Abstract
Nitrogen is a very common element, comprising approximately 78% of Earth's atmosphere, and is an important component of various electronic devices while also being essential for life. However, it is challenging to directly utilize dinitrogen because of the highly stable triple bond in this molecule. The present review examines the use of non-equilibrium plasmas to generate controlled electron impacts as a means of generating reactive nitrogen species (RNS) with high internal energy values and extremely short lifetimes. These species include ground state nitrogen atoms, excited nitrogen atoms, etc. RNS can subsequently react with oxygen and/or hydrogen to generate new highly reactive compounds and can also be used to control various cell functions and create new functional materials. Herein, plasma-processing methods intended to provide RNS serving as short-lived precursors for a range of applications are examined in detail.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
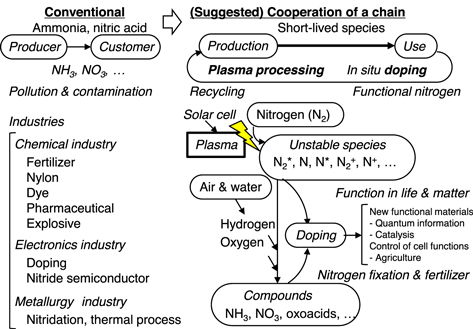
Nitrogen gas comprises 78% of Earth's atmosphere and has numerous uses in chemical industries, primarily as a component of fertilizers, nitric acid, nylons, dyes and explosives, based on the formation of ammonia via reaction with hydrogen. 1) This element also has applications in the electronics industry, in the production of transistors and diodes, and is employed when heating various steels. Many such technologies use ammonia and nitric acid as nitrogen-based raw materials (Fig. 1), as part of a value-added chain of processes involving production, industry, commerce, and use. We are currently consuming nitrogen resources generated by producers. Therefore, to ensure sustainable consumption, it is important to address the concerns of growing waste, pollution, and contamination. Sustainable consumption requires the use of nitrogen resources in cooperation of a chain of the processes. In the future, it is expected that the use of plasma technology will enable the generation of nitrogen sources, such as dopants and fertilizers, at the locations where these nitrogen materials are required. Essentially, a nitrogen cycle would be created with in situ functionalization at the point of use, employing plasma-generated reactive nitrogen species (RNS). Plasma technology will facilitate methods that produce nitrogen resources from atmospheric air at the consuming locations, thereby making recycling of the nitrogen resources possible. This would represent a revolutionary approach to the use of atmospheric nitrogen in various forms.
Fig. 1. (Color online) Nitrogen is an indispensable raw material in many industries. Conventionally, ammonia and nitric acid are used as nitrogen sources in essentially linear processes. However, plasma processing technology could allow the use of cooperative chains or cyclic systems based on functional nitrogen science that can provide short-lived unstable nitrogen-based precursors in situ on a real time basis.
Download figure:
Standard image High-resolution imageThe present review summarizes new research related to the cyclic use of nitrogen, involving novel plasma technologies capable of utilizing locally generated RNS. This paper addresses both the creation and functionalization of RNS, as shown in Fig. 2. Section 2 briefly discusses the background of functional nitrogen research, including the generation of a wide variety of RNS using plasmas. In Sect. 3, we review the various applications of functional nitrogen in materials science, such as the fabrication of quantum devices by incorporating nitrogen vacancy (NV) centers in diamonds, the use of nitrogen-doped titanium oxide as a photocatalyst, and the role of nitrogen in photosynthesis. In Sect. 4, we review the recent progress of air plasma for seed germination enhancement in plasma agriculture. In Sect. 5, we examine the numerical modeling of network reactions, especially for plasma-induced intracellular functions in cells. Section 6 examines the presence of nitrogen oxides in biological systems, including in plant defense and immune systems, cellular signaling, and the destruction of viruses and bacteria. Additionally, we discuss nitrogen oxide homeostasis that regulates phytohormone balance and promotes seed germination. To provide an understanding of the mechanisms associated with plant biosystems, the concepts of genetics, chemical biology and biochemistry are introduced. Section 7 discusses the development of plasma diagnostics and in situ analyses, with an emphasis on operando, real-time and multiple-point measurements. Lastly, this review looks at challenges related to the development and functionalization of RNS. This incorporates scientific knowledge from the transdisciplinary field of quantum electronics, informatics and mathematics, chemistry in material and life sciences, and mechanics and system engineering. Specifically, recent advances in the application of functional nitrogen to various material and biological systems show the importance of the atomic localization of nitrogen and the potential of functional nitrogen science.
Fig. 2. Overview of the present review regarding the science and technology of functional nitrogen.
Download figure:
Standard image High-resolution image2. Background
Nitrogen molecules, otherwise known as dinitrogen (N2), are extremely unreactive as a result of the strength of the triple bond between the two atoms. As early as the 1770s, Carl Wilhelm Schiele referred to nitrogen as "foul air" but to oxygen as "fire air." 2) At present, inorganic nitrogen is primarily used in the form of either ammonia or nitric acid. As an example, synthetic fibers such as nylon as well as a variety of electronic devices based on nitride semiconductors are synthesized from ammonia.
2.1. Past and present production of ammonia and nitric acid
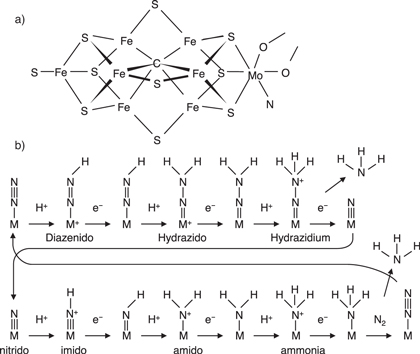
The Haber–Bosch (HB) process, initially developed in the 1910s, is based on the reaction N2 + 3H2 → 2NH3 at pressures above 10 MPa and temperatures between 400 °C and 500 °C with an iron-based catalyst bed. This has since become a common nitrogen fixation method but has the drawback of requiring extreme conditions. Thus, the synthesis of ammonia at ambient temperature and atmospheric pressure would be highly beneficial and has been widely researched. Bacterial nitrogenase enzymes are known to have a Fe–Mo complex center, as shown in Fig. 3(a) and the conversion of dinitrogen to ammonia occurs through a catalytic cycle involving reactive intermediates, such as diazenido (–N=NH), hydrazido (–N–NH2), hydrazidium (–N–NH3), nitrido (≡N), imido (=NH), amido (–NH2), and ammonium (–NH3) complexes. 3,4) The Nishibayashi group recently demonstrated a molybdenum-based catalyst that promotes the formation of ammonia from dinitrogen at temperatures and pressures close to ambient. 5) In addition, Legare et al. reported that a solid phase reductant and acid reagents such as B(OH)3 can be used as protonating agents in the reduction of dinitrogen to ammonia. Legare et al. demonstrated a complex series of reactions involving protonation to form hydraziono diradicals in a one-pot synthesis. 6)
Fig. 3. (a) Molecular structure of the FeMo complex center in nitrogenase. (b) Conversion of dinitrogen to ammonia, involving the formation of dinitrogen complexes as intermediates, in a process known as the Chatt cycle. 3) Cooperation of reductants (electron donor) and oxidants (acid reagent) play a key role in the transformation of dinitrogen to ammonia.
Download figure:
Standard image High-resolution imageIn plants, rhizobium bacteria act to fix nitrogen to generate nitrite nitrogen (NO2–N) or nitrate nitrogen (NO3–N), both of which are essential nutrients. However, the direct use of dinitrogen as a raw material for fertilizer has not yet been achieved. The enzymatic reactions involving nitrogenases by which rhizobium bacteria fix nitrogen are believed to be catalyzed by iron-sulfur clusters or molybdenum metal complexes. 7–9) (Fig. 3) In this process, an iron site binds with dinitrogen and catalyzes the formation of ammonia via the generation of a hydraziono diradical and distal nitride (Fe≡N + NH3). (Fig. 3)
Prior to the invention of the HB process, electrical discharges that generated plasmas were used as a means of nitrogen fixation. 10) After the HB process was introduced, the electrical discharge-based nitrogen fixation became obsolete, since this technique could not be efficiently scaled up and also required a significant amount of electrical energy. 11,12) In 1900s, gaseous electrical discharges were limited by the use of simple direct current sources to control electrical currents. Discharge technologies developed over time, with the use of short-pulsed high-voltage, high-frequency, and microwave discharge power sources. At present, the energy produced by plasma generation has become efficient. However, the economic cost of the plasma-based process is higher than that of the HB process, which is directly powered by fossil fuels. An electrical discharge-based process is required to use costly electricity; an indirect use of primary energy sources. From another viewpoint, the plasma-based process can be used at any location where nitrogen is necessary. Therefore, this review discusses the in-situ utilization of nitrogen using atmospheric air plasma technology.
2.2. Atmospheric air plasma generation
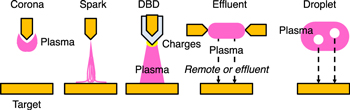
Briefly, the electric breakdown of gases results in an avalanche of ionization due to accelerating primary electrons in a static electrical force caused by applying high electric voltages. 13) Figure 4 shows the schematics of the plasma sources. The ionized charges cause coronal plasma and transient sparks, forming filamentary discharges. An electrode is at least covered by a low-electrical-conductivity material. Electric current flows only when the polarity of the applied voltage changes and a plasma discharge is ignited. This basic mechanism is called the dielectric barrier discharge (DBD). In addition, plasma is generated independently and remotely from the target, and reactive species transfer indirectly to the target surface through effluent gases. Moreover, there is another configuration in which processed droplets flow inside the plasma region and the processed droplet or mist transfers to the surface. At atmospheric pressure, the electric breakdown is not stabilized, and a transition of the thermal arc occurs as the discharge current increases. Therefore, the prevention of thermalization, which is caused by the higher electron collision-induced momentum transfer than the lower cooling mechanisms on the ambient and surrounding walls outside the discharge region, is extremely important. Meanwhile, electron-related phenomena, including ionization, dissociation, and excitation, produce reactive species at lower temperatures than thermal processes. In atmospheric air, plasmas, reactive oxygen, and nitrogen species (Table I) are produced. 14) A detailed list has been provided by Ishikawa. 15)
Fig 4. (Color online) Schematics of plasma sources and configurations of treatments. DBD stands for dielectric barrier discharge.
Download figure:
Standard image High-resolution imageTable I. A list of representative reactive oxygen and nitrogen species (RONS), as previously listed in Ref. 14. A detailed list is presented in Ref. 15.
| Reactive oxygen species (ROS) |
| Superoxide (O2), hydroxyl (OH), hydroperoxyl (HO2) |
| H2O2, ozone (O3), singlet oxygen (O2 1Δg), etc |
| Reactive nitrogen species (RNS) |
| Nitric oxide (NO), nitrogen dioxide (NO2), nitrate radical (NO3), nitrous acid (HNO2), nitrosyl cation (NO+), nitroxyl anion (NO−), dinitrogen trioxide (N2O3), dinitrogen tetroxide (N2O4), dinitrogen pentoxide (N2O5), peroxynitrite (ONOO−), peroxynitrate (O2NOO−), peroxynitrous acid (ONOOH), etc. |
Even so, plasma discharge may be a viable approach to enhancing catalytic reactions at low temperatures because such discharges can promote the dissociation of the stable N≡N bond (bond dissociation energy of 9.76 eV) and also generate ultraviolet radiation. As such, the concept of plasma-assisted nitrogen fixation at low or moderate temperatures and atmospheric pressure has recently attracted much attention, especially with regard to plasma catalysis and plasma-treated solutions.
