Abstract
We have deloveloped plastic scintillators loaded with perovskite quantum dots (QDs) to obtain efficient scintillation and enhanced detection efficiency of high-energy photons such as X-rays and gamma rays. In previous studies, the loading of the perovsite QDs was not successful to achieve efficient scintillation owing to the severe self-absorption of the QD emission, which is caused by small Stokes shift of the QDs. In the present study, we added wavelength shifting molecules to suppress the self-absorption. Among three samples, we have succeeded in enhancement in the scintillation light yield and shortening of the scintillation decay by the suppression of the self-absorption in polustyrene-based plastic scintillators added with butyl-PBD, QD-P450, and Coumarin6, and butyl-PBD, QD-P510, and Nile red. This result indicates that it is necessary to avoid the self-absorption to fully exploit the fast and efficient emission of the perovskite QDs.
Export citation and abstract BibTeX RIS
1. Introduction
Scintillators are phosphors used for real-time radiation detection and used in scintillation detectors in combination with photon detectors such as photomultipliers (PMTs) or photodiodes. Scintillation detectors operated in a pulse counting mode can detect individual ionizing radiation quanta (high-energy photons or particles) to obtain the information on their energies and arrival timings. Scintillation detectors equipped with scintillators having fast scintillation decay are required in the applications where detection capability at high counting rate or in an excellent timing resolution are necessary.
In many applications which require excellent timing resolution, scintillators based on Ce3+- or Pr3+-doped insulators have been used. 1) They have scintillation decay time constants of several tens of ns. Even faster decay is required in some applications and achieved in some insulator crystals. Among them, Auger-free luminescence (AFL) of some insulators have been used as fast scintillation. The AFL is caused by electronic transition of a valence electron to the outermost core level where holes are generated by ionizing radiation. 2) Among them, BaF2 has been used as fast scintillators owing to its subnanosecond scintillation decay around 200 nm. 3,4) Recently, our group and other groups have developed fast scintillators using CsCl-based compounds, such as CsCaCl3 5) or mixed crystals based on CsCaCl3, 6–8) Cs2ZnCl4, 9–11) Cs3ZnCl5, 10,11) CsMgCl3, 8) CsSrCl3, 8) and Cs2BaCl4, 8,12) owing their advantage of scintillation wavelengths longer than that of BaF2. Unfortunately, their scintillation lights of the AFL component are less than 2000 photons MeV−1, which limits the applications.
On the contrary to the inorganic scintillators, organic scintillators have fast scintillation decay of less than 10 ns and relatively high scintillation yields of typically 10 000 photons MeV−1. 13) Among them, plastic scintillators are suitable as fast scintillators with low cost and excellent scalability. One drawback of plastic scintillators is the low atomic numbers of the constituent elements (C, H, N, O, etc.), which results in a low interaction probability with high-energy photons such as X-rays or gamma rays and consequently low detection efficiency. One effective method of enhancing detection efficiency of high-energy photons is loading high atomic number elements. 14,15) Recently, loading of insulator nanoparticles of compounds containing heavy elements has been proven to be advantageous over loading of organometallic compounds of heavy elements. 16) To achieve the fast response of the plastic scintillators loaded with nanoparticles, non-emissive nanoparticles of ZrO2, 17,18) HfO2, 19–22) Gd2O3, 23) YbF3, 24) Bi2O3, 25–28) and HfO2-SiO2 mixed amorphous phase, 29–31) have been loaded, which results in a reduced scintillation light yield. Plastic scintillators loaded with emissive nanoparticles having a slow scintillation decay component would have worse detection capability at high counting rate. On the contrary, if we load emissive nanoparticles exhibiting fast scintillation decay, the reduction in the scintillation light yield may be avoided or the scintillation light yield may even be enhanced by the loading.
