diameter wafer exfoliation.
Export citation and abstract BibTeX RIS
The availability of high-quality AlN templates on sapphire and bulk AlN substrates has led to the development of high-performance AlGaN-based UV-LEDs. 1) In the wide wavelength range of 250–365 nm, which corresponds to the UV-A (wavelength range: 315–380 nm, according to ISO 21348), UV-B (280–315 nm), and UV-C (100–280 nm) regions, the above AlGaN-based UV-LEDs have achieved an external quantum efficiency of ∼10%. Similarly, the development of AlGaN-based UV laser diodes (LDs) has progressed considerably, and RT oscillation from UV-A 2,3) to UV-B 4,5) and UV-C 6,7) has been demonstrated.
These devices have potential applications in sterilization, biotechnology, medicine, and curing processes. Many of these applications require high light output; hence, high-current operation is essential. For high-current operation, vertical devices, which require placing p-type and n-type electrodes in opposite directions across the semiconductor layer with current flowing vertically to the device, are advantageous because current can flow uniformly compared to horizontal devices, where current flows horizontally to the device. Additionally, it is beneficial for heat dissipation. However, sapphire and AlN, which are used as substrates for the above UV-LEDs, are insulating, making it difficult to obtain electrical conduction. Consequently, a method for removing the insulating substrate and AIN layer is essential. Among these methods, laser lift-off (LLO), 8–15) electrochemical etching, 16) and grinding and polishing 17) have been reported to exfoliate sapphire substrates. These methods have been employed in operating vertical devices. 8,13–17) Furthermore, an approach that combines mechanical polishing and inductively coupled plasma (ICP) etching with SF6 gas has also been reported as a method to exfoliate UV-C LEDs on a SiC substrate. 18) Meanwhile, the method for exfoliating these substrates has many issues. For example, LLO requires the wavelength of the laser source to be adjusted according to the AlN molar fraction of AlGaN, and the Al droplets generated 14) during laser irradiation make achieving a large aperture and low reproducibility difficult. Other methods also have issues with large diameters, versatility in application to AlGaN with various AlN mole fractions, and reproducibility.
We reported a method for exfoliating AlGaN with a wafer size of 1 cm2 fabricated on a sapphire substrate using deionized water under heated-pressurized saturated vapor pressure of 170 kPa and 115 °C using an autoclave apparatus. 19) This method involves exfoliating AlGaN templates formed on periodic AlN nanopillars via nanoimprinting and ICP etching with Cl2 gas. These AlGaN templates are used as an underlying layer for UV-B LDs. 20–22) Previously, it has been reported that AlOOH, Al(OH)3, and Al2O3 are formed by reaction with water for AlN powders and polycrystalline substrates. 23–26) In the report, 19) an alteration layer is formed by heating and pressurizing water, and the alteration layer is identified as AlOOH using the X-ray diffraction method. 27) Nevertheless, the details of the exfoliation mechanism of the AlGaN film using heated-pressurized water have not yet been clarified. The key point is that if wafers are exfoliated by mechanical stress, it is difficult to achieve high reproducibility due to the cracking of the wafer caused by stress concentration. However, for example, if wafers are exfoliated via chemical etching, stress concentration is less likely and reproducibility and large-diameter wafer exfoliation are expected.
Therefore, understanding the detailed exfoliation mechanism of the proposed method using saturated vapor pressure water is vital to a versatile and reproducible process for exfoliating large-diameter wafers and applying the proposed method to AlGaN with different AlN mole fractions. In this study, we investigated the crystallographic plane dependence of the reaction of AlN and AlGaN using heated-pressurized water. Consequently, it was confirmed that the reaction of AlN and AlGaN using heated-pressurized water has strong crystallographic plane dependence, i.e., it exhibits no reaction, altered layer formation, and etching, depending on the crystallographic plane. The exfoliation mechanism of the proposed method is discussed based on the results.