2.3. Plasma catalysis
Plasma discharges have been applied to chemical reactions in several contexts. Plasma can dissociate molecules by providing bond-breaking energy through stepwise vibrational excitation. This leads to (1) the formation of pre-dissociative species in the gaseous phase and (2) radical-enhanced reactions. The surface of the catalysts is exposed by electrons, ions, and the chemically activated neutral species generated in the plasma. Impingement activates the surface, leading to (3) activation of the catalytic surface induced by thermal energy, (4) photocatalytic activation, (5) build-up of electric field and electronic charges on the surface to assist in reduction and (6) acid-base pair formation. The effective catalytic formation of ammonia can be regarded as acid hydrolysis of the intermediate complexes. (Fig. 3)
As an example, Kim et al. reported that a ruthenium-based catalyst could be used for ammonia synthesis in conjunction with a plasma-assisted catalytic reactor. 16) The Haruyama group also demonstrated ammonia production using ultraviolet radiation at the interface between a plasma and an aqueous phase. 17) In 2015, Patil et al. published a review of plasma-assisted nitrogen fixation 18) and further extensive reviews of nanocatalysts for plasma-assisted nitrogen fixation were published in 2020. 19,20) Hong et al. demonstrated that the electric field associated with a plasma may enhance catalytic surface reactivity by accelerating the donation and acceptance of electrons. 21–23) Zhang and Oehrlein reviewed plasma-assisted catalytic surface reactions and compared these with thermal processes, 24) and another review provided a summary of progress in the study of plasma-assisted catalytic reactions. 25–27) Kambara et al. reported the plasma-assisted decomposition of ammonia for the production of hydrogen using a plasma membrane reactor. 28) Plasma catalysis is gaining interest as a sustainable method for material synthesis with lower energy input. However, research on the topic has not yet assessed any industrialization issues, such as catalyst life and economic cost. The advantage of plasma catalysis is that the catalytic surface is effectively activated by facilitating chemically reactive radicals and other energetic particle irradiations, leading to activation of the catalytic reaction on the surface via the enhancement of electron transfer from sources to products.
2.4. Plasma-treated solutions
Plasma treatment of water under ambient air has attracted attention, based on the use of atmospheric air plasma sources. Ikawa et al. reported that plasma-activated water (PTW) is able to kill bacteria at low pH values due to the formation of hydroperoxy (HOO·) radicals. 29) In addition, Lukes et al. established that plasma-activated water (PAW) contained H2O2 and HNO2 in addition to peroxynitrite (ONOO−) ions. 30) Kurake et al. demonstrated that applying a plasma to cell culture media generated both H2O2 and HNO2, which killed cancer cells through a synergistic process. 31) The reaction mechanisms of these species in the gaseous and aqueous phases were also examined 32) and it was determined that reactive oxygen species (ROS) were able to selectively kill cancer cells in the presence of normal cells. The synergistic effects of ROS and RNS together with other organic compounds play an essential role in this selective phenomenon, suggesting that plasma-based cancer therapies may be viable. Tables II and III tabulate aqueous concentrations and primary routes of representative ROS and RNS. Bradu et al. reviewed the use of PTW in agriculture. 33) RNS have been found to activate various physiological processes in plants, such as seed germination and plant growth. Progress in the study of the PTW and PAW has been reviewed elsewhere. 34,35)
Table II. List of aqueous concentrations of representative reactive oxygen and nitrogen species (RONS) in the plasma-activated water (PTW) and PAW. DBD stands for dielectric barrier discharge.
| References | Machala 36) | Lukes 37) | Chauvin 38) | Heirman 39) | Kruszelnicki 40) |
|---|---|---|---|---|---|
| Plasma source | Spark | Corona | DBD | Effluent (kINPen) | Droplet |
| H2O2 | 600 μM | 0.22 mM | 1.6 mM | 600 nM | 570 mM |
O2
 − −
| — | — | — | 40 nM | 1.1 μM |
OH
| — | — | — | — | 0.3 μM |
| NO2 − | 300 μM | 0.10 mM | 0.5 mM | 50 nM | — |
| NO3 − | 1.2 mM | 0.14 mM | 0.4 mM | 20 nM | 570 mM |
| ONOO− | — | — | — | 1 nM | 0.09 μM |
Table III. Primary route for loss of a part of ROS and RNS. 41)
| Species | Primary route for loss | Lifetime |
|---|---|---|
| H2O2 | 2H2O2 → O2 + 2H2O | Stable |
| O2 .− | H+ + O2
 − ↔ HO2 − ↔ HO2
| 10–6 s |
NO + O2 + O2
 − → ONOO− − → ONOO−
| ||
OH
| OH + OH + OH → H2O2 → H2O2
| 10–10 s |
| NO2 − | H+ + NO2 − ↔ HNO2 | Stable |
NO + NO2
− → N2O + O2
– + NO2
− → N2O + O2
–
| ||
| 2 NO2 − → N2 + 2 O2 – | ||
| NO3 − | H+ + NO3 − ↔ HNO3 | Stable |
| ONOO− | H+ + ONOO− ↔ ONOOH | 10–3 s |
2.5. Plasma generation of RNS
Electron collisions are able to rupture the stable triple bond of nitrogen under some conditions. This process involves excitation, dissociation, ionization and recombination and is capable of synthesizing a variety of RNS together with metastable forms of excited state nitrogen and vibrationally excited nitrogen molecules.
Recent progress in the plasma generation of RNS has shown that a combination of two plasma sources in conjunction with humid conditions can efficiently generate N2O5. 42) Takashima et al. reported that spraying water droplets into an atmospheric air plasma suppressed the formation of ozone 43) and other groups have developed different plasma sources on this basis. 44–46) The low-temperature-operated source generates a high level of ozone while the other high-temperature-operated source can provide high NO concentrations. A plasma that is rich in N2O5 can also be used as an RNS source because the dissolution of N2O5 in water immediately generates nitrite and nitrate anions (NO2 −, and NO3 −). This PTW can be used for the sterilization of bacteria and inactivation of viruses, as well as the activation of plant immune systems. 47) Furthermore, although a different plasma source was used, another study demonstrated that NH4NO3 was generated in plasma-treated ammonia water. 48) There have been reports of the formation of a wide variety of RNS using atmospheric air plasma discharges.
Presently, various types of plasma sources have been developed by many researchers and engineers. Among the requirements for this development, one is the selective formation of selected species, as described above in the case of N2O5. Uchida et al. reported that the stoichiometry of N2 and O2 changed the concentration ratio of NO2 − to H2O2. 49–51) However, a comprehensive understanding of this process and sophisticated control of RNS generation have not yet been achieved. In such systems, the RNS that reach the reaction surface adhere to or penetrate into the substrate, and this process is termed nitrogen doping. This doping, in turn, can lead to functionalization that modifies properties such as hardness, electrical resistance and magnetism. Considering this situation, the directionality of plasma source development should be to precisely control any quantitative dosages for each reactive species at any spatial location in material applications. As described in Sect. 3.3, nitrogen-doped in a lattice system is sensitive to the location of nitrogen atoms, for example, whether substitutional or interstitial sites modify the photocatalytic activity of TiO2. Further developments are required to improve the atomic control of nitrogen localization in lattice systems. In biological applications, the homeostatic responses of living organisms play a key role in the dosing of reactive species generated in plasma. Even as the homeostatic regulation of living organisms is retained under natural environmental stresses, mixed concentrations of ingredients or constituents should be dosed. As described in Sect. 6.1, RNS in the plant defense system and phytohormone balance for seed germination are seen in natural responses. At the molecular level, artificial RNS also affects these regulations in plant and cell systems. The challenge arises from the complexity of biological responses that are represented by reaction networks, as described in Sect. 5. In other words, macroscopic physiology as phenotypes in plant immune responses and seed germination promotion may control the mesoscopic reconnection of biochemical reaction networks through microscopic modifications induced by molecular level reactions. Thus, novel methods that combine measurements and manipulations are required for plasma sources.
3. Material applications
In this section, we introduce the applications of nitrogen in the field of materials science.
3.1. Quantum devices
Quantum information technology has become a hot topic. Quantum bits (qubits) are based on the superposition of multiple states, which is equivalent to the quantum entanglement associated with the Einstein, Podolsky and Rosen paradox. 52) In quantum mechanics, quantum states can be represented by the superposition of basis vectors. As an example, if the vectors ∣0> and ∣1> are defined, these vectors can undergo coherent superposition to produce a qubit. Qubits have been realized by controlling the spin of electronic charges, based on employing superconductor junctions, ion-traps, photons or quantum dots. 53)
Fullerenes are a family of mesh-like carbon allotropes, and endohedral fullerenes can be obtained by incorporating nitrogen atoms into C60, to isolate the electronic spin of single nitrogen atoms.
54) Coupling between this endohedral fullerene (N@C60) of spin S = 3/2 and C60
 − radical anion (S = 1/2), for instance, offers implementation of quantum spin manipulation.
55,56) Since multiple quantum states can be superposed, this configuration can act as a qubit with a long quantum decoherence time. It has been reported that nitrogen can be incorporated into fullerenes using plasma technology,
57–60) although it should be noted that this process is different from technologies that incorporate nitrogen into various other molecules. The isolated spin of a nitrogen atom and the nuclear spins of isotopes such as 31P, 29Si and 13C in a suitable matrix can be used as qubits, as described in the section below.
− radical anion (S = 1/2), for instance, offers implementation of quantum spin manipulation.
55,56) Since multiple quantum states can be superposed, this configuration can act as a qubit with a long quantum decoherence time. It has been reported that nitrogen can be incorporated into fullerenes using plasma technology,
57–60) although it should be noted that this process is different from technologies that incorporate nitrogen into various other molecules. The isolated spin of a nitrogen atom and the nuclear spins of isotopes such as 31P, 29Si and 13C in a suitable matrix can be used as qubits, as described in the section below.
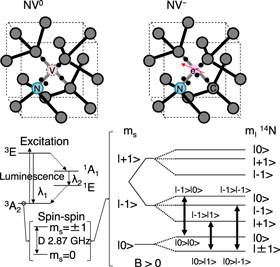
Recently, diamond has attracted attention as a potential component of quantum computing devices 61–64) because dangling bonds and nitrogen complex centers in this substance can provide extremely long coherence times. These NV centers can have negative, neutral or positive charges, and negatively charged NV centers are the most effective at storing quantum information. Figure 5 shows a schematic illustration of the neutral NV center (NV0) and negatively charged NV center (NV−). For NV0, transitions between the triple electronic ground state 3A2 and the excited state 3E (λ1 ∼ 637 nm), and the singlet 1E and 1A1 (λ2 ∼ 1064 nm), are optically active. Applying the external magnetic field of B, Zeeman shifts are observed by the degeneracy of the spin momentum sublevels, ms = ±1. Furthermore, as an electron is trapped at the vacancy site, coupling between the nuclear spin I of nitrogen and an electron spin is split into sublevels of the degeneracy of spin quantum numbers, ms and mI. 65) This two-spin system holds quantum states and the optically active transitions (thick arrows) can be used for both the operation and observation of qubits.
Fig 5. (Color online) The quantum properties of the nitrogen-vacancy (NV) center defect in diamond. The negatively charged NV center (NV−, right) is suitable for the operation and detection of quantum entanglement of two qubits with a long coherence time by using the optical active transitions.
Download figure:
Standard image High-resolution imageNeumann et al. reported that the optical detection of magnetic resonance analysis of NV centers is sensitive to temperature and external magnetic and electric fields. 61) Using the environmental sensitivity of the NV centers in diamond, nano-quantum sensors can be realized and this phenomenon has been widely researched, especially with regard to quantum magnetometry. 61) The prolonged coherence of two qubits can also be used for the secure transmission of data, and the formation of EPR pairs from multiple qubits is referred to as a quantum network. The information on such networks can be stored and transmitted securely.
3.1.1. Formation of nitrogen-vacancy center in artificial diamond
"Man-made diamond" was firstly reported by using the so-called high-pressure high-temperature method, in which diamond stable state was realized with catalytic metals. 66) Nowadays, by using this method, high-purity single crystal diamond with 10 × 10 mm2 is commercially available. 67) However, this method has been considered hard to achieve inch size substrate. Chemical vapor deposition (CVD) is another option. 68) Various plasma and radical sources were verified to be an alternative method to prepare inch-sized wafers. 69–73) Among them, microwave plasma CVD is nowadays widely adopted to prepare quantum as well as electronic grade samples where impurity control is severely required, because one may not have to place electrodes around the top surface of the substrate.