Recently, it has been reported that perovskite quantum dots (QDs) with Pb as a main constituent exhibit fast photoluminescence emission. Inclusion of Pb as the main constituent is advantageous for enhancing the detection efficiency of high-energy X-rays because of its high atomic number of 82. Utilizing fast and efficient emission of the perovskite QDs, several research groups have reported the development of plastic scintillators loaded with perovskite QDs. 32–37) However, owing to the small Stokes shift of the perovskite QDs and consequent self-absorption, the scintillation yield was reduced by the loading. 37) Also, slow decay components appeared by the loading. 37) To avoid the self-absorption, in this study, we added wavelength shifting molecules to the perovskite QD-loaded plastic scintillators.
2. Experimental methods
Perovskite QDs having peak emission wavelengths of 450, 480, and 510 nm (denoted as QD-P450, QD-P480, and QD-P510, respectively) were loaded in plastic scintillators. The perovskite QDs were supplied by Quantum Solutions, and the specifications of the perovskite QDs provided by the supplier are summarized in Table I. First, polystyrene (average Mw ≈ 350 000 and average Mn ≈ 170 000, Sigma Aldrich) was dissolved in tetrahydrofuran. Subsequently, 2-(4-tert-Butylphenyl)−5-(biphenyl-4-yl)-1,3,4-oxadiazole (butyl-PBD; Sigma Aldrich) and wavelength shifting molecules were dissolved in the solution. The quantity of butyl-PBD was 0.5 mol% to the monomer unit of polystyrene, and the weight ratio of butyl-PBD and the wavelength shifting molecules was 80:1. Butyl-PBD was added in the plastic scintillators because the efficient scintillation cannot be obtained without butyl-PBD. If we assume that the QDs were homogeneously dispersed in the plastic scintillators, the average distance between the nearest neighbor QDs is estimated to be >30 nm, which is significantly longer than general Förster radius of several nm. Hence, it is expected that direct energy transfer from the polystyrene host to the QDs is not efficient. Subsequently, the perovskite QDs were dispersed in the solutions. Finally, we obtained solid scintillators after evaporation of the solvent at RT for 1 week. We used Coumarin 6, 9,10-bis(2-phenylethynyl)anthracene (BPEA), and Nile red as the wavelength shifting molecules for QD-P450, QD-P480, and QD-P510, respectively, on the basis of the good overlap between the emission spectra of the perovskite QDs and the absorption spectra of the wavelength shifting molecules. The emission wavelengths of Coumarin 6, BPEA, and Nile red are 510, 540 nm (in film), and 600 nm, respectively. The samples are denoted as QDS-450, QDS-480, and QDS-510, respectively.
Table I. Specifications of perovskite QDs.
| Composition | Average size | Emission peak wavelength | PL quantum yield | |
|---|---|---|---|---|
| QD-P450 | CsPb(Cl/Br)3 | 7 nm | 450 nm | >40% |
| QD-P480 | CsPb(Cl/Br)3 | 9 nm | 480 nm | >50% |
| QD-P510 | CsPbBr3 | 10 nm | 510 nm | >70% |
The microstructure of the scintillators was characterized using SEM (S-4800, Hitachi). The photoluminescence excitation and emission spectra were obtained using a spectrofluorometer (F-7000, Hitachi). Photoluminescence decay behavior was analyzed using a time-correlated single photon counting spectrometer (Delta Flex 3000U–TMK2, Horiba).
