Characterizing the Variation of Dissolvable PAHs in Receiving Water in a Reclaimed Water Irrigation Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Analysis
3. Results and Discussion
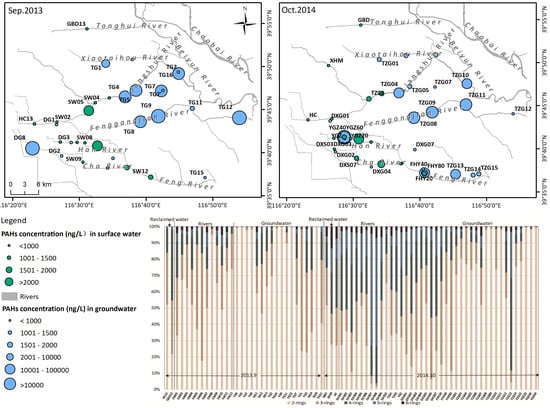
3.1. Characteristics of Dissolved PAHs Concentration in Rivers and Aquifers
3.2. Temporal Variation of Dissolved PAHs
3.3. Physio-Chemical Indices Associated with PAHs Concentration
3.4. The Influences of Reclaimed Water at Reclaimed Water Irrigation Region
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.; Fu, J.; Zhang, H.; Li, Z.; Ma, Y.; Wu, M.; Liu, X. Spatial and seasonal variations of occurrences and concentrations of endocrine disrupting chemicals in unconfined and confined aquifers recharged by reclaimed water: A field study along the Chaobai River, Beijing. Sci. Total Environ. 2013, 450, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiang, X.; Li, M.; Ma, Y.P.; Wang, J.H.; Liu, X. Occurrence and risk assessment of pharmaceuticals and personal care products and endocrine disrupting chemicals in reclaimed water and receiving groundwater in China. Ecotoxicol. Environ. Saf. 2015, 119, 74–80. [Google Scholar] [CrossRef]
- Ma, W.; Nie, C.; Chen, B.; Cheng, X.; Lun, X.; Zeng, F. Adsorption and biodegradation of three selected endocrine disrupting chemicals in river-based artificial groundwater recharge with reclaimed municipal wastewater. J. Environ. Sci. 2015, 31, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, X.; Li, B.; Ma, Y.; Zhang, Y.; Yang, L.; Bu, H.; Holm, P.E. Temporal variation in groundwater hydrochemistry driven by natural and anthropogenic processes at a reclaimed water irrigation region. Hydrol. Res. 2018, 49, 1652–1668. [Google Scholar] [CrossRef] [Green Version]
- Loganathan, B.G.; Lam, K.S. Global Contamination Trends of Persistent Organic Chemicals; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Chen, L.; Jin, S.; Liu, Y.; Liu, F. Presence of Semi-Volatile Organic Contaminants in Shallow Groundwater of Selected Regions in China. Ground Water Monit. R. 2014, 34, 33–43. [Google Scholar] [CrossRef]
- Han, D.; Currell, M.J. Persistent organic pollutants in China’s surface water systems. Sci. Total Environ. 2017, 580, 602–625. [Google Scholar] [CrossRef] [PubMed]
- Weihong, D.; Wei, X.; Xiaosi, S.; Chuanlei, W.; Zhipeng, C.; Yuyu, W. Micro-organic Contaminants in Groundwater in China. Hydrogeol. J. 2018, 26, 1351–1369. [Google Scholar]
- Manamsa, K.; Crane, E.; Stuart, M.; Talbot, J.; Lapworth, D.; Hart, A. A national-scale assessment of micro-organic contaminants in groundwater of England and Wales. Sci. Total Environ. 2016, 568, 712–726. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.E.; Overton, E.B.; Meyer, B.M.; Miles, M.S.; Hooper-Bui, L. Changes in the concentration and relative abundance of alkanes and PAHs from the Deepwater Horizon oiling of coastal marshes. Mar. Pollut. Bull. 2014, 86, 291–297. [Google Scholar] [CrossRef]
- Wania, F.; Mackay, D. Tracking the distribution of persistent organic pollutants. Environ. Sci. Technol. 1996, 30, A390–A396. [Google Scholar] [CrossRef]
- Breivik, K.; Armitage, J.M.; Wania, F.; Sweetman, A.J.; Jones, K.C. Tracking the Global Distribution of Persistent Organic Pollutants Accounting for E-Waste Exports to Developing Regions. Environ. Sci. Technol. 2016, 50, 798–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhao, Y.; Sun, J.; Zhang, Y.; Liu, C. The distribution and sources of polycyclic aromatic hydrocarbons in shallow groundwater from an alluvial-diluvial fan of the Hutuo River in North China. Front. Earth Sci. 2019, 13, 33–42. [Google Scholar] [CrossRef]