Single crystal diamond is a cubic crystal, whose cleavage surface is {111}. Because twins are easily formed on {111} surface, {100} surface is usually adopted as the top surface for the CVD growth. Because of the enhancement of the growth rate and stabilization of {100} surfaces, nitrogen is sometimes adopted as a part of the source gas mixture. 74) Nitrogen is incorporated into the CVD layer during the growth and forms various kinds of defect centers. Among them, nitrogen-vacancy center with spin, which is called as NV−, has attracted in the field of quantum metrology, computing, and communications. 75)
NV− itself is a quantum element with an outstanding spin property of long coherence time and offers access to isolated quantum systems that can be initialized and read out optically even at room temperature. Such spin coherency is based on a higher Debye temperature of around 1840 K of diamond crystal, which can suppress decoherence factors associated with diamond lattice deformation and distortion. Diamond, consists of carbon, is a biocompatible material, which can easily spread into application fields of healthcare, medical, and food hygiene as well as solid-state semiconductors.
Basically, the NV is excited by a 532 nm green laser and emits red fluorescence around 637 nm, and there are two relaxation processes depending on the initial spin state based on a triplet ground state with zero-field splitting around 2.87 GHz. Electrons at a ground state of ms = 0 can emit red fluorescence and relax back to the ground state ms = 0. On the other hand, magnetically resonated electrons on ms = ±1, these electrons are also excited by a green laser but relax down to the ground state by a non-radiative process. These two relaxation passes (radiative and non-radiative) are basic of NV quantum sensing by optically detected magnetic resonance. Moreover, the spin initialization by optical pumping, in which electrons always return back to a ground state of ms = 0 by excitation and relaxation process, is a unique advantage for high sensitivity even at room temperature.
Methods to prepare NV− center are used in electron beam irradiation, ion implantation, and CVD. The electron beam irradiation can introduce vacancies in diamond and the post-annealing process can efficiently create NV centers in nitrogen-doped diamond bulk. In the case of ion implantation, one may be able to control location and density precisely, mainly at surface. 76) In case of CVD, while several methods have been confirmed as candidate methods, such as direct current discharge and plasma jet, microwave plasma CVD has been widely studied. The tuning of the growth condition and use of appropriate crystal surfaces realized enhancement of the spin-coherence time and doping efficiency.
3.1.2. Plasma CVD engineering of diamond
Achard et al. reviewed the various plasma-assisted CVD methods, which can allow the growth of diamonds having NV centers with long-coherence time for use in highly sophisticated quantum devices. 77) Basically, using CVD, nitrogen can be incorporated directly into a highly crystalline matrix by in situ doping. Work to date has addressed the need to reduce impurities so as to obtain long coherence times due to availability of single isolated spins. The spatial localization of nitrogen atoms within the diamond matrix is also a key technology, based on controlling the positions of nitrogen on the atomic scale while considering the surrounding spins. 77) Thus, CVD engineering is indispensable to the future fabrication of quantum devices.
Watanabe et al. reported the growth of diamond with CH4, N2 and H2 mixtures in grooves that were formed artificially on the crystalline faces of the substrate. This technique allowed the selective distribution of NV centers in the grooves. 78) Lobaev et al. determined that the efficiency of nitrogen incorporation depended on the gas pressures, methane, nitrogen and hydrogen flow rates, and crystal miscut angle. As the nitrogen flow rate increased, the growth rates of diamond decreased but layers with a greater degree of nitrogen doping were grown. 79,80) Meynell et al. assessed crystal miscuts to evaluate the in situ doping of nitrogen based on a CVD diamond growth mechanism in which carbon adatoms were incorporated at step-edge sites, and determined that nitrogen incorporation was enhanced by the formation of hillock defects. 81)
Tallaire et al. reported that N2O gas could be used to generate nitrogen-related defects in diamond, and fabricated a single diamond crystal with a thickness on the millimeter scale and a nitrogen concentration of 26 ppm. The subsequent irradiation of this specimen with high energy electrons and in situ annealing converted the nitrogen into NV centers at a concentration of 5 ppm, with a simultaneous change in the color of the crystal to pink. 82,83) De Oliveira et al. demonstrated that helium ion irradiation followed by thermal annealing could be used to control the formation of NV centers in diamond, and reported spin coherence times up to 50 μs at depths less than 5 nm. 84) There have also been other publications related to the fabrication of NV centers. 85–92) Plasma-assisted CVD is a useful means of forming NV centers while controlling the density, size and location of the centers so as to produce various quantum devices.
3.2. Application of nitrogen in electronics
The doping of impurities into a crystal lattice can change the characteristics of a material. Such effects are based on adjusting the stoichiometry of the substance being processed and can tune the acoustic, elemental, chemical, electrical (such as resistance, piezo and pyro characteristics), magnetic, mechanical, optical and thermal properties of the material.
Materials such as semiconductors are vital to the fabrication of ultra-small, ultra-high speed, ultra-low power electronic devices, and these may also require nitrogen doping. Nitrogen doping in a silicon dioxide film used as a gate insulator can effectively suppress the diffusion of boron originating from a doped poly-silicon gate through the insulator film. In the case of ultra-large-scale integrated semiconductor devices, nitrogen-doped silicon dioxide films serve as gate-insulating materials. 93–95) These films are referred to as silicon oxynitride (SiON) layers. The concentration profile of nitrogen in the film is localized at the interface between the metal or semiconductor and the bulk insulating SiON film, as exampled by a stack of 0.5 nm-level SiN layer and bulk 1.0 nm-level bulk SiON. Optimization of this profile can allow the realization of low power and high drivability transistors. In future devices, nitrogen doping will need to be controlled at the sub-nanometer scale.
3.3. Visible light-responsive photocatalysts
Nitrogen-doped TiO2 acting as a visible light-responsive photocatalyst has been prepared via N+ implantation and found to promote the photocatalytic degradation of methylene blue (MB) under visible light. X-ray absorption near-edge structure (XANES) analyses indicated that the nitrogen in this material had two possible chemical states: photocatalytically active N substituted at active O sites and inactive NO2 and/or N–N species. Investigations using the depth-resolved N K-edge energy loss near edge structure (ELNES) technique confirmed these two types of N, which varied depending on the N concentration, and the local N concentration that provided an effective visible light response was estimated to be less than 1.8 atom%.
3.3.1. Nitrogen ion-implanted TiO2 photocatalysts
TiO2 is one of the most well-known photocatalysts, and the photocatalytic reactions at the surface of this oxide have numerous practical applications, such as environmental remediation. 96–101) Being a wide band gap oxide semiconductor, TiO2 exhibits photocatalytic activity under UV light although, unfortunately, this radiation accounts for only a small fraction of the solar spectrum. In 2001, Asahi et al. reported that doping TiO2 with nitrogen narrowed the band gap, thus providing a visible light response. 102) Furthermore, previous investigations have demonstrated that nitrogen doping generates new optical absorption bands in the visible light region and that the extent of absorbance increases with increasing nitrogen levels. 103–105) However, photo-absorbance is not always linearly proportional to photocatalytic activity 106,107) and so it is important to understand the chemical state and optimum concentration of nitrogen to obtain the most effective visible light response. In order to investigate these factors, an ion implantation technique has been developed as a means of nitrogen doping, because this process can easily control the depth and concentration of the implanted ions by varying the ion beam energy and fluence. Depth-selective chemical state analyses of N+-implanted TiO2 photocatalysts have also been performed by measuring the XANES and ELNES spectra.
3.3.2. Nitrogen-implanted TiO2
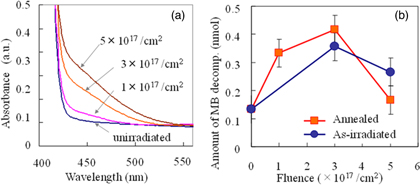
The samples were comprised of TiO2(100) single crystals (5 × 5 × 0.5 mm3). Mass analyzed 100 keV N2 + ions (50 keV/N+ ion−1) were injected into the samples at room temperature, perpendicular to the sample surface at an N+ fluence ranging from 1 to 5 × 1021 m−2. After ion implantation, some samples were heat-treated at 573 K for 2 h in air. Optical absorption spectra of the samples before and after N+ implantation were acquired at room temperature using a spectrometer (JASCO V-670). A typical photocatalytic reaction experiment consisted of placing an N+-implanted sample in 0.5 ml of an aqueous MB solution (9.8 μ mol l−1) followed by exposure to visible light generated using a 15 W Xe lamp with a cut filter for λ > 430 nm. Figure 6(a) presents the optical absorption spectra of the TiO2 samples before and after the N+ implantation process. The absorption edge corresponding to the bandgap of TiO2 was observed at approximately 410 nm prior to implantation and a new absorption band in the visible light region became more intense as the nitrogen dose was increased. The photocatalytic activity of this material was maximized at a fluence of 3 × 1021 m−2 and then decreased with further increases in the fluence, suggesting that there was an optimum nitrogen concentration [Fig. 6(b)]. The sample doped using a fluence of 5 × 1021 m−2 followed by heat treatment at 573 K was almost photocatalytically inactive under visible light. This confirmed that visible light responsiveness was not directly connected to the photo-absorbance of the material.
Fig 6. (Color online) (a) Variations in the optical absorption spectra of N+-implanted samples with changes in N+ fluence. (b) Methylene blue (MB) decomposition during 2 h of visible light irradiation as a function of N+ fluence.
Download figure:
Standard image High-resolution image3.3.3. Active and inactive nitrogen sites in TiO2
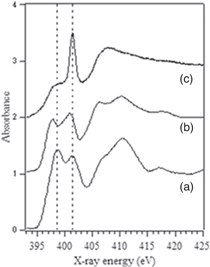
N K-edge XANES spectra of the N+-implanted TiO2 samples were obtained on the UVSOR-II BL-8B1 station at the Institute for Molecular Science and on the BL-7U station of the Aichi Synchrotron Radiation Center. Data were recorded at room temperature in the total electron yield mode to probe near the surface of the samples. 108) Figure 7 provides N K-edge XANES spectra of the N+-implanted TiO2 samples and of TiN powder. The similar features of these spectra suggest that the N in the sample treated using a fluence of 3 × 1021 m−2 (the active photocatalyst) was in a chemical environment similar to that in TiN. More thorough observations showed that a double peak at approximately 400 eV generated by the sample was shifted to lower energy compared with that for TiN. In contrast, the XANES spectrum of the sample treated with an N+ fluence of 5 × 1021 m−2 followed by heat treatment (the inactive photocatalyst) showed a distinct single peak at approximately 401 eV. Therefore, a double-peak structure around 400 eV corresponds to an active nitrogen species while a single peak at 401 eV represents an inactive nitrogen species. These spectral features were well reproduced by theoretical calculations using the FEFF code. 109) The double peak obtained from the active photocatalyst was reproduced using a model in which one O atom in TiO2 was substituted with an N, while the single peak at 401 eV generated by the inactive photocatalyst was attributed to the formation of NO2 and/or N–N bonds. These results indicated that the occupation of the oxygen sites by nitrogen effectively provided visible light responsiveness.
Fig 7. (a) N K-edge XANES spectra of N+ implanted TiO2 at a fluence of (a) 3 × 1021 m−2 and (b) 5 × 1021 m−2 and (c) after heating at 573 K for 2 h.