X-ray-induced radioluminescence (XRL) spectra were obtained using a CCD-based spectrometer (QE Pro, Ocean Insight) and an X-ray generator (2300-HK, Rigaku) equipped with Cu target and operated at 40 kV and 40 mA. Pulse height spectra of the scintillation detectors equipped with the sample scintillators or a commercially available plastic scintillator, NE-142, were obtained for 59.5 keV gamma rays from 241Am source. The scintillators were wrapped with a Teflon tape and attached to a PMT (R7600U-20, Hamamatsu) having relatively high sensitivity at long wavelength. The output signal of the PMT was amplified using a preamplifier (113, Ortec) and a main amplifier (572 A, Ortec). The output signal of the main amplifier was fed to a multichannel analyzer (MCA; MCA-8000D, Amptek) to obtain the pulse height spectra. Scintillation time profiles were obtained using a time-correlated single photon counting method, which is called delayed coincidence method, with gamma rays as the excitation source: a 22Na source (∼100 kBq) was used as the gamma-ray source. The gamma-ray source emits a positron accompanying emission of a gamma-ray photon of 1.27 MeV. An emitted positron annihilates with a nearby electron to emit two gamma-ray photons of 511 keV in several hundreds of picoseconds. Hence, one radioactive decay event emits three gamma-ray photons in less than 1 ns. We used a fast scintillation detector equipped with a Pilot U plastic scintillator to determine the timing of the generation of gamma-ray photons. A PMT (R7400P, Hamamatsu) was used to detect the scintillation photons from the samples excited by the gamma-ray photons. The generation timings of the gamma-ray photons and the scintillation photons were determined using a constant fraction discriminator (935, Ortec), and the timing signals were used, respectively, as the start and stop signals of time-to-amplitude converter (TAC; 566, Ortec). Finally, the output signal of the TAC is supplied to the MCA to obtain the scintillation time profiles.
3. Results and discussion
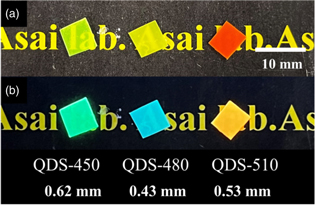
The photographs of the sample scintillators under room lighting and UV light at 365 nm are presented in Fig. 1. The thicknesses of the scintillators are also presented. In room lighting, QDS-450, QDS-480, and QDS-510 are yellow green, yellow, and orange, respectively, and the colors are consistent with those of the loaded QDs. The emission colors of QDS-450, QDS-480, and QDS-510 are light green, light blue, and yellow orange, respectively. The emission color of QDS-480 is similar to that of plastic scintillators loaded with QD-P480 without the wavelength shifting molecules, whereas those of QDS-450 and QDS-510 are different from those of plastic scintillators loaded with QD-P450 and QD-P510 without wavelength shifting molecules. 37)
Fig. 1. Photographs of sample scintillators under (a) room lighting and (b) UV light at 365 nm.
Download figure:
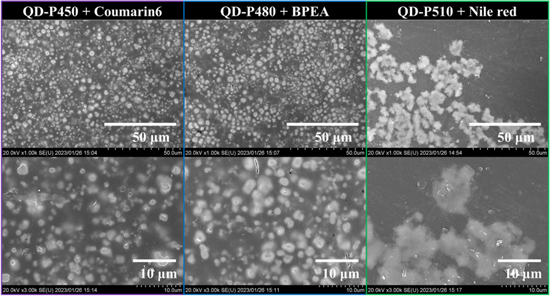
Standard image High-resolution imageThe SEM images of the sample scintillators with different magnifications are shown in Fig. 2. QDs were partly precipitated in all samples. The typical size of the precipitations was several μm in QDS-450 and QDS-480, whereas that in QDS-510 was ∼10 μm. Because the fabrication process was similar for all samples, the difference in the microstructure may be attributed to the difference in the surface modification of the QDs, which results in a difference in the aggregation behavior. Because typical range of photoelectrons of ∼60 keV is ∼100 μm, 38) the samples are considered to be quasi-homogeneous from the viewpoint of the photoelectron range.
Fig. 2. SEM images of sample scintillators with different magnifications.
Download figure:
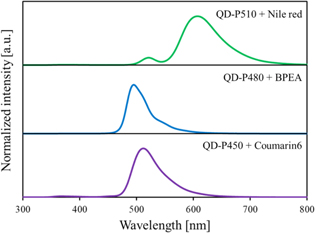
Standard image High-resolution imageThe photoluminescence excitation and emission spectra of the sample scintillators are presented in Fig. 3. In the emission spectra of all the samples, emission bands of butyl-PBD were observed at 350–420 nm. Additional emission bands of the perovskite QDs were observed at 450, 480, and 510 nm for QDS-450, QDS-480, and QDS-510, respectively. In addition, emission bands of the wavelength shifting molecules are seen at 510 and 600 nm for QDS-450 and QDS-510, respectively. For QDS-480, the emission bands of BPEA were hardly seen, possibly owing to inefficient emission in the polymer matrix.