Next, we describe the experimental method. In this study, samples with four crystallographic planes of AlN [group-III polar plane (0001) (hereafter called +c-plane), nitrogen-polar plane  (hereafter called −c-plane),
(hereafter called −c-plane),  (hereafter called m-plane), and
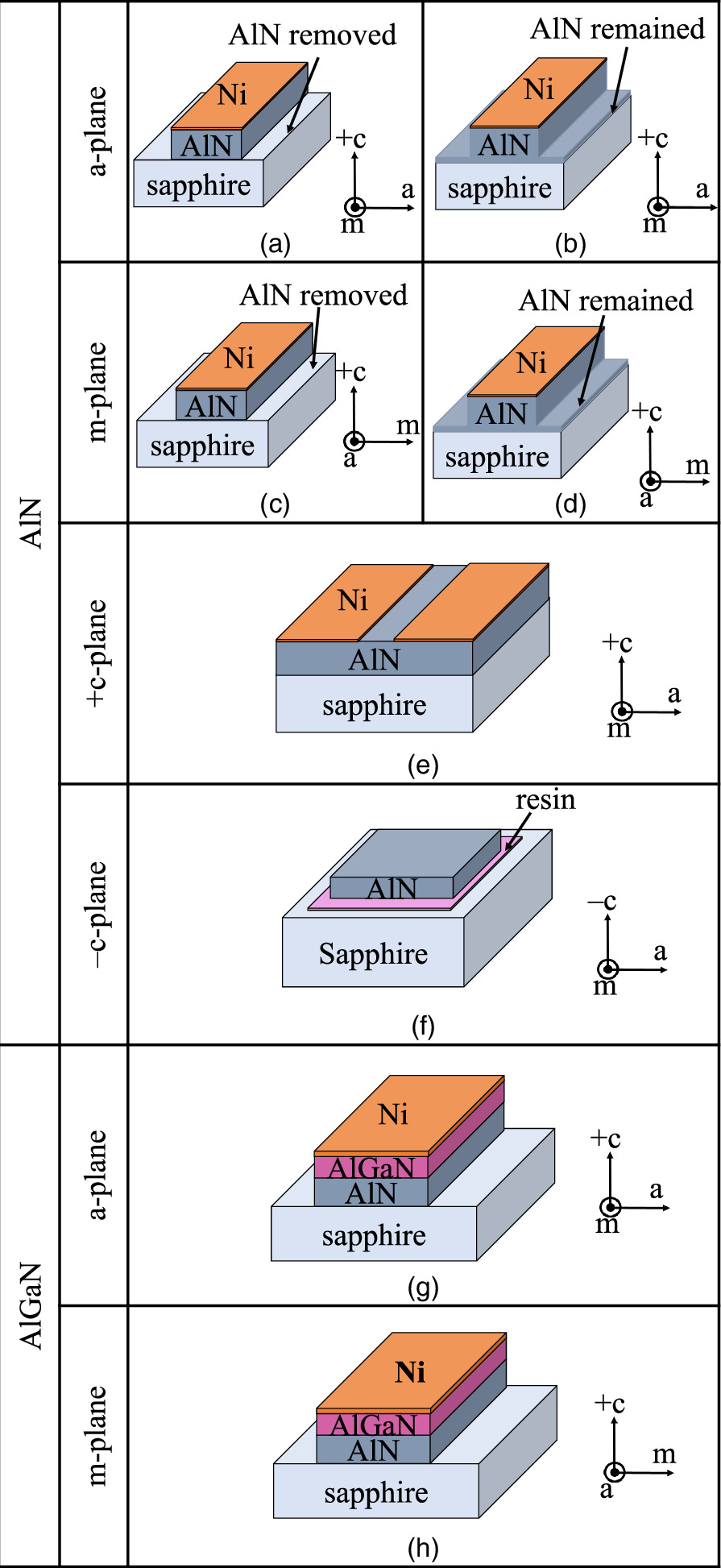
(hereafter called m-plane), and  (hereafter called a-plane)] were prepared, and we examined the reaction of each plane using heated-pressurized water. Samples of AlGaN on AlN templates with a- and m-planes were also prepared and simultaneously investigated. Figures 1(a)–1(h) illustrate the schematic overhead view of the samples. +c-, a-, and m-plane AlN samples were obtained by processing 1.2 μm thick AlN crystal-grown templates by the metalorganic vapor phase epitaxial (MOVPE) method on high-temperature annealed high-quality sputtered AlN fabricated on sapphire substrates.