Download figure:
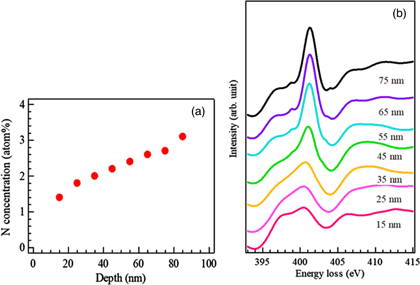
Standard image High-resolution imageThe optimum nitrogen concentration required to generate photocatalytically active nitrogen was investigated via quantitative analysis of the depth-resolved ELNES spectra of the active photocatalyst. Depth-resolved EEL spectra of sample cross-sections were recorded using a Gatan ENFINA 1000 spectrometer attached to a JEM200CX transmission electron microscope operated at 200 kV. 110) The local N concentrations were also evaluated, as shown in Fig. 8(a). A Monte Carlo calculation (SRIM code 111)) indicated that the implanted N atoms were distributed to depths of 180 nm from the surface, with the peak concentration at approximately 90 nm. Figure 8(b) presents the depth-resolved profiles extracted from the N K-edge ELNES data for the active photocatalyst. A double-peak structure around 398–401 eV was again observed near the surface region, in good agreement with the XANES spectrum of the same sample. A distinct single peak at approximately 401 eV gradually increased in intensity with increasing depth, while a single peak feature was observed during the XANES analysis of the photocatalytically inactive sample. These results suggested that the two different spectral profiles corresponded to variations in the local nitrogen concentrations as well as the photo-catalytic responsiveness. The single peak at 401 eV was predominant at depths below approximately 25 nm, and so the local concentration of doped nitrogen that most effectively provided a visible light response was estimated to be less than 1.8 atom%.
Fig 8. (Color online) (a) Depth distribution of the concentration of implanted nitrogen atoms as calculated from ELNES data. (b) Depth-resolved N K-edge ELNES data for a N+-implanted TiO2 sample treated at a fluence of 3 × 1021 m−2.
Download figure:
Standard image High-resolution image3.3.4. Atomic-scale localization of nitrogen for effective photocatalysis
The implantation of nitrogen ions into TiO2 samples has been investigated with the aim of determining the chemical state and concentration of nitrogen that is most effective with regard to visible light-responsive photocatalysis. Two chemical states of nitrogen have been identified depending on the local nitrogen concentration, using XANES and ELNES. The photocatalytically active species were determined to be nitrogen atoms substituted at O sites in the TiO2, while the inactive states were associated with the formation of NO2 and/or N–N species at higher N concentrations. Depth-resolved N K-edge ELNES measurements showed that the most effective local N concentration was less than 1.8 atom%.
3.4. Free charge and free spin: functional nitrogen in materials science
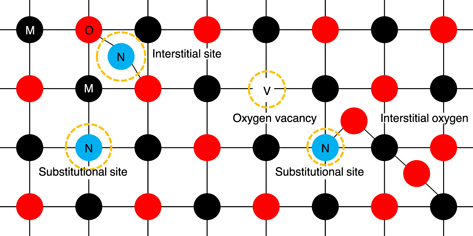
The preceding sections reviewed nitrogen doping into quantum devices and photocatalysts, in which the atomic position of nitrogen in the matrix lattice plays a significant role. Generally, metal oxides have a band gap between the valence and conduction bands. When an oxygen atom is eventually removed from the lattice position, an oxygen vacancy is formed (as schematically shown in Fig. 9), and unoccupied dangling orbitals create deep defect states in the band gap. By substituting the oxygen atom of the lattice with a nitrogen atom, the dangling bond of nitrogen generates a mid-gap energy state. In addition, interstitial nitrogen can be incorporated into the lattice and eventually, oxygen vacancies can be formed. For transition metals, atomic configurations become complex, owing to the d-orbitals. However, for simplicity, once electrons migrate from the valence band to the conduction band, for example, optical absorption, electron-hole (e−– h+) pairs are generated. In catalytic reactions, these electrons yield highly oxidative species. For example, redox reactions involving the reduction of O2 + e− to O2
 − and oxidation of OH− + h+ to OH
− and oxidation of OH− + h+ to OH can occur, based on nitrogen dangling bonds and electrons in the conduction band. Thus, the localization of doping nitrogen into the lattice is essential to obtain chemical activity.
can occur, based on nitrogen dangling bonds and electrons in the conduction band. Thus, the localization of doping nitrogen into the lattice is essential to obtain chemical activity.
Fig 9. (Color online) A schematic of substitutional and interstitial sites of metal oxide lattice. The black circles represent metal (M) atoms, the red circles represent oxygen (O), the void represents an oxygen vacancy (V), and the blue circle depicts nitrogen (N). Precise control of atomic localization of nitrogen in lattice matrixes and its neighboring environments is essential for the emergence of a new functional material property via a redox pair of nitrogen and oxygen (either oxidation or reduction takes place), as well as conjugate acid-base (H+–OH−) pairs.
Download figure:
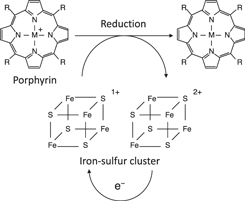
Standard image High-resolution imageIn addition to ammonia synthesis, the photosynthesis of carbohydrates from CO2 can occur, during which porphyrins (made of four pyrroles interconnected by methine bonds) are key molecular units (Fig. 10). In a porphyrin, the nitrogen is coordinated with a central metal atom, and these compounds undergo reactions with either ammonia or CO2 involving electron transfer. 112) Chlorophyll is a photosystem reaction center and nitrite reductase is a type of porphyrin that mediates electron transfer from iron-sulfur metalloprotein (4Fe-4S cluster center enzyme). Although porphyrins are easily oxidized or reduced, they are stable when generating the corresponding radical cations or radical anions. 113) This property of porphyrin is excellent in catalyzing redox reactions.
Fig 10. Structure of porphyrin units and an example of the electron transfer scheme of porphyrin-related redox reaction with the cofactor enzyme (4Fe-4S cluster), acting as an electron donor.
Download figure:
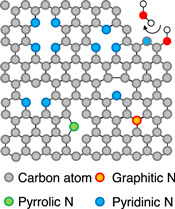
Standard image High-resolution imageThese systems are presently being studied in relation to oxygen reduction reactions, in particular the reactions occurring in fuel cells, and there has been significant progress in techniques for the incorporation of nitrogen into graphene-based catalysts. Pyridinic nitrogen on graphene sheets (Fig. 11) has been shown to catalyze efficiently the electrochemical oxygen reduction reaction. In this process, the pyridinic nitrogen modifies the system to generate a 4e− reduction pathway via quaternary nitrogen formation in place of a 2e− pathway. 114,115) Thus, the atomic localization of nitrogen in the matrix is vital to obtaining and controlling electron donation to the reactants. In the case of N2 plasma treatment of graphitic materials of carbon nanowall, electrical conductivity was increased by N doping. 116) As described in Sect. 3.1, the endohedral N@C60 encapsulate isolated electrons in the closed cage. The diamond NV center has emerged as a basic unit for quantum computing devices. Specifically, isolated electrons can act as either sources of free charges or as qubits having free spin. However, electronic or spintronic devices have not yet been realized based on this technology. Therefore, the doping of nitrogen atoms into various matrices with atomically localized control is necessary. Functional nitrogen can be provided only by atomically controlled doping based on the electron charge and spin. As a consequence, future research regarding the in situ doping of nitrogen will require interdisciplinary studies related to the development of new materials for device fabrication.
Fig 11. (Color online) Structure of pyridinic nitrogen on graphene sheets. The pyridinic nitrogen catalyzes the electrochemical reduction of oxygen. The atomic localization of nitrogen in the matrix is vital to obtain and control electron donation to the reactants.
Download figure:
Standard image High-resolution image4. Biological applications of plasma
4.1. Organic manure
The world's population will reach 8.6 billion in 2030, which will increase the demand for fertilizers, and so further developments in industrial nitrogen fixation processes are of great importance. 117) It is well-known that the HB process plays a dominant role in chemical nitrogen fixation and is used to produce large amounts of ammonia. However, this is an energy-intensive process that consumes 1%–2% of the total energy used worldwide and 2% of the total natural gas. 117) The HB process is also responsible for the emission of 300 million metric tons of CO2 per year. Considering the fast-growing human population and the ongoing depletion of natural resources, it would be highly beneficial, both economically and environmentally, if the energy efficiency of nitrogen fixation could be improved using greener technology. As an example, Koga et al. demonstrated an environmentally friendly plasma technique for generating nitrogen-enriched organic manure. 118) This was the first study in which the plasma was exposed on the soil to supplement soil with NH4 + and NO3 −. However, other studies have focused on the synthesis of NH3 or NOx using plasma technology with different gas mixtures at various pressures. 18) Earlier NH3 production was reported using N2 with H2 119) or H2O. 120) Plasma-catalytic processes can produce NH3 at low atmospheric pressure with N2/H2 gas. 121)
Recently, NH3 synthesis has been widely studied in plasma-electrochemical processes 122) and non-thermal plasmas using N2 and H2 gas at reduced pressure and low temperature. 123) For example, radio frequency excited (RF) plasma at low pressure (2 Pa) was used for NH3 formation with and without tungsten or stainless steel surfaces using an N2/H2 gas mixture. 124) In another study, RF plasma with and without a furnace was used to produce NH3 at low pressure (30–40 Pa), in the presence of a catalyst. 125,126) The RF plasma caused the adsorption of NHx and H radicals; in contrast, the microwave (MW) plasma caused the adsorption of N radicals. 127) It has been reported that RF and MW low-pressure plasma reactors cause a higher degree of vibrational excitation, which is useful for N2 activation before the dissociation of N2. 123)
Many studies have previously reported the production of NOx from air or N2/O2 mixtures using RF plasma, 128) MW plasma, 129) plasma jet, 130) DBD plasma, 131) and gliding arc discharge. 132) The energy consumption of these methods varies widely, from 0.3 to 1600 MJ mol−1. Mutel et al. synthesized NO using MW plasma at a pressure of 6600 Pa with a catalyst and N2–O2 gas mixture and the energy cost of this system was 0.084 MJ mol−1. 133) The energy cost for NO production using the microwave plasma at a reduced pressure after applying a magnetic field was the lowest at 0.30 MJ mol−1. 134) Although only plasma power was considered for low-value energy costs, the energy used for the cooling reactor was not included. On the other hand, gliding arc plasmas with N2/O2 gas mixtures have shown promising energy consumption rates of 3.6 MJ mol−1 with 1.5 % NOx yield. 135)
The energy cost of production of NH4NO3, as well as that of NO, NOx , and NH3, is still higher with plasma technology than with existing technology. It is essential to shift towards plasma technology by introducing small-scale units to reduce environmental damage. In addition, plasma processes have the significant advantage of directly using abundant resources, such as air and water, and can be powered by electricity generated from renewable resources. There have been no reports of using direct plasma on the plant soil to enrich the soil with nitrogen species for later use in agriculture.
The Koga group has developed a low-pressure plasma capable of producing nitrogen-rich fertilizer without the use of expensive H2 gas or a catalyst and has reported increased NH4 + and NO3 − concentrations in plasma-treated soil. Interestingly, the NO3 − concentration in this material was higher than that in commercially available nitrogen-based fertilizer. The results revealed a 40% increase in early germination when using plasma-treated soil and a 34% increase in the shoot length of radish sprouts along with 39% higher sprout weights. The authors concluded that plasma-treated soil shows similar or better results with regard to the germination and growth of radish seeds compared with commercial nitrogen fertilizer.
4.2. Enhancement of seed germination
The Koga group also demonstrated the effects of an atmospheric air plasma treatment on the germination and growth of radish seeds collected in two different harvest years with two different seed coat colors. 136,137) Various treatments, including electromagnetic waves, ionizing radiation and atmospheric air plasma were used to accelerate the rate of seed germination, enhance plant growth and increase agricultural yields. 138) Recently, the use of atmospheric air plasma to treat seeds has increased. 139) In one study, the effects of RONS produced from an air scalar DBD plasma 140) were compared in terms of the germination rate and early growth of radish seeds harvested in two different years. In this study, each seed lot was sorted into two groups (brown and grey) and RONS-induced changes in the physical and chemical states, as well as the phytohormone and antioxidant levels in the radish seeds, were assessed before and after plasma treatment. 140) The average length of the brown seed sprouts was 24 ± 0.5 cm for the 2017 harvest, whereas, after the plasma treatment, the value was 20 ± 0.4 cm on the fourth day after treatment. The average length of the sprouts from brown seeds for the 2018 harvest was not changed significantly after plasma treatment. The authors concluded that RONS produced by an air DBD had a substantial effect on older seeds but not new seeds and that the seed coat color influenced the germination speed and sprout length.