Fig. 3. Photoluminescence excitation (dashed lines) and emission (solid lines) spectra of sample scintillators.
Download figure:
Standard image High-resolution imageThe XRL spectra of the sample scintillators are shown in Fig. 4. The XRL spectrum of QDS-450 has a dominant band at 510 nm in addition to a small band at 350–420 nm, which are attributed to the emission of butyl-PBD. The emission from the perovskite QDs is not clearly seen, which indicates that it was absorbed by the wavelength shifting molecule, Coumarin 6. On the contrary, in the XRL spectrum of QDS-480, a dominant emission band of the perovskite QDs was observed at 480 nm with a shoulder at around 540 nm owing to the wavelength shifting molecule, BPEA. The emission of the perovskite QDs was partially absorbed by BPEA. In the XRL spectrum of QDS-510, a dominant band of the wavelength shifting molecule, Nile red, was observed at around 600 nm with a weak band of the perovskite QDs at around 510 nm. This result indicates that majority of the emission from the perovskite QDs were absorbed by Nile red. The XRL spectra were different from the PL emission spectra for all the samples. The difference can be attributed to the difference in the geometry of the measurements: the PL emission spectra were obtained in a reflection geometry. Hence, emission from a near-surface region was detected. On the contrary, the XRL spectra were obtained in a transmission geometry, and the emission photons from the bulk of the samples were detected. In the latter case, the probability of the self-absorption of the emission is significantly higher, and the emission of the wavelength shifting molecules was more pronounced. This result indicates that the shift in the emission wavelength was successful in QDS-450 and QDS-510.
Fig. 4. XRL spectra of sample scintillators.
Download figure:
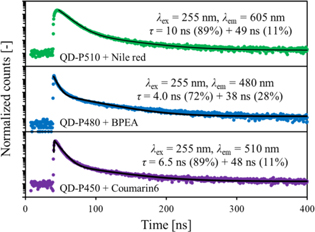
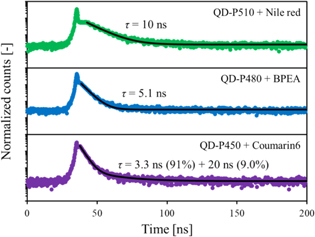
Standard image High-resolution imageThe photoluminescence decay curves of the sample scintillators are presented in Fig. 5. The excitation wavelength was 255 nm, which corresponds mainly to the excitation of polystyrene host. The emission wavelengths were at the emission maxima in the XRL spectra of the sample scintillators. The decay time constants of the sample scintillators in the present study and plastic scintillators loaded with perovskite QDs, which consist of polystyrene, butyl-PBD, and perovskite QDs (QD-P450, QD-P480, or QD-P510), in the previous study 37) are summarized in Table II. In both cases, the decay behaviors cannot be expressed as a single exponential one because of the emission from multiple emissive species (organic molecules and QDs) and non-radiative or radiative energy transfer between them. Because photoluminescence decay of butyl-PBD was ∼2 ns, the decay behaviors of the scintillators which consist of polystyrene, butyl-PBD, and perovskite QDs are attributed to the emission of the perovskite QDs. The PL decay was elongated by the self-absorption, i.e. the absorption of the QD emission and re-emission by the perovskite QDs. Based on the comparison of the decay behaviors of the samples with and without the wavelength shifting molecules, the addition of the wavelength shifting molecules significantly reduced the long component. This result strongly suggests that the addition of the wavelength shifting molecules suppressed the elongation of the PL decay by suppressing the self-absorption of the QD emission by the QDs.
Fig. 5. Photoluminescence decay curves of sample scintillators.