28) The crystal quality of these AlN templates is excellent, with the FWHM of the (0002) and
(hereafter called a-plane)] were prepared, and we examined the reaction of each plane using heated-pressurized water. Samples of AlGaN on AlN templates with a- and m-planes were also prepared and simultaneously investigated. Figures 1(a)–1(h) illustrate the schematic overhead view of the samples. +c-, a-, and m-plane AlN samples were obtained by processing 1.2 μm thick AlN crystal-grown templates by the metalorganic vapor phase epitaxial (MOVPE) method on high-temperature annealed high-quality sputtered AlN fabricated on sapphire substrates.
28) The crystal quality of these AlN templates is excellent, with the FWHM of the (0002) and  diffraction X-ray rocking curves representing the tilt and twist components of the crystal being approximately 12 and 130 arcsec, respectively, the dislocation density estimated from these values is as low as 108 cm−2. The a- and m-plane AlGaN samples were obtained by processing wafers with a 1.2 μm thick AlGaN film and an AlN mole fraction of 0.62 grown on the AlN template using the MOVPE method. The description of the processing of these samples is as follows. Using an electron beam evaporator (JEOL, JBS-Z0500EHA) and photolithographic lift-off process, 100 nm thick Ni masks were formed on the AlN and AlGaN films. The shape of the mask was a periodic rectangle, with a crystal m-axis length of approximately 200 μm, and a-axis length of approximately 700 μm, respectively. Samples that completed the process at this stage were used as +c-plane AlN samples. The a- and m-plane samples were obtained by etching the AlN and AlGaN samples using an ICP (ULVAC, CE-S) with Cl2 gas to form mesas, followed by additional etching with 25 at% tetramethylammonium hydroxide (TMAH) solution at 85 °C for 5 min. Etching with the TMAH solution was conducted because it forms nearly perfect a- and m-planes.
29) Two samples were prepared: one with etched +c-plane AlN remaining and the other etched up to sapphire and the complete removal of AlN from the etched surface. In this experiment, Ni was used as the mask was left as a guide to verify the effectiveness of the heated-pressurized water treatment. Furthermore, a 5 μm thick −c-plane AlN sample was obtained by exfoliating AlN from the sapphire substrate using the heated-pressurized water method described in Ref. 19 and by chemically mechanical polishing the exfoliated surface. The exfoliated AlN was fixed onto the sapphire support substrate using resin (NAMICS Corp., SUF1575-9).
diffraction X-ray rocking curves representing the tilt and twist components of the crystal being approximately 12 and 130 arcsec, respectively, the dislocation density estimated from these values is as low as 108 cm−2. The a- and m-plane AlGaN samples were obtained by processing wafers with a 1.2 μm thick AlGaN film and an AlN mole fraction of 0.62 grown on the AlN template using the MOVPE method. The description of the processing of these samples is as follows. Using an electron beam evaporator (JEOL, JBS-Z0500EHA) and photolithographic lift-off process, 100 nm thick Ni masks were formed on the AlN and AlGaN films. The shape of the mask was a periodic rectangle, with a crystal m-axis length of approximately 200 μm, and a-axis length of approximately 700 μm, respectively. Samples that completed the process at this stage were used as +c-plane AlN samples. The a- and m-plane samples were obtained by etching the AlN and AlGaN samples using an ICP (ULVAC, CE-S) with Cl2 gas to form mesas, followed by additional etching with 25 at% tetramethylammonium hydroxide (TMAH) solution at 85 °C for 5 min. Etching with the TMAH solution was conducted because it forms nearly perfect a- and m-planes.
29) Two samples were prepared: one with etched +c-plane AlN remaining and the other etched up to sapphire and the complete removal of AlN from the etched surface. In this experiment, Ni was used as the mask was left as a guide to verify the effectiveness of the heated-pressurized water treatment. Furthermore, a 5 μm thick −c-plane AlN sample was obtained by exfoliating AlN from the sapphire substrate using the heated-pressurized water method described in Ref. 19 and by chemically mechanical polishing the exfoliated surface. The exfoliated AlN was fixed onto the sapphire support substrate using resin (NAMICS Corp., SUF1575-9).