4.3. Other aspects of plasma-based agriculture
In addition to air DBD, seeds treated with other plasmas have been found to exhibit enhanced germination and growth. 141) Ito et al. reported that plasma-based agriculture can presently be divided into three categories. The first of these deals with plant seeds and bodies, while the second is water and nutrients containing short-lived species, and the last is the microbial environment in soil. 142) The electric field induces structural conformational changes in biomolecules, such as proteins. RNS participates in cell signaling pathways and mediates the biological responses to biotic and abiotic stress conditions in plants. The electromagnetic fields and RNS generated in plasma discharges can be used to directly stimulate the plant body and also to provide secondary effects by modifying water and soil. Yoshimura et al. reviewed the effects of the normothermic plasma irradiation of living organisms and reported that yeast cells must be irradiated at standard temperature. 143) Cells respond transiently to short-lived species such as ONOO− anions and this transient response was found to promote the entry of calcium ions into cells. 144) However, the associated mechanisms have not been assessed comprehensively. The applications of plasmas in agriculture have been reviewed elsewhere 145,146) and cover a wide range of research areas, including utilization during harvesting 47) and post-harvest 147) as well as in the food supply chains. 148)
5. Theoretical approaches to plasma-biological reaction networks
5.1. Networks in biochemistry
The biological processes of living organisms involve complex interactions between many signaling molecules. The balance between the intrinsic production and consumption of signaling species and external perturbation affects physiological homeostasis 149) and RNS function as major regulators of various signaling pathways. 150) For example, NO and ONOO− are known to play important roles in cell survival and death processes by regulating mitochondrial transmembrane permeability. 151,152) Intracellular and intercellular systems are based on many molecular compounds, proteins and signaling pathways. That is, biological functions do not stem from a single agent, but from collective interactions among components of a network. Network biology is therefore a systematic approach to understanding how these agents and their interactions trigger specific functions. 153,154) Network analysis based on mathematical graph theory has been applied in various fields, including physics, chemistry, biology, computer science, geography, economics and sociology. 154–156) In graph theory, the elements (reagents) of the system and the interactions between these elements (reaction pathways) are represented as nodes and edges, respectively, to model pairwise relationships. Therefore, a graphical and theoretical analysis can capture not only the unique characteristics of a particular network but also the statistical characteristics common to a wide range of networks.
5.2. Networks in plasma-induced chemistry
Both gas phase and liquid phase plasma-induced chemistry are sufficiently complex to form network structures. Recently, the basic characteristics of gas-phase chemical networks have been examined. 157–161) Sakai et al. applied graph theory to the study of CH4 plasma and SiH4 plasma chemistry to identify the species that play a central role in triggering subsequent reactions. They showed that the graph theory approach allows rapid visualization and classification of these complex chemical systems. 142,157) In addition, Sakai's group elucidated the growth mechanism associated with the SiH4 plasma reaction network. 159) Holmes et al. also used graph theory and proposed an algorithm to quantify the requirements for the target's chemical composition. 161)
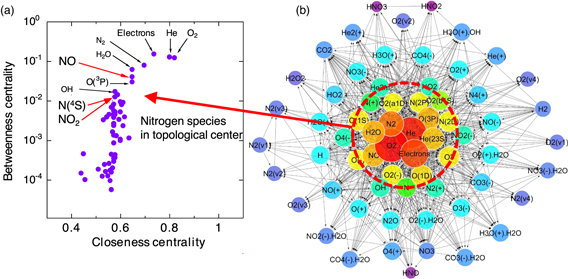
Murakami and Sakai extracted essential information from the complex reaction data obtained for a He + humid air plasma 162–164) and devised a method to rescale the reaction network based on graph theory analysis. Furthermore, they quantified the impact of network rescaling on a quasi-2D numerical simulation of a plasma jet. Figure 12 shows the topological characteristics of the chemistry within a plasma obtained from a He + N2 + O2 + H2O + CO2 mixture, together with the relationship between closeness centrality and betweenness centrality, and a network diagram of closeness centrality. 160) Closeness centrality is a measure of how quickly information spreads from one particular species in the network to other reachable species, and so those species with high closeness centrality are placed in the center of the network. Betweenness centrality represents the controllability, informativeness or importance of an intermediate that directly or indirectly bridges reactants and products. Figure 12 shows that oxygen molecules and helium atoms, acting as the primary background species, and electrons, acting as the reaction driver species, dominate the network topology. Ground state atomic nitrogen N (4S), NO2 and ·OH are also in the most central region because these species have relatively high betweenness centrality. Species with higher betweenness centrality interact with a wider range of other species, more intermediate reactions, and integrate the entire reaction system.
Fig 12. (Color online) (a) Relationships between topological properties and (b) a network diagram of He + N2 + O2 + H2O + CO2 plasma chemistry (modified from Ref. 162).
Download figure:
Standard image High-resolution imageDuring the biological and agricultural applications of atmospheric air plasmas, various reactive species are generated and applied to biological targets. Through a reaction network analysis, it is possible to predict the trajectory of functional nitrogen while obtaining an overview of the more general framework of the plasma-biology interactions.
5.3. Networks in plasma-induced intracellular functions
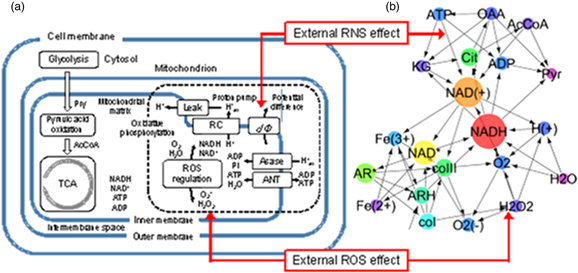
As an interdisciplinary numerical study of the interactions between plasma chemistry and molecular biology, Murakami developed a biochemical network model of mitochondrial redox homeostasis and energy metabolism. This work clarified the effects of reactive species from the plasma on the intracellular functions through a time-dependent 0D numerical simulation. 165) The study was motivated by recent experimental results showing that nitrogen-based plasma lowers mitochondrial membrane potentials and significantly affects cell survival rates. 166) Figure 13 shows a pathway map and a topology diagram of the modeled biochemical network. 165) The numerical model includes fundamental mitochondrial functions associated with pyruvic acid oxidation, the tricarboxylic acid cycle, and oxidative phosphorylation involving the respiratory chain, adenosine triphosphate/adenosine diphosphate (ATP/ADP) synthesis machinery, and ROS/RNS-mediated mechanisms. The functions of the cell membrane, cytosol and glycolysis are not considered. The effects of plasma irradiation are modeled based on the influx of H2O2 to an ROS regulation system and the change in mitochondrial transmembrane potential induced by the effect of NO/ONOO− on membrane permeability. Nicotinamide adenine dinucleotide (NAD; C21H27N7O14P2), its reduced form NADH and oxidized form NAD+ play a central role in oxidative phosphorylation in aerobic organisms. This work has demonstrated that the plasma-derived RONS stimuli greatly affect ROS oscillation, NAD redox and ATP/ADP conversion. This plasma-induced ROS influx changes the intrinsic intramitochondrial redox oscillatory rhythm from homeostatic to irreversible. It is also evident that the RNS-driven change in transmembrane permeability controls the redox regulation system and ATP/ADP metabolism through NAD-mediated reactions, that is the RNS-induced deterioration of the mitochondrial transmembrane potential promotes the ATP → ADP transformation.
Fig 13. (Color online) (a) Essential mechanisms simulated in the biochemical reaction model and (b) a graph of the reaction network, where the betweenness centrality value is indicated by circle size (modified from Ref. 165).
Download figure:
Standard image High-resolution image6. Plasma biology: toward molecular biology from phenomenology
6.1. Plant defense systems
We examine recent progress in research related to RNS in plant defense and phytohormones as signaling molecules and post-translational modification. The effects of RNS can be understood based on molecular modifications. Prior studies have focused on plant defense systems 167) and have found that S-nitrosylation (the attachment of NO to a cysteine thiol moiety) triggers the release of phytohormones related to seed germination and seedling growth. 168)
One of the basic responses of plants is defense against pathogens. In the event of infection, the plant encloses the pathogen in a limited area and responds with resistance. For this response to occur, the plant must recognize the pathogens, generate a signal and transmit this signal to the surrounding area, which involves microbial substances known as elicitors. Genes related to defense responses are expressed and cells in the enclosed region containing the pathogens are killed through hypersensitive cell death. As well, plants produce pathogenesis-related proteins and phytoalexins, which are low molecular weight antibacterial substances. It is known that ROS and RNS, in particular O2
 −, NO and ONOO−, act as signaling molecules in these systems, and that hypersensitive cell death is induced by elicitors produced from NO, O2 and H2O2.
−, NO and ONOO−, act as signaling molecules in these systems, and that hypersensitive cell death is induced by elicitors produced from NO, O2 and H2O2.
Monjil et al. found that a synthetic bis-aryl-methanone compound (NUBS-4190) and a methanol extract from a pathogen (the mycelia of P. infestans) that contained lipophilic compounds were elicited by potato leaves in response to NO. 169,170) The gene for Arabidopsis thaliana nitric oxide-associated 1 (AtNOA1) has been identified as a putative regulator of NOS activity in plants. 171) In N. benthamiana leaves, the pathogenesis-related 1a (NbPR1a) gene and nitric oxide associated 1 (NbNOA1) gene, a homolog of AtNOA1, were found to be associated with infection resistance. 172,173) Moreover, nitrate reductase (NR) has been shown to be responsible in part for INF1 elicitor-induced NO production. 174,175) Protein S-nitrosylation, a redox-related modification of cysteine thiol groups by NO, is known to be one of the most important post-translational modifications regulating the activity and interactions of proteins. The proteomic analysis of S-nitrosylated proteins in potato (Solanum tuberosum) using a modified biotin switch assay found that dehydroascorbate reductase 1 (DHAR1, EC 1.8.5.1) is modified by S-nitrosylation, inducing glutathione-dependent dehydroascorbate-reducing activity. 176) In addition, intracellular fluorescent imaging of ONOO− using aminophenyl fluorescein by Saito et al. identified tyrosine nitration in the defense responses of plants and showed that ONOO− reacts with tyrosine residues in proteins to form nitrotyrosine in tobacco BY-2 cells treated with INF1. 177) ONOO− seems to indirectly mediate the nitration of proteins and nucleic acids or phenolics. 178) The oxidative and nitrative modifications of proteins may also regulate some biological functions by evolving physiologically adaptive responses.
Kawakita et al. demonstrated that NO production is involved in plant-microbe interactions and is an effective invasion strategy for pathogens but is also essential for plant defense responses
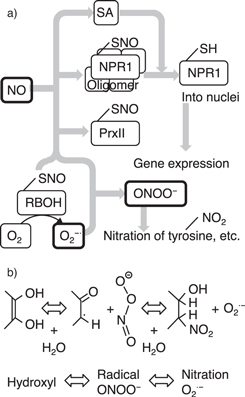
179) (Fig. 14). Systematic acquired resistance in plants is regulated by the non-expression of pathogenesis-related gene I (NPR1), which forms oligomers via S-nitrosylation (indicated by –SNO in Fig. 14) based on the NO concentration. Salicylic acid (SA) dissociates the NPR1 oligomers (forming the thiol moiety indicated by –SH in Fig. 14) that translocate to provide nuclei and gene expressions involved with plant defense genes. Peroxiredoxin (PrxII) is a reductase for ONOO− and this enzymatic activity is regulated by S-nitrosylation. Therefore, superoxide radicals (O2
− ) generated by respiratory burst oxidase homolog D (RBOHD) play an important role in generating ONOO−. Thus, the cellular signaling of NO is delivered and cascades through multiple molecular modification pathways in the plant defense system.
