Evaluation of the Abdala Vaccine: Antibody and Cellular Response to the RBD Domain of SARS-CoV-2
Abstract
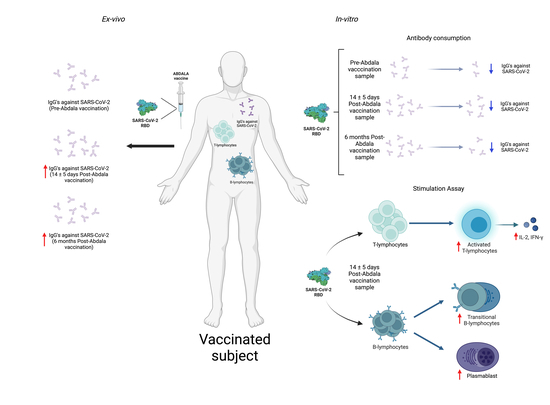
:1. Introduction
2. Materials and Methods
2.1. Subjects Studied
2.2. Blood and Serum Sample Collection
2.3. Anti-RBD Antibody Consumption
2.4. Detection of IgGs Related to SARS-CoV-2 by Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Stimulation Assay Using Whole Blood
2.6. Cytometric Bead Array (CBA)
2.7. Multi-Parametric Flow Cytometry Analysis
2.8. Statistical Analysis
3. Results
3.1. Abdala Vaccine Increases the OD of Specific IgGs against RBD after Two Weeks of Vaccination and Maintains It over Six Months
3.2. Consumption of IgGs before and after Abdala Vaccination and Six Months Post-Abdala Vaccination
3.3. RBD Stimulation Induces Plasmablast and Transitional B-Lymphocyte Increases
3.4. RBD Stimulation Induces Helper-T-Lymphocyte Activation, as well as IL-2 and IFN-γ Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Collier, A.Y.; Yu, J.; McMahan, K.; Liu, J.; Chandrashekar, A.; Maron, J.S.; Atyeo, C.; Martinez, D.R.; Ansel, J.L.; Aguayo, R.; et al. Differential Kinetics of Immune Responses Elicited by COVID-19 Vaccines. N. Engl. J. Med. 2021, 385, 2010–2012. [Google Scholar] [CrossRef] [PubMed]
- Polinski, J.M.; Weckstein, A.R.; Batech, M.; Kabelac, C.; Kamath, T.; Harvey, R.; Jain, S.; Rassen, J.A.; Khan, N.; Schneeweiss, S. Durability of the Single-Dose Ad26.COV2.S Vaccine in the Prevention of COVID-19 Infections and Hospitalizations in the US Before and During the Delta Variant Surge. JAMA Netw. Open 2022, 5, e222959. [Google Scholar] [CrossRef]
- Widge, A.T.; Rouphael, N.G.; Jackson, L.A.; Anderson, E.J.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N. Engl. J. Med. 2021, 384, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Doria-Rose, N.; Suthar, M.S.; Makowski, M.; O’Connell, S.; McDermott, A.B.; Flach, B.; Ledgerwood, J.E.; Mascola, J.R.; Graham, B.S.; Lin, B.C.; et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for COVID-19. N. Engl. J. Med. 2021, 384, 2259–2261. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 Vaccine up to 6 Months in a Large Integrated Health System in the USA: A Retrospective Cohort Study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- World Health Organization COVID-19 Vaccines. 2023. Available online: https://www.who.int/news/item/18-05-2023-statement-on-the-antigen-composition-of-covid-19-vaccines (accessed on 17 November 2023).
- Gobierno de México Política Nacional de Vacunación Contra El Virus SARS-CoV-2, Para La Prevención de La COVID-19 En México. Documento Rector. Versión 10.1. 2022. Available online: https://vacunacovid.gob.mx/wp-content/uploads/2022/12/2022.12.23-PNVxCOVID.pdf (accessed on 17 November 2023).