Fig. 1. (a)–(h) Schematic overhead view of the samples.
Download figure:
Standard image High-resolution imageThe dependence was investigated by treating the samples with water heated and pressurized via the autoclave system (Tomy Seiko Corp., LSX-300). In this experiment, temperature and pressure conditions were applied to treat the heated-pressurized water to keep it under saturated vapor pressure. First, deionized water and samples were placed in a beaker at RT (∼26 °C), which was then placed in the autoclave, heated and pressurized to saturated vapor pressure, 30) and held at that temperature and pressure to treat the samples. The samples were then cooled and depressurized back to RT and atmospheric pressure. The time required for heating/pressurization and cooling/decompression was roughly 50 and 70 min, respectively. This study considered the aforementioned holding time as the treatment time with heated-pressurized water, and its dependence is verified by varying it from 2 to 6 h. Three treatment conditions (115 °C/170 kPa, 125 °C/250 kPa, and 135 °C/313 kPa) under saturated vapor pressure were applied to investigate the dependence of the heated-pressurized water treatment. Treated samples were cleaved, cross-sectional scanning electron microscopy (SEM) was performed for appearance, and X-ray photoelectron spectroscopy (XPS) measurements were performed to verify the presence or absence of reactions.
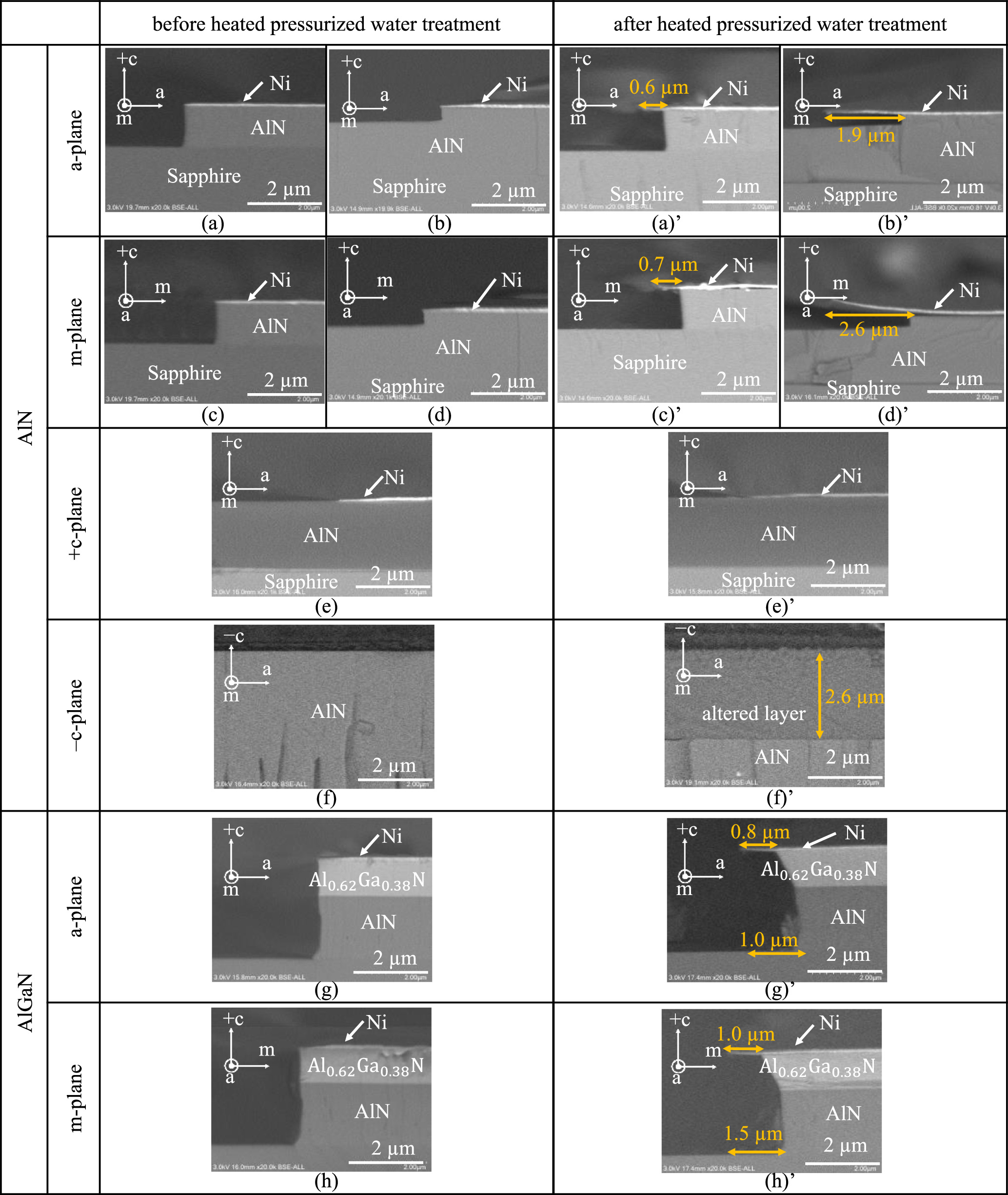
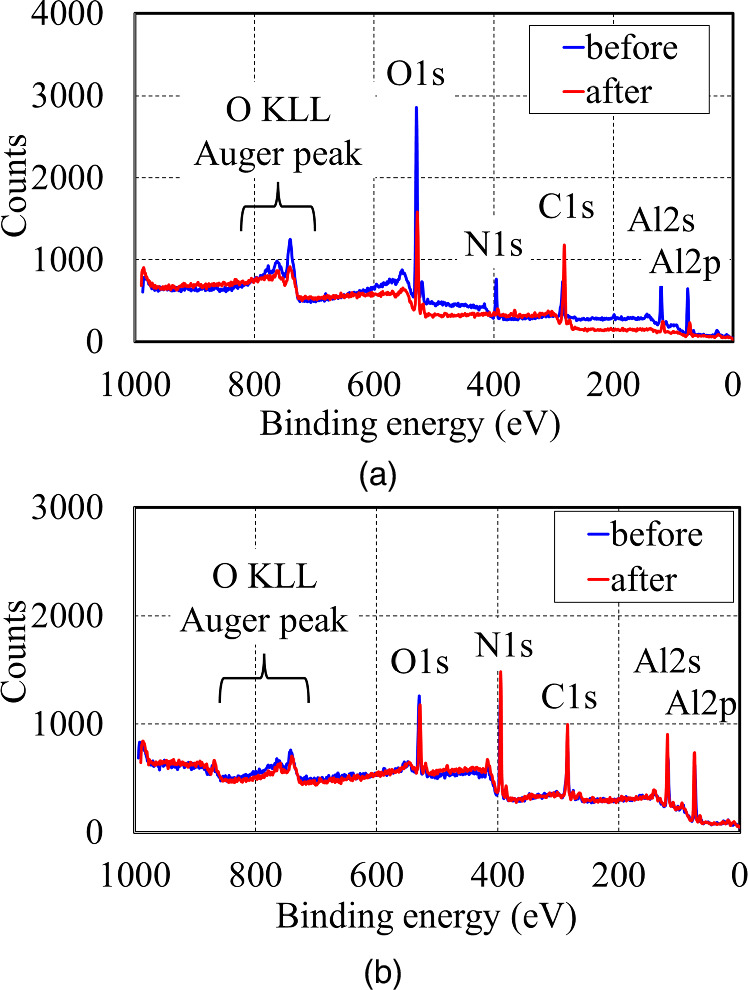
Figures 2(a)–2(h) and 2(a)'–2(h)' are the cross-sectional SEM images of each sample shown in Fig. 1 before and after treatment (treatment conditions: 135 °C, 313 kPa, and 2 h), respectively. These images show strong crystallographic plane dependence. Specific results are described below. For the −c-plane samples, a clear contrast change of ∼2.6 μm thickness was observed, as indicated by the arrow and numerical value in the figure, suggesting that the treatment formed an altered layer. Meanwhile, no clear contrast was observed in the +c-plane sample. Figures 3(a) and 3(b) show the XPS measurement results of the two samples before and after treatment. The figures clearly indicated the peaks, which are assigned based on Ref. 31. Based on the results of the −c-plane [Fig. 3(a)], the peaks attributed to nitrogen almost disappeared due to the heated-pressurized water treatment. However, based on the results of the +c-plane [Fig. 3(b)], there was almost no observed change in the peaks. X-ray diffraction peaks attributed to AlOOH were observed in the −c-plane samples treated with heated-pressurized water.