) generated by respiratory burst oxidase homolog D (RBOHD) play an important role in generating ONOO−. Thus, the cellular signaling of NO is delivered and cascades through multiple molecular modification pathways in the plant defense system.
Fig 14. (a) Plant defense functions based on NO in conjunction with post-translational modification. Legend: SA = a phytohormone of salicylic acid, NPR1 = non-expresser of pathogenesis-related gene I, PrxII = peroxiredoxin II, RBOH = respiratory burst oxidase homolog D. Modified from Ref. 179. (b) Hydroxyl groups are oxidized by dehydration or dehydrogenation. Once a radical is formed, peroxynitrite (ONOO−) reacts with it, forming nitro compounds (nitration) and a superoxide anion radical (O2
 −).
−).
Download figure:
Standard image High-resolution imageThe nitration of tyrosine is a key topic in biochemistry related to the oxidative modification of proteins.
180) This reaction is mediated by ONOO− via the substitution of a nitro group for a hydrogen atom.
181) As an example, in cytochromes iron sites cooperate to form ferrous superoxo (FeIIOO) and ferric nitrosyl (FeIIINO) intermediates.
182) These ONOO− intermediates dissociate homolytically into O2
 − and NO.
182) Lastly, biosynthetic gene clusters that function to promote the nitrosation of metalloenzymes are found in bacteria.
183) These metalloenzymes catalyze the oxidative rearrangement of the guanidine group.
183) NO production in mammalian cells is catalyzed by NO synthase (NOS), although no NOS-like enzymes have been identified in plants.
184) Even so, the actions of RNS with regard to NO metabolism, signaling and stress response are important.
− and NO.
182) Lastly, biosynthetic gene clusters that function to promote the nitrosation of metalloenzymes are found in bacteria.
183) These metalloenzymes catalyze the oxidative rearrangement of the guanidine group.
183) NO production in mammalian cells is catalyzed by NO synthase (NOS), although no NOS-like enzymes have been identified in plants.
184) Even so, the actions of RNS with regard to NO metabolism, signaling and stress response are important.
In addition to tyrosine nitration, these post-translational modifications are considered to be essential biological responses related to the collaborative effects of NO and O2
− radicals as well as ONOO− at the molecular level. Diazo (R–N=N), nitro (R–NO2) and nitrosyl (R–N=O) moieties are important. Diazo moieties have been shown to give rise to various reactive intermediates, including vinyl radicals, o-quinone methide (C7H6O), acylfulvene (C14H16O2), and a covalent adduct resulting from both reductive and nucleophilic activation of diazo groups.
185) Carbonyl groups and neighboring methylidene or hydroxyl groups work together to form diazo groups by combining with reactants such as ONOO−. In addition, the quinone moiety may undergo redox cycling to produce ROS.
186,187) [Figure 14(b)] Once hydroxyl groups are oxidized by dehydration and dehydrogenation forming radical, ONOO− reacts with the radical forming nitro moiety and O2
−
radicals as well as ONOO− at the molecular level. Diazo (R–N=N), nitro (R–NO2) and nitrosyl (R–N=O) moieties are important. Diazo moieties have been shown to give rise to various reactive intermediates, including vinyl radicals, o-quinone methide (C7H6O), acylfulvene (C14H16O2), and a covalent adduct resulting from both reductive and nucleophilic activation of diazo groups.
185) Carbonyl groups and neighboring methylidene or hydroxyl groups work together to form diazo groups by combining with reactants such as ONOO−. In addition, the quinone moiety may undergo redox cycling to produce ROS.
186,187) [Figure 14(b)] Once hydroxyl groups are oxidized by dehydration and dehydrogenation forming radical, ONOO− reacts with the radical forming nitro moiety and O2
− radical. This nitration plays a pivotal role in post-translational modifications. Although it has not yet been completely proven, it has been hypothesized that the RNS generated in plasma, involving NO, NO2
−, and ONOO−, may directly and indirectly affect the biochemical processes of multiple cell signaling and communication pathways.
radical. This nitration plays a pivotal role in post-translational modifications. Although it has not yet been completely proven, it has been hypothesized that the RNS generated in plasma, involving NO, NO2
−, and ONOO−, may directly and indirectly affect the biochemical processes of multiple cell signaling and communication pathways.
6.2. Regulation of seed germination
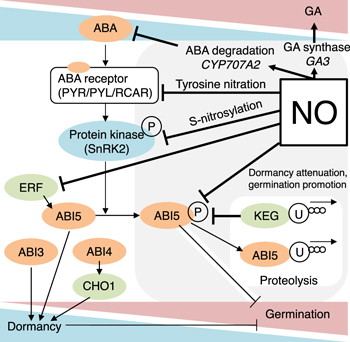
Phytohormones control and regulate plant growth and development by acting as signal molecules produced within plants at extremely low concentrations. 188) These compounds include abscisic acid (ABA), auxin (OC), gibberellin (GA), jasmonic acid (JA), SA, cytokinins, strigolactone, ethylene, peptides and polyamines. As a result of recent advances in functional genomics, the metabolism of ABA (a plant hormone that regulates seed dormancy and germination) involving biosynthesis and catabolism has been widely elucidated. 189,190) Figure 15 shows the mechanisms of the regulation of dormancy attenuation and germination promotion of seeds. ABA levels promote seed dormancy and germination; during seed germination, ABA levels decrease and GA levels increase. To attenuate this dormancy level, NO-related metabolism in relation to ABA contributes to the promotion of seed germination. In response to internal and external cues, seeds undergo changes in ABA levels, and large-scale comparative studies have been conducted on seeds under different environmental conditions. 191,192) The variants of this compound include ABA1 and ABA2 acting as protein phosphatases, and ABA3, ABA4 and ABA5, all of which are related to plant dormancy. ABA4 also plays a role in response to NO2 and NH3. 193) Using two ABA metabolism mutants, an ABA-deficient mutant (ABA2) and an ABA over-accumulation mutant (CYP707A1A2A3 triple mutant), Okamoto et al. studied transcriptomes in seeds for endogenous ABA. 194) Yamagishi et al. reported that the CHO1 mutation acts as a strong enhancer of the ABI5 mutant, whereas the CHO1 ABI4 double mutant shows ABA resistance similar to the ABI4 single mutant. 195)
Fig 15. (Color online) A phytohormone balance between abscisic acid (ABA) and gibberellin (GA) regulates to attenuate dormancy and promote the germination of seeds. Seed imbibition results in an increase in NO levels, meaning ABA controlled dormancy can be attenuated by (i) inhibition of ABA receptors by tyrosine nitration, (ii) inactivation of protein kinase (SnRK2) activity, (iii) enhancement of the proteasome of the transcription factor, ABSCISIC ACID INSENSITIVE5 (ABI5), and (iv) promotion of expression gene of ABA degradation, CYP707A2. ERF is Ethylene response factor; SnRK2 is SUCROSE NON-FERMENTING1 (SNF1)-RELATED PROTEIN KINASE2; KEG is CULLIN4-based and KEEP ON GOING E3 Ligases. P is phosphorylation and U is ubiquitination to the proteasome.
Download figure:
Standard image High-resolution imageSimilar to lettuce seeds, the germination of Lactuca sativa seeds is regulated by alterations in the levels of phytohormones, such as ABA and GA. 196) The imbibition responses of Arabidopsis seeds were analyzed by acquiring transcriptomic and hormone profiles. 197) In the case of dormant Arabidopsis seeds, nitrate levels were reduced but exogenous nitrate concentrations increased with ABA uptake by the seeds. The expression of the ABA catabolic gene CYP707A2 was also regulated by exogenous nitrate. In addition, the CYP707A2–1 mutant failed to reduce seed ABA levels in response to either endogenous or exogenous nitrate. 198) The ABA level in seeds evidently controls germination and seedling growth throughout the expression of the gene for the leucine zipper transcriptional factor ABI5, with is counteracted by NO. ABI5 is therefore accumulated in conjunction with impaired NO homeostasis. The S-nitrosylation of ABI5 at cysteine-153 facilitates its degradation through CULLIN4-based and KEEP ON GOING E3 ligases and promotes seed germination. Conversely, the mutation of ABI5 at cysteine-153 deregulates protein stability and the inhibition of seed germination by NO depletion. An inverse molecular link between NO and ABA hormone signaling through distinct posttranslational modifications of ABI5 during early seedling development has also been identified. 168) Hypothetically, plasma-generated RNS may contribute to the regulation of the ABA signaling network by promoting post-translational modifications, such as tyrosine nitration, S-nitrosylation. Notably, extracellular NO gas is known to be affected exogenously, which explains why intracellular NO acting as the signaling molecule should be regulated at the desired location inside a cell. It has also attracted the plasma-based RNS, which is clearly recognized as an intracellular signaling molecule; however, the mechanisms underlying the regulation of NO have not yet been elucidated.
6.3. Perspectives for plasma biology
In plant science, genomic screening using gene mutants is often employed for dormant germination control. The mutation of genes is possible by base transformation using the reactions of specific compounds. Gene-editing techniques such as CRISPR/Cas9 are also used and an experimental system to quantitatively measure the effects of RNS on living organisms (that is, plants) can be constructed. The comprehensive disruption of candidate genes using genome editing methods has shown that genetic mutations may be involved in the effects of RNS. Screening of the mutant libraries can also identify genes that have been found to have physiological effects. A compound library can be screened to identify drugs that have been shown to have these effects, and the identification of molecules involved in this process can proceed in combination with the selection of mutants with different sensitivities to these compounds.
In chemical biology in contrast to genetic studies, the screening of bioactive compounds produced by RNS can be used to elucidate target molecules and signaling pathways. A comprehensive analysis of the nitrosylation (–NO) and nitration (–NO2) of amino acid residues after the translation of gene expressions is required, using post-translational modification omics (PTM-omics). This technique can assist in elucidating the effects of NO and NO2 on proteasome and gene transcription regulation.
In biochemistry, the relationship between metabolome and phenotypes involving epigenetic and post-translational modifications is an important area of study. There are so-called direct effects of RNS compounds on seeds and plants through biological transients and indirect effects through plant physiological substances. ABI5 was first identified as a transcriptional regulator of ABA related to NO, based on the symbiotic expression of serine from cysteine. ABI5 mutants exhibit S-nitrosylation, in which the SH group of the cysteine residue binds with NO, and this reaction is accompanied by oxygen metabolism and ubiquitination.
In plant immunity, Ochi et al. demonstrated that the plasma irradiation of water-soaked rice seeds stimulated the generation of ROS that were correlated with the suppression of plant disease. 199) Both RNS and ROS function as important signaling factors in plant immunity and it is assumed that the RNS produced by plasma may act directly or indirectly on plant immune mechanisms. 200) Therefore, the involvement of plasma-derived RNS in signal transduction due to pathogen infection and the epigenetic regulation of plant immune mechanisms should be analyzed. A recent study revealed that irradiation of Arabidopsis thaliana with different active species, including plasma-derived RNS, selectively activated the expression of marker genes for defense responses.
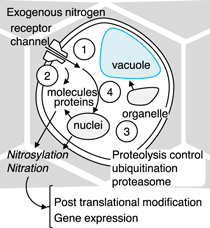
In the context of plasma-based agriculture, the development of RNS doping techniques, the effects on biological materials, and the lifecycle phase in which functionalization is achieved are all important. Possible reaction pathways may involve receptors, post-translational modification, proteolysis control with poly-ubiquitination and proteasome, or regulation of gene expression with the nitration of nucleic acids (Fig. 16). The effects of RNS are distinguished by primary and secondary actions. In the simplest case, RNS directly regulate biological responses. However, we should consider that the secondary effects of the nitrosylation and nitration of amino acid residues and proteolysis control also regulate the biological responses of RNS. The timing of RNS doping of the cell may also be important for obtaining functional responses.