- Ozaki, N.; Takamura, Y.; Kojima, K.; Kindaichi, T. Loading and removal of PAHs in a wastewater treatment plant in a separated sewer system. Water Res. 2015, 80, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Qi, W.; Liu, H.; Qu, J. Occurrence, behavior and removal of typical substituted and parent polycyclic aromatic hydrocarbons in a biological wastewater treatment plant. Water Res. 2014, 52, 11–19. [Google Scholar] [CrossRef]
- Qiao, M.; Bai, Y.; Cao, W.; Huo, Y.; Zhao, X.; Liu, D.; Li, Z. Impact of secondary effluent from wastewater treatment plants on urban rivers: Polycyclic aromatic hydrocarbons and derivatives. Chemosphere 2018, 211, 185–191. [Google Scholar] [CrossRef]
- Zhang, G.; Li, J.; Cheng, H.; Li, X.; Xu, W.; Jones, K.C. Distribution of organochlorine pesticides in the northern South China Sea: Implications for land outflow and air-sea exchange. Environ. Sci. Technol. 2007, 41, 3884–3890. [Google Scholar] [CrossRef]
- Gustafson, K.E.; Dickhut, R.M. Gaseous Exchange of Polycyclic Aromatic Hydrocarbons across the Air−Water Interface of Southern Chesapeake Bay. Environ. Sci. Technol. 1997, 31, 1623–1629. [Google Scholar] [CrossRef]
- Fernandes, M.B.; Sicre, M.A.; Boireau, A.; Tronczynski, J. Polyaromatic hydrocarbon (PAH) distributions in the Seine River and its estuary. Mar. Pollut. Bull. 1997, 34, 857–867. [Google Scholar] [CrossRef]
- Montuori, P.; Triassi, M. Polycyclic aromatic hydrocarbons loads into the Mediterranean Sea: Estimate of Sarno River inputs. Mar. Pollut. Bull. 2012, 64, 512–520. [Google Scholar] [CrossRef] [Green Version]
- Shaw, M.; Tibbetts, I.R.; Muller, J.F. Monitoring PAHs in the Brisbane River and Moreton Bay, Australia, using semipermeable membrane devices and EROD activity in yellowfin bream, Acanthopagrus australis. Chemosphere 2004, 56, 237–246. [Google Scholar] [CrossRef]
- Malik, A.; Verma, P.; Singh, A.K.; Singh, K.P. Distribution of polycyclic aromatic hydrocarbons in water and bed sediments of the Gomti River, India. Environ. Monit. Assess. 2011, 172, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Khim, J.S.; Chen, C.; Naile, J.E.; Lu, Y.; Kannan, K.; Park, J.; Luo, W.; Jiao, W.; Hu, W.; et al. Perfluorinated compounds in surface waters from Northern China: Comparison to level of industrialization. Environ. Int. 2012, 42, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; He, M.; Yang, Z.; Lin, C.; Quan, X.; Wang, H. Distribution of polycyclic aromatic hydrocarbons in water, suspended particulate matter and sediment from Daliao River watershed, China. Chemosphere 2007, 68, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Xia, X.; Shen, Z.; Zhou, Z. Distribution and sources of polycyclic aromatic hydrocarbons in Wuhan section of the Yangtze River, China. Environ. Monit. Assess. 2007, 133, 447–458. [Google Scholar] [CrossRef]
- Luo, X.J.; Mai, B.X.; Yang, Q.S.; Fu, J.M.; Sheng, G.Y.; Wang, Z.S. Polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides in water columns from the Pearl River and the Macao harbor in the Pearl River Delta in South China. Mar. Pollut. Bull. 2004, 48, 1102–1115. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, H.; Lydy, M.J.; You, J. Occurrence, seasonal variation and inhalation exposure of atmospheric organophosphate and pyrethroid pesticides in an urban community in South China. Chemosphere 2014, 95, 363–369. [Google Scholar] [CrossRef]