27) Accordingly, it is considered that the treatment formed layers consisting of AlOOH. According to Ref. 23, the chemical reaction in which AlN and H2O react to form AlOOH is expressed as AlN + 2 H2O → AlOOH + NH3. In addition, when the water after treatment used in this experiment was measured with litmus test paper, the pH increased slightly from 7, which is neutral before treatment, to approximately 8, which indicates alkalinity, suggesting that ammonia was produced in the above chemical reaction and that low-concentration ammonia water was obtained. Therefore, the aforementioned chemical reaction is considered to have occurred in the −c-plane sample. Meanwhile, no treatment-induced changes were observed on the +c-plane sample, either in appearance by SEM or at the XPS level. Further differences were observed in the a- and m-plane samples compared to the +c- and −c-plane samples. After treating the a- and m-planes, etched areas were observed under the Ni that remained as a guide in the AlN and AlGaN samples, as indicated by the orange arrows and numerical values in each figure. The shape of the a- and m-plane remained almost unchanged, indicating that etching progressed while the a- and m-plane facets were almost maintained. Furthermore, it was confirmed that both planes have almost the same etching amounts. Meanwhile, a large difference in the amount of etching was observed between the samples with and without residual-etched AlN (i.e., those not etched down to the sapphire substrate and those etched down to the sapphire substrate), with the amount of etching in the sample with residual-etched AlN being approximately four times greater than that in the sample without residual AlN. Additionally, in the case of a- and m-plane AlGaN, both planes were also observed to be etched, although the planes formed by the etching were different from those of AlN,  and
and  planes, respectively. Opposing mesa edge surfaces and several different mesas were also observed by SEM, but almost no difference in etching shape was observed. In addition, the distribution of etching amounts was not large, as detailed by the error bars in Fig. 4. Although there is a need to further investigate the chemical reaction mechanisms to ascertain the reason for the larger etching amount in the case of residual AlN, it was confirmed that at least the a- and m-planes of AlN and AlGaN were etched using heated-pressurized water. In addition, investigations are underway for AlN mole fraction 0, i.e., GaN, although details are omitted since this is different from the exfoliation mechanism of AlGaN, which is the subject of this manuscript, the details will be reported in Ref. 27. Only a brief summary of the results obtained are described hereafter. For GaN, the +c-plane was also confirmed to be non-reactive at the XPS level, while the −c-plane was confirmed to be oxidized at the XPS level, similar to the results for AlN. Meanwhile, no clear etching was observed for the a- and m-planes, in spite of slight shape changes observed by SEM observation, and a trend clearly different from AlN and AlGaN appeared in GaN. Further investigation of the dependence on AlN mole fraction and reaction conditions is needed.
planes, respectively. Opposing mesa edge surfaces and several different mesas were also observed by SEM, but almost no difference in etching shape was observed. In addition, the distribution of etching amounts was not large, as detailed by the error bars in Fig. 4. Although there is a need to further investigate the chemical reaction mechanisms to ascertain the reason for the larger etching amount in the case of residual AlN, it was confirmed that at least the a- and m-planes of AlN and AlGaN were etched using heated-pressurized water. In addition, investigations are underway for AlN mole fraction 0, i.e., GaN, although details are omitted since this is different from the exfoliation mechanism of AlGaN, which is the subject of this manuscript, the details will be reported in Ref. 27. Only a brief summary of the results obtained are described hereafter. For GaN, the +c-plane was also confirmed to be non-reactive at the XPS level, while the −c-plane was confirmed to be oxidized at the XPS level, similar to the results for AlN. Meanwhile, no clear etching was observed for the a- and m-planes, in spite of slight shape changes observed by SEM observation, and a trend clearly different from AlN and AlGaN appeared in GaN. Further investigation of the dependence on AlN mole fraction and reaction conditions is needed.