Download figure:
Standard image High-resolution imageTable II. Photoluminescence decay time constants of sample scintillators in the present study and plastic scintillators loaded with perovskite QDs in a previous study. 37)
| Sample | Decay time constants and relative intensities |
|---|---|
| Polystyrene + butyl-PBD + QD-P450 | 5.4 ns (45%), 20 ns (44%), 54 ns (11%) |
| QDS-450 (Polystyrene + butyl-PBD + QD-P450 + Coumarin 6) | 6.5 ns (89%), 48 ns (11%) |
| Polystyrene + butyl-PBD + QD-P480 | 2.6 ns (57%), 18 ns (27%), 104 ns (16%) |
| QDS-480 (Polystyrene + butyl-PBD + QD-P480 + BPEA) | 4.0 ns (72%), 38 ns (28%) |
| Polystyrene + butyl-PBD + QD-P510 | 3.8 ns (45%), 16 ns (40%), 93 ns (15%) |
| QDS-510 (Polystyrene + butyl-PBD + QD-P510 + Nile red) | 10 ns (89%), 49 ns (11%) |
The scintillation decay curves of the sample scintillators are presented in Fig. 6. A cusp structure around the time origin is attributed to the Cherenkov radiation produced in the samples or in the window of the PMT. The decay time constants were estimated by fitting the decay curves after the Cherenkov radiation peak. The scintillation decay time constants of the sample scintillators in the present study and plastic scintillators loaded with perovskite QDs, which consist of polystyrene, butyl-PBD, and perovskite QDs (QD-P450, QD-P480, or QD-P510), in the previous study 37) are summarized in Table III. The decay behaviors of the former and the latter samples were measured using different measurement systems; nevertheless, their time resolutions of both measurement systems are less than 1 ns and they do not affect the estimation of the decay time constants.
Fig. 6. Scintillation decay curves of sample scintillators.
Download figure:
Standard image High-resolution imageTable III. Scintillation decay time constants of sample scintillators in the present study and plastic scintillators loaded with perovskite QDs in a previous study. 37)
| Sample | Decay time constants and relative intensities |
|---|---|
| Polystyrene + butyl-PBD + QD-P450 | 11 ns (50%), 40 ns (50%) |
| QDS-450 (Polystyrene + butyl-PBD + QD-P450 + Coumarin 6) | 3.3 ns (91%), 20 ns (9%) |
| Polystyrene + butyl-PBD + QD-P480 | 12 ns (81%), 53 ns (19%) |
| QDS-480 (Polystyrene + butyl-PBD + QD-P480 + BPEA) | 5.1 ns (100%) |
| Polystyrene + butyl-PBD + QD-P510 | 8.6 ns (36%), 45 ns (64%) |
| QDS-510 (Polystyrene + butyl-PBD + QD-P510 + Nile red) | 10 ns (100%) |
The scintillation decay is discussed in the following based on the comparison of the scintillators with or without wavelength shifting molecules. For the plastic scintillators loaded with QD-P450, a fast component of 3.3 ns was observed with the addition of Coumarin 6. Also, a long component of 20 ns was observed with the addition of Coumarin 6 and is faster than the long component without Coumarin 6. Similarly to the PL decay, the scintillation decay became faster with the addition of Coumarin 6. The scintillation decay also became much faster with the addition of BPEA and Nile red for the plastic scintillators loaded with QD-P480 and QD-P510, respectively. This is possibly owing to the suppression of the elongation the PL decay by suppressing the self-absorption of the QD emission by the QDs.
The scintillation decay of QDS-450, QDS-480, and QDS-510 was significantly faster than the PL decay behaviors. This is also the case of the plastic scintillators loaded with QD-P480 and QD-P510 without the wavelength shifting molecules, as summarized in Tables II and III. This is not a general trend in scintillators because scintillation process includes additional energy transfer process in the scintillators containing the wavelength shifting molecules. The cause of the faster scintillation decay is difficult to be discussed. A possible reason is the difference in the initial excitation in PL and scintillation: in the case of PL with the excitation wavelength of 255 nm, polystyrene host is mainly excited. In this case, the emission occurs via energy transfer from polystyrene to butyl-BPD and subsequent radiative or nonradiative energy transfer to the perovskite QDs (and the wavelength shifting molecules). In the case of scintillation, because of the substantial weight fraction and high atomic numbers of the constituent elements, a substantial fraction of energy of ionizing radiation is deposited on the perovskite QDs, which accelerate the scintillation process and decay behavior.