- Qi, W.; Liu, H.; Pernet-Coudrier, B.; Qu, J. Polycyclic aromatic hydrocarbons in wastewater, WWTPs effluents and in the recipient waters of Beijing, China. Environ. Sci. Pollut. Res. 2013, 20, 4254–4260. [Google Scholar] [CrossRef] [Green Version]
- Jin, A.; He, J.; Chen, S.; Huang, G. Distribution and transport of PAHs in soil profiles of different water irrigation areas in Beijing, China. Environ. Sci. Processes Impacts 2014, 16, 1526–1534. [Google Scholar] [CrossRef]
- Han, D.M.; Tong, X.X.; Jin, M.G.; Hepburn, E.; Tong, C.S.; Song, X.F. Evaluation of organic contamination in urban groundwater surrounding a municipal landfill, Zhoukou, China. Environ. Monit. Assess. 2013, 185, 3413–3444. [Google Scholar] [CrossRef]
- Bahr, A.; Fischer, A.; Vogt, C.; Bombach, P. Evidence of polycyclic aromatic hydrocarbon biodegradation in a contaminated aquifer by combined application of in situ and laboratory microcosms using 13C-labelled target compounds. Water Res. 2015, 69, 100–109. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Ochonogor, A. Groundwater contamination by polycyclic aromatic hydrocarbon due to diesel spill from a telecom base station in a Nigerian City: Assessment of human health risk exposure. Environ. Monit. Assess. 2018, 190, 249. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, F.; Liu, Q. PAHs behavior in surface water and groundwater of the Yellow River estuary: Evidence from isotopes and hydrochemistry. Chemosphere 2017, 178, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, F. Polycyclic aromatic hydrocarbons in the Yellow River estuary: Levels, sources and toxic potency assessment. Mar. Pollut. Bull. 2017, 116, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.J.; Yuan, D.; Jiang, Y.J.; Sun, Y.; Li, Y.; Xu, X. Sources and transports of polycyclic aromatic hydrocarbons in the Nanshan Underground River, China. Environ. Earth Sci. 2014, 71, 1967–1976. [Google Scholar] [CrossRef] [Green Version]
- Lan, J.; Sun, Y.; Xiao, S.; Yuan, D. Polycyclic aromatic hydrocarbon contamination in a highly vulnerable underground river system in Chongqing, Southwest China. J. Geochem. Explor. 2016, 168, 65–71. [Google Scholar] [CrossRef]
- Wu, C.; Zhu, H.; Luo, Y.; Wang, J. Concentrations and potential health hazards of polycyclic aromatic hydrocarbon in shallow groundwater of a metal smelting area in Southeastern China. Sci. Total Environ. 2016, 569, 1561–1569. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Xu, X.; Wu, X.; Jiang, Z.; He, S.; Qian, K. Occurrence and source apportionment of PAHs in highly vulnerable karst system. Sci. Total Environ. 2014, 490, 153–160. [Google Scholar] [CrossRef]
- Jin, A.; He, J.; Chen, S.; Huang, G. Impact of reclaimed water irrigation on PAHs in agricultural soil and groundwater. AIP Conf. Proc. 2010, 1251, 37–40. [Google Scholar]