Fig. 2. (a)–(h) and (a)'–(h)' Cross-sectional SEM images of each sample before and after treatment (135 °C, 313 kPa, and 2 h). In these figures, (a)–(h) and (a)'–(h)' represent the images before and after treatment, respectively. Additionally, (a)–(h) correspond to the structure shown in Fig. 1.
Download figure:
Standard image High-resolution imageFig. 3. XPS measurement results of the (a) −c-plane and (b) +c-plane samples with and without treatment. Descriptions in the graph are assigned peak energies based on Ref. 31.
Download figure:
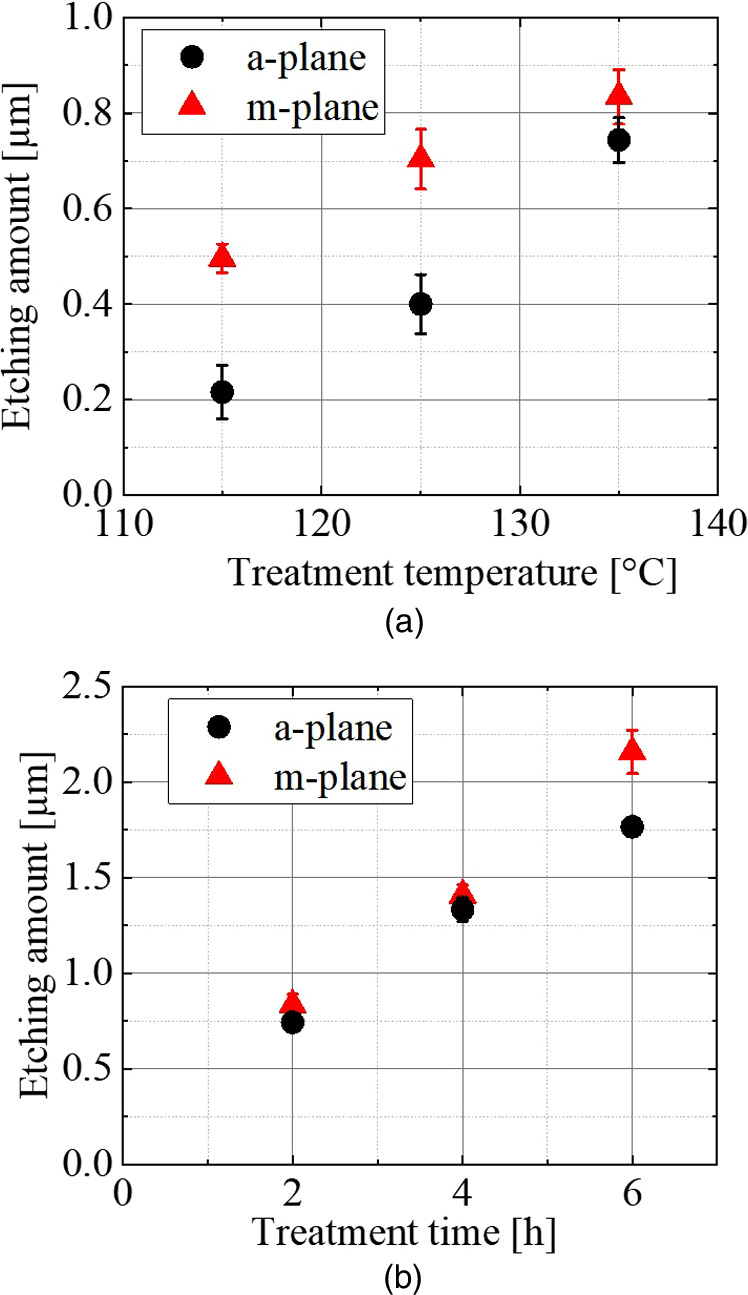
Standard image High-resolution imageFig. 4. Temperature (treatment time fixed at 2 h) and treatment time dependence (saturated vapor pressure water conditions fixed at 135 °C/313 kPa) of the etching amount.