- Hernández-Bernal, F.; Ricardo-Cobas, M.C.; Martín-Bauta, Y.; Navarro-Rodríguez, Z.; Piñera-Martínez, M.; Quintana-Guerra, J.; Urrutia-Pérez, K.; Urrutia-Pérez, K.; Chávez-Chong, C.O.; Azor-Hernández, J.L.; et al. Safety, Tolerability, and Immunogenicity of a SARS-CoV-2 Recombinant Spike RBD Protein Vaccine: A Randomised, Double-Blind, Placebo-Controlled, Phase 1-2 Clinical Trial (ABDALA Study). eClinicalMedicine 2022, 46, 101383. [Google Scholar] [CrossRef]
- Hernández-Bernal, F.; Ricardo-Cobas, M.C.; Martín-Bauta, Y.; Rodríguez-Martínez, E.; Urrutia-Pérez, K.; Urrutia-Pérez, K.; Quintana-Guerra, J.; Navarro-Rodríguez, Z.; Piñera-Martínez, M.; Rodríguez-Reinoso, J.L.; et al. A Phase 3, Randomised, Double-Blind, Placebo-Controlled Clinical Trial Evaluation of the Efficacy and Safety of a SARS-CoV-2 Recombinant Spike RBD Protein Vaccine in Adults (ABDALA-3 Study). Lancet Reg. Health—Am. 2023, 21, 100497. [Google Scholar] [CrossRef]
- Más-Bermejo, P.I.; Dickinson-Meneses, F.O.; Almenares-Rodríguez, K.; Sánchez-Valdés, L.; Guinovart-Díaz, R.; Vidal-Ledo, M.; Galbán-García, E.; Olivera-Nodarse, Y.; Morgado-Vega, I.; Dueñas-Carrera, S.; et al. Cuban Abdala Vaccine: Effectiveness in Preventing Severe Disease and Death from COVID-19 in Havana, Cuba; A Cohort Study. Lancet Reg. Health—Am. 2022, 16, 100366. [Google Scholar] [CrossRef] [PubMed]
- Islas-Vazquez, L.; Cruz-Aguilar, M.; Velazquez-Soto, H.; Jiménez-Corona, A.; Pérez-Tapia, S.M.; Jimenez-Martinez, M.C. Effector-Memory B-Lymphocytes and Follicular Helper T-Lymphocytes as Central Players in the Immune Response in Vaccinated and Nonvaccinated Populations against SARS-CoV-2. Vaccines 2022, 10, 1761. [Google Scholar] [CrossRef] [PubMed]
- Moulana, A.; Dupic, T.; Phillips, A.M.; Chang, J.; Roffler, A.A.; Greaney, A.J.; Starr, T.N.; Bloom, J.D.; Desai, M.M. The Landscape of Antibody Binding Affinity in SARS-CoV-2 Omicron BA.1 Evolution. eLife 2023, 12, e83442. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, P.J.; Ruan, Q.; Grieshaber, J.L.; Swift, K.M.; Taylor, R.E.; Prostko, J.C.; Tetin, S.Y. Affinity of Anti-Spike Antibodies in SARS-CoV-2 Patient Plasma and Its Effect on COVID-19 Antibody Assays. eBioMedicine 2022, 75, 103796. [Google Scholar] [CrossRef]
- Rogliani, P.; Chetta, A.; Cazzola, M.; Calzetta, L. SARS-CoV-2 Neutralizing Antibodies: A Network Meta-Analysis across Vaccines. Vaccines 2021, 9, 227. [Google Scholar] [CrossRef]
- Muruato, A.E.; Fontes-Garfias, C.R.; Ren, P.; Garcia-Blanco, M.A.; Menachery, V.D.; Xie, X.; Shi, P.-Y. A High-Throughput Neutralizing Antibody Assay for COVID-19 Diagnosis and Vaccine Evaluation. Nat. Commun. 2020, 11, 4059. [Google Scholar] [CrossRef]
- Pape, K.A.; Dileepan, T.; Kabage, A.J.; Kozysa, D.; Batres, R.; Evert, C.; Matson, M.; Lopez, S.; Krueger, P.D.; Graiziger, C.; et al. High-Affinity Memory B Cells Induced by SARS-CoV-2 Infection Produce More Plasmablasts and Atypical Memory B Cells than Those Primed by mRNA Vaccines. Cell Rep. 2021, 37, 109823. [Google Scholar] [CrossRef]
- Fryer, H.A.; Hartley, G.E.; Edwards, E.S.J.; O’Hehir, R.E.; Van Zelm, M.C. Humoral Immunity and B-Cell Memory in Response to SARS-CoV-2 Infection and Vaccination. Biochem. Soc. Trans. 2022, 50, 1643–1658. [Google Scholar] [CrossRef]
- Cromer, D.; Juno, J.A.; Khoury, D.; Reynaldi, A.; Wheatley, A.K.; Kent, S.J.; Davenport, M.P. Prospects for Durable Immune Control of SARS-CoV-2 and Prevention of Reinfection. Nat. Rev. Immunol. 2021, 21, 395–404. [Google Scholar] [CrossRef]
- Hartley, G.E.; Edwards, E.S.J.; Aui, P.M.; Varese, N.; Stojanovic, S.; McMahon, J.; Peleg, A.Y.; Boo, I.; Drummer, H.E.; Hogarth, P.M.; et al. Rapid Generation of Durable B Cell Memory to SARS-CoV-2 Spike and Nucleocapsid Proteins in COVID-19 and Convalescence. Sci. Immunol. 2020, 5, eabf8891. [Google Scholar] [CrossRef]