Fig 16. (Color online) Possible reaction pathways through (i) receptors and channels, (ii) post-translational modification, (iii) proteolysis, and (iv) regulation of gene expression via nitrosylation and nitration of molecules.
Download figure:
Standard image High-resolution image7. Spatial and temporal localization of atomic nitrogen
7.1. In situ measurements at multiple points in real-time
The previous sections examined the transportation of nitrogen in living systems and the hierarchical analysis of spatiotemporal phenomena associated with RNS, as illustrated in Fig. 17. Table IV lists the representative results of the in-situ measurements of plasma processing. In this process, nitrogen compounds are synthesized from air and electricity. As an example, RNS in the gas phase are transported into aqueous or solid phases via boundaries. In the first case, solid-state materials can be functionalized by the doping of nitrogen into the bulk matrices of inorganic substances such as catalysts and ceramics, or of organics such as polymers. In this manner, in situ plasma and ion irradiation can provide nitrogen doping to create new functionalities without the use of ammonia or acid.
Fig 17. (Color online) Diagram showing the hierarchical analysis of the spatiotemporal distribution of RNS for the functionalization of various materials. Legend: LTS = laser Thomson scattering, E-FISH = electric field induced second harmonic generation, LIF = laser induced fluorescence, ESR = electron spin resonance, LC-MS = liquid chromatography mass spectrometry, XAFS = X-ray absorption fine structure, ABA = abscisic acid, PTM = post-translational modification.
Download figure:
Standard image High-resolution imageTable IV. A list of typical methods used in the hierarchical analysis.
| Physical parameters (Electron behaviors) |
| Plasma density, electron temperature, laser Thomson scattering |
| Gas temperature (Raman) |
| Electric field, E-FISH, Stark broadening |
| Chemical parameters (Gaseous chemistry) |
| Atomic and molecular species, QMS, LIF, VUVAS, IR&Raman, CARS, SFG |
| Chemical parameters (Aqueous chemistry) |
| LC-MS, ion chromatography, ESR, NMR, UV/Vis, electrochemistry |
| Material characterizations |
| X-ray analysis, electron microscopy, solid-state spectroscopy |
| Biological tests |
| Biochemistry (Omics), crystallography, circular dichroism |
| Theoretical simulation and calculation |
| Molecular dynamics, quantum chemistry |
At present, we do not have an atomically resolved understanding of the mechanism related to the localization of nitrogen during in situ nitrogen doping. Thus, advancements in techniques to measure and analyze the transportation of various species are required. Spatiotemporal and simultaneous observations of multiple species at various points should be conducted.
7.1.1. Electron behavior
Electrons in plasma can be characterized using various methods, including invasive optical diagnostics. Table V provides a list of measurements of the electron behavior of atmospheric air plasmas. Laser Thomson scattering (LTS) is one of the most reliable diagnostic techniques 201–204) for measuring the electron density and electron energy distribution function (EEDF) in various types of plasmas including non-thermal atmospheric pressure plasmas. 205,206) One of the weak points of LTS is its small scattering cross-section. 207) In addition, strong rotational Raman scattering (RRS) signals from N2 are inevitably superimposed on the weak LTS spectrum in the case of discharges produced under nitrogen-rich conditions, including in air. Therefore, to obtain an LTS spectrum with a sufficient signal-to-noise ratio, tens of thousands of LTS signals must be accumulated. It should also be noted that separation of the LTS signal and the RRS signal has been already demonstrated in trials using an atmospheric pressure plasma jet under noble gases. 208,209)
Table V. Measurements of electron behaviors. LTS, laser Thomson scattering; OES, optical emission spectroscopy; CARS, coherent anti-Stokes Raman scattering; E-FISH, electric field induced second harmonics; and Doppler, Doppler broadening absorption spectroscopy.
| Target | Gas | Pressure | Method | Value | References |
|---|---|---|---|---|---|
| Electron density | He/Air | 1 atm | LTS | 2 × 1019 m−3 | 220 |
| Ne | 53 kPa | Stark | 15.0 × 1022 m−3 | 206 | |
| Ar/N2/O2 | 1 atm | LTS | 4.6 × 1022 m−3 | 208 | |
| EEDF | Ne | 53 kPa | LTS | Te ∼ 1.0 eV | 206 |
| Gas temperature | Ar/N2/O2 | 1 atm | Raman | 308 K | 208 |
| Air | 1 atm | Raman | 1200 K | 209 | |
| Electric field | N2 | 1 atm | OES | 25 kV cm−1 | 221 |
| He/Air | 1 atm | Polarimetry | 27 kV cm−1 | 222,223 | |
| Air | 1 atm | CARS | 40 kV cm−1 | 214 | |
| He/N2 | 1 atm | E-FISH | 35 kV cm−1 | 219 | |
| H2 | 6.3 kPa | Doppler | 600 V cm−1 | 224 |
Recently, the LTS technique has been applied to the analysis of streamer discharges under ambient air. 210) As predicted before these experiments, a very intense RRS signal was inevitably superimposed on the LTS spectra. Therefore, spectra were accumulated over 20 000 laser shots to obtain suitable data. As a result, the EEDF in the air streamer plasma was experimentally obtained for the first time and was found to clearly deviate from the Maxwellian distribution predicted by the Boltzmann equation. 211) The experimental and calculated EEDF results for a reduced electric field value of 150 Td showed good agreement, although the consistency between these data within the high energy tail part of the distribution was uncertain due to the measurement error caused by large statistical fluctuations. For more precise measurements, it will be necessary to reduce the intense RRS signal, which may be possible by improving the spectral resolutions associated with the spectrometer. 212)
The electric fields in plasmas are also uncertain. The discharge apparatus typically applies a high voltage between two electrodes such that an intense electric field is generated and propagates through space. Ito et al. reported electric field measurements based on field-induced coherent anti-Stokes Raman scattering (CARS) of nitrogen molecules, monitoring the coherent infrared radiation intensity at 2331 cm−1 produced by the CARS process in the presence of an electric field. 213) A dynamics of atmospheric-pressure air plasma was revealed by the measured results of the electric field. 214) Recently, Dogariu et al. reported that the second harmonic signal exhibits a quadratic dependence on the Laplacian electric field; this phenomenon is referred to as electric field induced second harmonic generation. 215,216) The Adamovich group used this principle to achieve a temporal resolution of 200 ps and a spatial resolution of approximately 0.5 mm. 217,218) The Czarnetcki group reported a temporal resolution of 100 ps and a spatial resolution of 50 μm. 219)
7.1.2. Transportation of reactive species in the gas phase
Electron collisions in both dry and humid air generate a rich variety of chemical species, and the atoms and small molecules in the plasma can be examined using optical diagnostics. 225–227) Table VI lists the measurements of representative reactive species in atmospheric air plasmas. As an example, laser-induced fluorescence (LIF) techniques have been used to determine the micrometer scale distributions of the densities of N, NO, O and OH in streamer discharges in air. 228,229) Other species, such as NH, NH2 and H, can also be assessed. In a prior study of the UV photolysis of hydrazoic acid, NH (X 3Σ) and NH2 (A 2A1) were all monitored by LIF. 230) Moreover, NH2 + NO and NH + NO reactions were studied using LIF in conjunction with quantum chemistry methods. 231) The grafting of nitrogen-based functional groups onto polymers and biological surfaces was reviewed by Meyer-Plath et al. 232) and a discussion of the application of this technique to fluoropolymers was published by Sarra-Bournet et al. 233) Analyses using two-photon laser-induced fluorescence (TALIF) and tunable infrared diode laser absorption spectroscopy were able to detect atomic N, NH radicals and NH3. 232) X-ray photoelectron spectroscopy and secondary ion mass spectrometry were able to detect surface amino (–NH2) and amine (>NH) groups. Van holden et al. reported the use of cavity ring-down spectroscopy (CRDS) to monitor NH and NH2 radicals. 234) NH2 radicals were observed at approximately 597.5 nm in conjunction with the transition A 2A1 ← X 2B1, while NH radicals appeared at 340 nm as a result of the transition A 3Π ← X 3Σ−. 234) Teramoto et al. reported that TALIF data showed an N atom signal at 750 nm based on the transition 4S ← 4P, 235) while Yatom et al. used TALIF to detect an H atom signal at 656.3 nm in association with the transition 2D ← 2P. 236)
Table VI. Measurements of gaseous species. APPJ, atmospheric pressure plasma jet; SBD, surface barrier discharge. CRDS, cavity ring-down spectroscopy; TALIF, two-photon absorption laser-induced fluorescence; FTIR, Fourier transformed infrared spectroscopy; VUVAS, vacuum ultraviolet absorption spectroscopy.
| Target | Plasma source | Gases | Pressure | Method | Density (cm−3) | Lifetime | References |
|---|---|---|---|---|---|---|---|
| NH | dc arc plasma jet | N2/H2 | 20 kPa | CRDS | 5 × 10(12) | n/a | 234 |
| NH2 | dc arc plasma jet | N2/H2 | 20 kPa | CRDS | 7 × 10(12) | n/a | 234 |
| N | pulsed streamer | N2 | 1 atm | TALIF | 1 × 10(16) | 300 μs | 235 |
| H | ns-pulsed APPJ | Ar/H2O | 1 atm | TALIF | 2 × 10(16) | 40 μs | 236 |
| NO | SBD | air | 1 atm | LIF | 6 × 10(15) | n/a | 237 |
| NO2 | rf plasma jet | Ar | 1 atm | FTIR | 4 × 10(12) | n/a | 238 |
| N2O | rf plasma jet | Ar | 1 atm | FTIR | 8 × 10(11) | n/a | 238 |
| N2O5 | rf plasma jet | Ar | 1 atm | FTIR | 3 × 10(12) | n/a | 238 |
| HNO3 | rf plasma jet | Ar | 1 atm | FTIR | 4 × 10(12) | n/a | 238 |
| N | ac-excited APPJ | Ar | 1 atm | VUVAS | 1 × 10(14) | n/a | 241 |
Dickenson et al. studied the generation and transport of RNS in an atmospheric air plasma, 237) and used the LIF technique to determine the NO density. Because NO is highly reactive, its fluorescent signal decay could be calibrated based on known quenching species. Subsequently, comparing data acquired using two power conditions and steady-state or pulsed operations, concentrations of O2, H2O and O3 species could be estimated. Computations using a plasma and fluid-flow model together with a chemistry model allowed the spatiotemporal concentration contours of N atoms, NO2 and N2O to be calculated. 237) In this manner, the reaction pathways and transport mechanisms of key RNS were studied.
Schmidt-Bleker et al. described measurements of the concentrations of O3, NO2, N2O, N2O5 and H2O2 in an atmospheric pressure Ar plasma with a combination of laser absorption spectroscopy, Fourier transformed infrared spectroscopy and plasma kinetics simulations. 238) The behavior of atomic nitrogen during the plasma processing of semiconductor devices has also been studied using vacuum ultraviolet absorption spectroscopy (VUVAS). 239) The other diagnostics in the semiconductor fabrication were reviewed elsewhere. 240) In addition, the line-of-sight concentrations of atomic nitrogen and NO in an atmospheric air plasma were systematically examined using a combination of the VUVAS and LIF techniques. 241)
Plasma chemistry is distinct from combustion chemistry. Specifically, nitrogen reaction products associated with the equilibrium phase are observed during combustion, whereas a pulsed electrical discharge generates a non-equilibrium phase containing high-temperature electrons but low-temperature ions and neutral species. A pulsed discharge also produces a very high density of reactive species on the nanosecond scale, after which a cascade of secondary reactions involving RNS occurs.
7.1.3. Transportation of reactive species in the aqueous phase
Gaseous species can dissolve in a bulk liquid or solid-state material through contact boundaries. Plasma-liquid interactions have been analyzed with a focus on radical generation using spin-trapping electron spin resonance (ESR). 242) As a plasma discharge contacts a water surface, a significant amount of ·OH radicals is generated. However, this process does not produce high concentrations of H2O2 because the ·OH radicals are very short-lived and tend not to combine. A wide variety of gaseous species is also generated in the plasma and subsequently dissolves in the water, after which the ·OH reacts with these dissolved species. Advanced oxidation of these compounds occurs to generate peroxides and other products that remain in the liquid phase.