Download figure:
Standard image High-resolution imageFigures 4(a) and 4(b) show the temperature (treatment time fixed at 2 h) and etching time dependence (saturated vapor pressure water conditions fixed at 135 °C/313 kPa) of the etching amount, respectively. Note that in this experiment, the amount of etching at each data point was measured by SEM at five different locations under the same conditions, and their average values were plotted. The variation of the data is indicated by error bars. In this experiment, samples with AlN completely removed were used. The amount of etching tends to increase with increasing temperature and etching time of saturated vapor pressure water, strongly suggesting that AlN is etched through the chemical reaction.
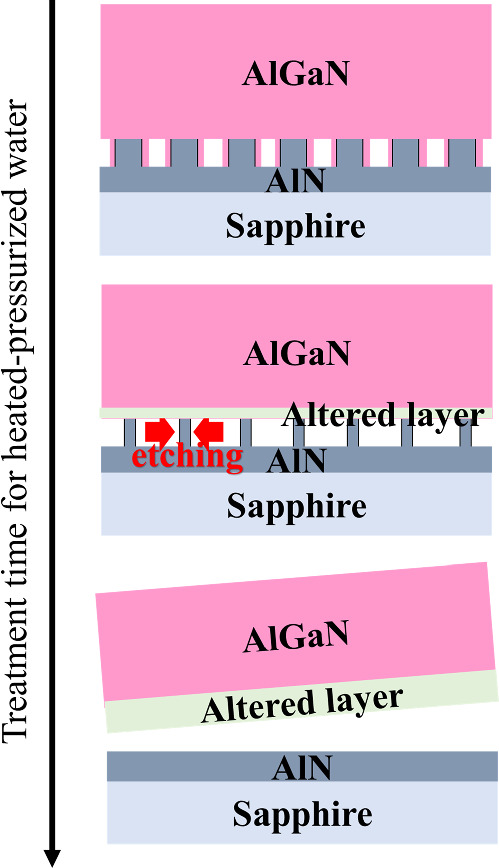
Based on these results, a schematic of the exfoliation mechanism of AlGaN fabricated on periodically formed AlN nanopillars using saturated vapor pressure water is shown in Fig. 5. It was confirmed that the +c-plane was almost chemically unreactive in the range of conditions under which the experiment was conducted; however, altered layers containing AlOOH were formed on the −c-plane, and the a- and m-planes were etched. Thus, it was deduced that the main mechanism of this exfoliation is the etching of the a- and m-plane due to the penetration of water under saturated vapor pressure into the AlN nanopillar area. 19) Moreover, the etching rate is relatively fast even for AlGaN with an AlN mole fraction of 0.62, suggesting that this method applies to AlGaN with various AlN mole fractions and is highly versatile. Furthermore, it is suggested that the proposed method applies to large-diameter AlGaN wafers if saturated vapor pressure water is allowed to penetrate the infiltrating AlN pillars.
Fig. 5. Schematic diagram of the exfoliation mechanism of AlGaN fabricated on periodically formed AlN nanopillars via saturated vapor pressure water.
Download figure:
Standard image High-resolution imageIn this study, we investigated the crystallographic plane dependence in the reaction of AlN and AlGaN with heated-pressurized water using the autoclave apparatus. Consequently, a strong crystallographic plane dependence was observed in the reaction of AlN and AlGaN using heated-pressurized water under saturated vapor pressure. The results confirmed that the main mechanism of exfoliation of AlGaN on periodically formed AlN nanopillars was the chemical reaction and etching of the a- and m-plane with heated and pressurized water. The etching rates of the a- and m-plane were fast even for AlGaN with an AlN mole fraction of 0.62. Thus, the proposed method applies to AlGaN with various AlN mole fractions and is highly versatile. The proposed method is also suitable for large-diameter wafers because etching AlGaN on the a- and m-planes causes the substrate to exfoliate.
Acknowledgments
This study was partially supported by the MOE program for the implementation of innovative infection-control and digital technologies with low CO2 emissions, JSPS KAKENHI (22H00304), NEDO Feasibility Study Program, and JST A-STEP Project (JPMJTR201D).