- Bi, E.; Liu, Y.; He, J.; Wang, Z.; Liu, F. Screening of Emerging Volatile Organic Contaminants in Shallow Groundwater in East China. Ground Water Monit. R. 2012, 32, 53–58. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Z.; Niu, J. Temperature-dependent sorption of polycyclic aromatic hydrocarbons on natural and treated sediments. Chemosphere 2011, 82, 895–900. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Zhang, Q.; Zhao, H.; Yang, Y.; Zhang, Y.; Xie, Q.; Chen, J. Seasonal variation, air-water exchange, and multivariate source apportionment of polycyclic aromatic hydrocarbons in the coastal area of Dalian, China. Environ. Pollut. 2019, 244, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.P.R.; Lapworth, D.J.; Nkhuwa, D.C.W.; Stuart, M.E.; Gooddy, D.C.; Bell, R.A.; Chirwa, M.; Kabika, J.; Liemisa, M.; Chibesa, M.; et al. Emerging contaminants in urban groundwater sources in Africa. Water Res. 2015, 72, 51–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Wang, X.; Jia, J.; Chen, Z. Spatial variation and sources of polycyclic aromatic hydrocarbons influenced by intensive land use in an urbanized river network of East China. Sci. Total Environ. 2018, 627, 671–680. [Google Scholar] [CrossRef]
- Hinga, K.R. Degradation rates of low molecular weight PAH correlate with sediment TOC in marine subtidal sediments. Mar. Pollut. Bull. 2003, 46, 466–474. [Google Scholar] [CrossRef]
- Rothermich, M.M.; Hayes, L.A.; Lovley, D.R. Anaerobic, sulfate-dependent degradation of polycyclic aromatic hydrocarbons in petroleum-contaminated harbor sediment. Environ. Sci. Technol. 2002, 36, 4811–4817. [Google Scholar] [CrossRef]
- Shang, J.; Chen, J.; Shen, Z.; Xiao, X.; Yang, H.; Wang, Y.; Ruan, A. Photochemical degradation of PAHs in estuarine surface water: Effects of DOM, salinity, and suspended particulate matter. Environ. Sci. Pollut. Res. 2015, 22, 12374–12383. [Google Scholar] [CrossRef]
- Xiao, Y.; Gu, X.; Yin, S.; Pan, X.; Shao, J.Y. Investigation of Geochemical Characteristics and Controlling Processes of Groundwater in a Typical Long-Term Reclaimed Water Use Area. Water 2017, 9, 800. [Google Scholar] [CrossRef] [Green Version]
- Renau-Pruñonosa, A.; García-Menéndez, O.; Ibáñez, M.; Vázquez-Suñé, E.; Boix, C.; Ballesteros, B.B.; Hernández García, M.; Morell, I.; Hernández, F. Identification of Aquifer Recharge Sources as the Origin of Emerging Contaminants in Intensive Agricultural Areas. La Plana de Castellón, Spain. Water 2020, 12, 731. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Espinosa, L.G.; Daesslé, L.W. Consolidating the use of reclaimed water for irrigation and infiltration in a semi-arid agricultural valley in Mexico: Water management experiences and results. J. Water Sanit. Hyg. Dev. 2018, 8, 679–687. [Google Scholar] [CrossRef]
- Deh-Haghi, Z.; Bagheri, A.; Fotourehchi, Z.; Damalas, C.A. Farmers’ acceptance and willingness to pay for using treated wastewater in crop irrigation: A survey in western Iran. Agric. Water Manag. 2020, 239, 106262. [Google Scholar] [CrossRef]






| Compounds | MQL (ng·L−1) | Recovery% | SD% (n = 6) |
|---|---|---|---|
| Nap | 0.02 | 65.3 | 2.03 |
| Acy | 0.01 | 60.2 | 5.38 |
| Ace | 0.07 | 65.8 | 9.41 |
| Fluo | 0.20 | 77.2 | 8.57 |
| Phe | 0.34 | 96.6 | 6.33 |
| Ant | 0.70 | 80.2 | 6.72 |
| Flua | 0.88 | 106.1 | 0.69 |
| Pyr | 1.00 | 110.8 | 1.66 |
| BaA | 3.20 | 115.6 | 1.39 |
| Chry | 3.41 | 112.5 | 3.40 |
| BbF | 1.85 | 100.3 | 2.50 |
| BkF | 3.32 | 89.5 | 0.41 |
| BaP | 2.90 | 88.5 | 3.86 |
| IcdP | 3.68 | 118.4 | 2.56 |
| DBA | 4.50 | 119.2 | 0.46 |
| BghiP | 3.56 | 101.7 | 2.51 |
| p Values | Nap | Acy | Ace | Fl | Phe | Ant | 3-Rings | Flu | Pry | BaA | Chr |