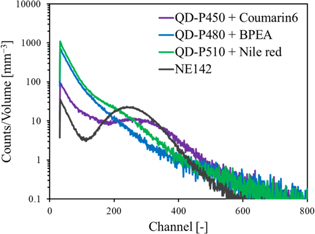
The pulse height spectra of the scintillation detectors equipped with the sample scintillators or NE-142 for 59.5 keV gamma rays from 241Am are presented in Fig. 7. The full-energy peak was broad, and the spectra was structureless, on the contrary to the case of the samples without the wavelength shifting molecules. 37) A full-energy peak was observed at around 260 channels for QDS-450. In comparison to the peak channel of NE-142 and considering the difference in the quantum efficiency of the PMT for the different scintillation spectra, the scintillation light yield of QDS-450 is estimated to be 4200 photons MeV−1. The quantum efficiency of the PMT for the measurements of the pulse height spectra was estimated based on the integral of the quantum efficiency as a function of wavelength weighed by the scintillation spectrum (i.e. scintillation intensity as a function of wavelength). For QDS-510, a small shoulder is seen at around 220 channels. If we assume that this is the full-energy peak, the scintillation light yield of QDS-510 is estimated to be 3200 photons MeV−1. No peak or shoulder was seen in the pulse height spectrum of QDS-480 possibly owing to the wavelength shifting was only partially successful as shown in the XRL spectrum in Fig. 4. The scintillation light yields of the sample scintillators in the present study and the plastic scintillators loaded with perovskite QDs in a previous study 37) are summarized in Table IV. For the plastic scintillators loaded with QD-P450, the addition of Coumarin 6 significantly enhanced the scintillation light yield. Also, the scintillation light yield was slightly enhanced for the plastic scintillators loaded with QD-P510 by the addition of Nile red.
Fig. 7. Pulse height spectra of scintillation detectors equipped with sample scintillators or NE-142 for 59.5 keV gamma rays from 241Am.
Download figure:
Standard image High-resolution imageTable IV. Scintillation light yields of sample scintillators in the present study and plastic scintillators loaded with perovskite QDs in a previous study. 37)
| Sample | Scintillation light yield (photons MeV−1) |
|---|---|
| Polystyrene + butyl-PBD + QD-P450 | 2500 |
| QDS-450 (Polystyrene + butyl-PBD + QD-P450 + Coumarin 6) | 4200 |
| Polystyrene + butyl-PBD + QD-P480 | 2900 |
| QDS-480 (Polystyrene + butyl-PBD + QD-P480 + BPEA) | — |
| Polystyrene + butyl-PBD + QD-P510 | 2900 |
| QDS-510 (Polystyrene + butyl-PBD + QD-P510 + Nile red) | 3200 |
4. Conclusions
We have developed plastic scintillators loaded with perovskite QDs with different emission wavelengths and wavelength shifting molecules to suppress the self-absorption of the QD emission by the QDs. The suppression of the self-absorption was successful for the plastic scintillators which consist of polystyrene host added with butyl-PBD, QD-P450, and Coumarin 6, and polystyrene host added with butyl-PBD, QD-P510, and Nile red, which is manifested in the suppressed contribution of long decay components in the photoluminescence and scintillation decay and the significant enhancement of the scintillation light yield. This result indicates that the suppression of the self-absorption of the QD emission by the QDs is necessary to fully exploit the fast and efficient emission of the perovskite QDs in the plastic scintillators loaded with perovskite QDs.
Acknowledgments
This research was supported in part by the Grant-in-Aid for Scientific Research (A) (No. 18H03890, 2018-2021; 22H00308, 2022-2025) and Grant-in-Aid for Scientific Research (B) (No. 21H01853, 2021-2023). Part of this research is based on the Cooperative Research Project of the Research Center for Biomedical Engineering, Ministry of Education, Culture, Sports, Science, and Technology.