NO generated by the plasma in the gas phase is transported to the liquid surface and plasma irradiation of the surface of the liquid may also produce NO. Spin-trapping ESR can be used to detect NO using 2-(4-carboxyphenyl)-4,4,5,5-tetramethyl imidazoline-loxyl-3-oxide [carboxy PTI (C-PTIO)]. This reagent reacts with NO according to the reaction C-PTIO + NO → C-PTI + NO2. Typically, minimal aqueous NO is detected during plasma irradiation, because gas-phase NO does not readily dissolve and the Henry's constants for NO and NO2 are very low. 32) Intracellular ONOO− has been observed using fluorescent probe analyses with 1,3,5,7-tetramethyl-2,6-dicyano-4,4-difluoro-8-(2-[2-hydroxy-2-oxoethoxy]−4-hydroxyphenyl)−3a,4-dihydro-3a-aza-4a-azonia-4-bora(IV)-s-indacene (NiSPY-3) and the scavenging agent 5,10,15,20-tetrakis(4-sulfonatophenyl)−21H,23H-porphyrin iron (III) chloride (FeTPPS). 243) In addition, HeLa cells incubated in plasma-activated media have shown increased levels of intracellular ONOO−. 243)
The species that are generated and dissolved at the liquid surface can flow within the bulk liquid, and the complex flow dynamics in the liquid have been analyzed. Kanazawa et al. studied the spread over the liquid surface from a pin electrode and reported that the ionic wind created a convection flow from the plasma irradiation point to the outer surface, with a flow that moved downward and upward at the outer and inner portions of the liquid vessel, respectively. 244) Kawasaki et al. also examined the laminar-like and circulating flows from the plasma irradiation point into the bulk liquid. 245,246) Brubaker et al. measured temporal changes in RONS concentrations throughout the depth of a liquid vessel and established that the plasma-induced liquid flow played an important role in determining reactive species concentrations at various locations in the liquid. 247,248) The flow of gases associated with the plasma irradiation forms a cavity on the liquid surface, and the depth of this cavity is determined by the balance between the electrohydrodynamic (EHD) force and the buoyancy of the liquid. 249) Studies have indicated that the plasma EHD force changes the mass transport inside the liquid, 248–250) such that unusual phenomena can appear in plasma-irradiated liquids.
7.1.4. Atomic localization of functional nitrogen
In the previous section, we reviewed methods of assessing the chemical states of nitrogen in solids, including XAFS, EELS and ELNES, all of which are very useful for this purpose. 251) The chemical states depend on the method of nitrogen incorporation in the crystalline state. Characterization of the functionality of the incorporated nitrogen should be performed at the atomic scale. Thus the atomic structure of nitrogen and the electronic configurations in the crystalline state are necessary to understand the chemical activity in the emergence of catalytic reactions and quantum spin characters.
Furthermore, there are computational studies for understanding the atomic localization and its functionalities. Molecular dynamics (MD) simulations of amine group formation were reported by the Hamaguchi group. 252–259) In addition, experimental work in which a plasma comprising a mixture of N2 and H2 was applied to organic substrates found the generation of functional groups, dangling bonds and desorbed species. 260–263) MD calculations have also shown the injection of NH3 ions and NH2 radicals into a polystyrene surface. 259) Considering the massive improvements in computing power, even complicated atomic localization problems can be solved using calculation techniques, capable of computing a large set of reaction systems.
7.2. Problems and prospects of functional nitrogen sciences
Before the suggested development of in situ utilization of nitrogen, i.e. the functional nitrogen method, one problem is the environmental issues responsible for residual risks. Conventional methods that use long-lived substances, such as drugs and fertilizers, are raised on environmental issues, and thus we should consider reuse, recycling, and recovery; otherwise, discarding forms of pollution and contamination. This is due to long-lived species. Indeed, the impact of residual pesticides on the biosphere and chemical fertilizers pollutes rivers and lakes to indirectly impact ecosystems. To solve these problems, we propose the use of short-lived species precisely at optimal locations and at suitable timing. A variety of short-lived species, particularly RNS generated in plasma, can be potentially used in many applications. In this view, it is advantageous that an environmentally benign method, using only air, water, and electricity, can be offered by the plasma-based in situ functionalization of RNS. To realize this, we should scientifically solve the responses seen in materials and living organisms, with respect to unstable nitrogen dosages. In dose dependences, it is common for the optimum dose to appear. (Fig. 18) The functionalization depends on the dose with optima. Therefore, there are several technological problems that need to be solved, including ways to control the localization of atomic nitrogen, gain the functionality of localized nitrogen, and stimulate living organisms using plasma-based RNS.
Fig 18. (Color online) Problems of plasma-based functional nitrogen are found in controlling responses versus responsibilities for the nitrogen dosages or RNS stresses. The plasma-based production of reactive species can be prospectively used at locations that are in demand. The in situ functionalization of nitrogen in both materials and living organisms is also essential for beneficial treatments using short-lived reactive species. Solving these problems requires assessments of atomic localization of the reactive nitrogen, responsible for being capable of controlling the functionalization through microscopic and mesoscopic responses seeing in electronic configurations on lattice systems and networking connections of metabolisms.
Download figure:
Standard image High-resolution imageNext, the atomic localization of nitrogen in the lattice system presents a problem. The localization of atomic nitrogen to provide various functions is a common process in both materials science and living systems. One example is the formation of NV centers in diamond and nitrogen atoms having isolated electronic spins in fullerene, which can function as qubits. The fabrication of these qubits must not damage the diamond crystal matrix or the substrate material during the localization of the atomic nitrogen, because small fluctuations in the periodic potential of the crystal generate spatially optimal positions with energy minima. This process is analogous to the doping of semiconductor devices.
The irreversible processes associated with the doping of living systems or inanimate materials are associated with the lifecycles of these substances (Fig. 19). Various events such as fracture, damage and fatigue must be included when assessing a lifecycle, and phenomena such as senescence, healing and disease are also important in biological systems. For example, in each phase of the lifecycle, the hierarchically trans-scale representation of the atomic localization of nitrogen and the network of observed parameters with hidden states can be examined to bridge between macroscopic and microscopic dynamics.
Fig 19. Diagram summarizing a real-time series of measurements at each phase of the lifecycle. The functional emergence of living systems and matter is assessed on the microscopic, mesoscopic and macroscopic levels. This trans-scale modeling is vital to assessing the kinetics of the states of the chain reaction network.
Download figure:
Standard image High-resolution imageThe plant lifecycle stages include seed germination, seedling, plant growth, mature plant and flower, and fruit with seed. (Fig. 19) In each phase, the plant uptakes and assimilates nitrogen as a nutrient. However, the timing of the nitrogen uptake and any effect of environmental RNS on the plant assimilation process has not yet been clarified. During the plasma activation of seeds and plants, the timing and duration of exposure to the RNS are certainly essential. That is, the network controls the functionalization of RNS during plasma processing.
In summary, we encompass an outlook for the future. (Fig. 20) Conventional processing is designed by the application of plasma treatments to both materials and living organisms. This is regarded as a linear cascade from plasma generation to process outcomes, which has previously been proven by many innovative emerging technologies. However, as the linear cascade model of plasma effects has been developed, it is necessary to increase our understanding of the constituents and compositions of the plasma-generated species, and the individual effects of each species need to be deconvolved. Moreover, it has been revealed to be essential to gain the plasma effects through the evolution of multiple pathways of reactions and synergisms of multiple species. In other words, the present research has already realized the preparation of the selected reactive species and decomposes the complex reaction paths into its primary and secondary impacts on hierarchical systems, such as living organisms, and to reveal hidden or latent reactions. Based on this knowledge, we may understand the relationship between the selected reactive species and the emergence of functions in materials and living organisms. This is insufficient to engineer the functionality of the reactive species. Since we should analyze all the multiple pathways quantitatively, we emphasize the requirement for multiple in situ and real-time measurements during this process.
Fig 20. (Color online) (a) A conventional model of the plasma effects by the linear cascade reaction responses. (b) The present status of the modeling of the plasma-induced effects by the wide-spreading synergisms on the multiple reactions pathways. (c) A future goal of the modeling by the complicated reaction network with communication between the clusters of bacteria, organelle, cells, etc.
Download figure:
Standard image High-resolution imageAs a consequence, our outlook for the future is the sciences of the clustering networks that are connected in a complicated manner among plasma, liquid, solid, and living organisms. An ensemble of multiple states is analyzed. For either materials or living organisms, changes remain in the irreversible life cycle. Similar to plasma stimulation, it is believed that the system responds by modifying the connectivity of the clustering networks. In other words, the stimulated states of the network are temporarily relaxed by losing their excited energy. This relaxation process can be decomposed into an ensemble of kinetically controlled reactions on the network. Lastly, a trans-scale understanding of the organized network state at each phase of the lifecycle would be possible by a series of in situ real-time measurements of multiple species with spatiotemporal resolutions (Fig. 19 bottom).
8. Conclusion
State-of-the-art plasma technology is capable of synthesizing RNS through excitation, dissociation, ionization and recombination processes in association with collisions between a high density of electrons and nitrogen molecules. The first stage in this process is the production of RNS, after which these species are utilized as precursors for further reactions to provide functionalization. In other scenarios, functional nitrogen can be produced initially. Starting from the doping of nitrogen into a suitable matrix, the controlled localization of nitrogen atoms with atomic-level precision can provide long-term functionalization of nitrogen in both inorganic and biological materials. RNS can also be generated in situ by a plasma discharge. Functional nitrogen science based on plasma processing covers a wide range of applications. Indeed, functional nitrogen can be employed for the fabrication of quantum devices and photocatalysts and also in the stimulation of plant metabolism and the treatment of manure, water and biological clusters.
Hierarchical multiple measurements, analyses and modeling involving network analysis of the lifecycles of RNS are important in this field. This means that the translocation from the production of RNS to localization is analyzed by integrating multiple plasma diagnostics. It is also important to determine the density distributions of molecular and vibrationally-excited nitrogen with high spatiotemporal resolution using laser-induced fluorescence, as a means of studying aqueous radicals in liquids. In this manner, artificial nitrogen cycles can be created. Functional nitrogen science and technology are closely associated with advances in plasma processing technology and therefore will likely continue to progress.
Acknowledgments
The authors would like to thank Dr. Kazuhito Kawakita, Dr. Kiyoshi Takamatsu, Dr. Keiji Imoto, Dr. Akio Komori, Dr. Masaru Hori, Dr. Masaharu Shiratani, Dr. Masafumi Ito, Dr. Keisuke Takashima, and Dr. Shota Sasaki for fruitful discussions regarding the effects of plasmas on biological systems, Dr. Noriyasu Ohno, Dr. Hirotaka Toyoda, Dr. Masaaki Mizuno and Dr. Shinya Toyokuni for encouraging this work, and Dr. Katsutoshi Nagaoka (Nagoya University), Dr. David B. Graves and Dr. A. Fridman for helpful discussions concerning catalytic reactions. The authors are also grateful to the Program Committee of the 13th International Symposium on Advanced Plasma Science and its Applications for Nitrides and Nanomaterials and the 14th International Conference on Plasma Nanotechnology and Science (ISPlasma2021/IC-PLANTS2021). T. K. declares that this research was partly supported by JSPS KAKENHI Grant No. 18H03687, Frontier Research in Duo (FRiD) in Tohoku University (Project Number 1904), and Plasma-bio consortium (Grant Number 01222001). K. I. was supported in part by KAKENHI Grants-in-Aid (nos. 19H05462, 17H02805, 20H00142 and 21H04451) from JSPS and by the Center for Low Temperature Plasma Science, Nagoya University.