| RW | 0.006 | 0.000 | 0.551 | 0.793 | 0.907 | 0.344 | 0.089 | 0.008 | 0.016 | 0.165 | 0.372 |
| SW | 0.267 | 0.822 | 0.547 | 0.421 | 0.574 | 0.383 | 0.344 | 0.603 | 0.372 | 0.016 | 0.002 |
| GW | 0.004 | 0.069 | 0.001 | 0.152 | 0.796 | 0.030 | 0.004 | 0.633 | 0.045 | 0.207 | 0.014 |
| p Values | 4-rings | BbF | BKF | BaP | DBA | 5-rings | InP | BghiP | 6-rings | ∑16PAHs | |
| RW | 0.339 | 0.219 | 0.346 | 0.061 | 0.110 | 0.168 | 0.108 | 0.190 | 0.057 | 0.601 | |
| SW | 0.014 | 0.000 | 0.000 | 0.002 | 0.002 | 0.000 | 0.002 | 0.000 | 0.000 | 0.840 | |
| GW | 0.640 | 0.037 | 0.127 | 0.073 | 0.001 | 0.015 | 0.078 | 0.019 | 0.016 | 0.004 |
| Oct. 2014 | 2-Rings | 3-Rings | 4-Rings | 5-Rings | 6-Rings | ∑16PAHs | Sep.2013 | 2-Rings | 3-Rings | 4-Rings | 5-Rings | 6-Rings | ∑16PAHs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 0.19 | −0.03 | 0.19 | 0.46 | 0.49 | 0.47 | pH | −0.22 | −0.23 | −0.09 | 0.18 | 0.44 | −0.28 |
| DO | −0.37 | −0.60 * | −0.15 | 0.11 | 0.09 | −0.18 | DO | −0.26 | 0.21 | −0.32 | −0.34 | −0.03 | −0.28 |
| EC | 0.38 | 0.61 * | −0.21 | −0.28 | −0.28 | 0.01 | EC | 0.10 | 0.23 | 0.15 | −0.03 | −0.58 * | 0.17 |
| Cl− | 0.56 * | 0.82 ** | −0.04 | −0.16 | −0.16 | 0.23 | Cl− | 0.16 | −0.10 | −0.17 | −0.10 | −0.27 | 0.09 |
| SO42− | 0.07 | 0.16 | −0.51 | −0.44 | −0.40 | −0.32 | SO42− | −0.02 | −0.03 | 0.07 | −0.07 | −0.22 | −0.03 |
| HCO3− | 0.49 | 0.61 * | −0.11 | −0.12 | −0.10 | 0.20 | HCO3− | 0.09 | 0.07 | −0.19 | −0.18 | −0.58 * | 0.08 |
| NO3− | −0.53 * | −0.74 ** | 0.16 | 0.19 | 0.15 | −0.18 | NO3− | −0.22 | 0.02 | 0.06 | −0.16 | −0.31 | −0.21 |
| TN | −0.41 | −0.22 | 0.18 | 0.10 | 0.03 | −0.16 | TN | 0.51 * | 0.51 * | 0.47 * | 0.27 | 0.14 | 0.69 ** |
| Oct. 2014 | 2-Rings | 3-Rings | 4-Rings | 5-Rings | 6-Rings | ∑16PAHs | Sep.2013 | 2-Rings | 3-Rings | 4-Rings | 5-Rings | 6-Rings | ∑16PAHs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 0.28 | 0.39 * | 0.17 | 0.04 | −0.13 | 0.28 | pH | −0.07 | −0.15 | 0.14 | −0.31 | −0.40 | −0.07 |
| EC | −0.37 | −0.20 | −0.04 | 0.00 | −0.02 | −0.38 | EC | 0.31 | 0.25 | −0.09 | 0.12 | 0.48 * | 0.31 |
| Cl− | 0.00 | −0.15 | 0.09 | 0.12 | 0.09 | 0.00 | Cl− | 0.32 | 0.17 | −0.07 | 0.10 | 0.39 | 0.32 |
| SO42− | −0.32 | −0.11 | −0.12 | −0.08 | −0.09 | −0.32 | SO42− | 0.02 | 0.16 | −0.24 | −0.04 | 0.18 | 0.02 |
| HCO3− | −0.36 | −0.25 | −0.09 | −0.02 | −0.01 | −0.36 | HCO3− | 0.23 | 0.25 | −0.38 | 0.00 | 0.23 | 0.23 |
| NO3− | −0.25 | −0.29 | 0.27 | −0.16 | −0.47 | −0.26 | NO3− | −0.05 | 0.07 | 0.26 | 0.64 ** | 0.72 ** | −0.05 |
| TN | −0.19 | 0.22 | −0.18 | −0.15 | −0.14 | −0.19 | TN | 0.53 * | 0.59 * | −0.01 | −0.01 | 0.10 | 0.53 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, B.; Ma, Y.; Yang, L.; Song, X. Characterizing the Variation of Dissolvable PAHs in Receiving Water in a Reclaimed Water Irrigation Region. Water 2020, 12, 2766. https://doi.org/10.3390/w12102766
Wang Y, Li B, Ma Y, Yang L, Song X. Characterizing the Variation of Dissolvable PAHs in Receiving Water in a Reclaimed Water Irrigation Region. Water. 2020; 12(10):2766. https://doi.org/10.3390/w12102766
Chicago/Turabian StyleWang, Yajun, Binghua Li, Ying Ma, Lihu Yang, and Xianfang Song. 2020. "Characterizing the Variation of Dissolvable PAHs in Receiving Water in a Reclaimed Water Irrigation Region" Water 12, no. 10: 2766. https://doi.org/10.3390/w12102766