- Byazrova, M.; Yusubalieva, G.; Spiridonova, A.; Efimov, G.; Mazurov, D.; Baranov, K.; Baklaushev, V.; Filatov, A. Pattern of Circulating SARS-CoV-2-specific Antibody-secreting and Memory B-cell Generation in Patients with Acute COVID-19. Clin. Transl. Immunol. 2021, 10, e1245. [Google Scholar] [CrossRef]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep Immune Profiling of COVID-19 Patients Reveals Distinct Immunotypes with Therapeutic Implications. Science 2020, 369, eabc8511. [Google Scholar] [CrossRef]
- Kratzer, B.; Trapin, D.; Ettel, P.; Körmöczi, U.; Rottal, A.; Tuppy, F.; Feichter, M.; Gattinger, P.; Borochova, K.; Dorofeeva, Y.; et al. Immunological Imprint of COVID-19 on Human Peripheral Blood Leukocyte Populations. Allergy 2021, 76, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Hartley, G.E.; Edwards, E.S.J.; O’Hehir, R.E.; Van Zelm, M.C. New Insights into Human Immune Memory from SARS-CoV-2 Infection and Vaccination. Allergy 2022, 77, 3553–3566. [Google Scholar] [CrossRef] [PubMed]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; et al. Robust Neutralizing Antibodies to SARS-CoV-2 Infection Persist for Months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef]
- Braun, J.; Loyal, L.; Frentsch, M.; Wendisch, D.; Georg, P.; Kurth, F.; Hippenstiel, S.; Dingeldey, M.; Kruse, B.; Fauchere, F.; et al. SARS-CoV-2-Reactive T Cells in Healthy Donors and Patients with COVID-19. Nature 2020, 587, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef]
- Huang, W.-T.; Weng, S.-W.; Tzeng, H.-T.; Yen, F.-C.; Chiang, Y.-S.; You, H.-L. Lymphocyte Subpopulations Associated with Neutralizing Antibody Levels of SARS-CoV-2 for COVID-19 Vaccination. Vaccines 2022, 10, 1550. [Google Scholar] [CrossRef]
- Rodda, L.B.; Morawski, P.A.; Pruner, K.B.; Fahning, M.L.; Howard, C.A.; Franko, N.; Logue, J.; Eggenberger, J.; Stokes, C.; Golez, I.; et al. Imprinted SARS-CoV-2-Specific Memory Lymphocytes Define Hybrid Immunity. Cell 2022, 185, 1588–1601.e14. [Google Scholar] [CrossRef]
- Rodda, L.B.; Netland, J.; Shehata, L.; Pruner, K.B.; Morawski, P.A.; Thouvenel, C.D.; Takehara, K.K.; Eggenberger, J.; Hemann, E.A.; Waterman, H.R.; et al. Functional SARS-CoV-2-Specific Immune Memory Persists after Mild COVID-19. Cell 2021, 184, 169–183.e17. [Google Scholar] [CrossRef]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Strålin, K.; Gorin, J.-B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020, 183, 158–168.e14. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.D.C.R.; Vasconcelos, G.S.; De Melo, A.C.L.; Matsui, T.C.; Caetano, L.F.; De Carvalho Araújo, F.M.; Fonseca, M.H.G. Influence of Age, Gender, Previous SARS-CoV-2 Infection, and Pre-Existing Diseases in Antibody Response after COVID-19 Vaccination: A Review. Mol. Immunol. 2023, 156, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Sandoval, R.; Nieto-Patlán, A.; Carballo-Uicab, G.; Montes-Luna, A.; Jiménez-Martínez, M.C.; Vallejo-Castillo, L.; González-González, E.; Arrieta-Oliva, H.I.; Gómez-Castellano, K.; Guzmán-Bringas, O.U.; et al. Development and Evaluation of a Set of Spike and Receptor Binding Domain-Based Enzyme-Linked Immunosorbent Assays for SARS-CoV-2 Serological Testing. Diagnostics 2021, 11, 1506. [Google Scholar] [CrossRef] [PubMed]







| Pre-Abdala Vaccine | Post-Abdala Vaccine | Six Months Post-Abdala Vaccine | |
|---|---|---|---|
| n | 25 | 25 | 17 |
| Sex Male/Female | 3/22 | 3/22 | 3/14 |
| Age: Years (Range) | 40 (25–58) | 40 (25–58) | 39 (25–58) |
| COVID-19 History (Last 6 month) | 0 | 0 | 0 |
| Last Booster Dose: Months (Range) | 10 (5–13) | N/A | 6 (6) |
| AEFI 1 | N/A | 0 | N/A |
| Initial scheme: | |||
| Pfizer-BioNTech (BNT162b2) | 5 | 5 | 3 |
| Astra Zeneca (AZD1222) | 17 | 17 | 11 |
| Sputnik V (Gam-COVID-Vac) | 3 | 3 | 3 |
| First Booster dose: | |||
| Pfizer-BioNTech (BNT162b2) | 3 | 3 | 2 |
| Astra Zeneca (AZD1222) | 12 | 12 | 7 |
| Sputnik V (Gam-COVID-Vac) | 10 | 10 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islas-Vazquez, L.; Alvarado-Alvarado, Y.C.; Cruz-Aguilar, M.; Velazquez-Soto, H.; Villalobos-Gonzalez, E.; Ornelas-Hall, G.; Perez-Tapia, S.M.; Jimenez-Martinez, M.C. Evaluation of the Abdala Vaccine: Antibody and Cellular Response to the RBD Domain of SARS-CoV-2. Vaccines 2023, 11, 1787. https://doi.org/10.3390/vaccines11121787
Islas-Vazquez L, Alvarado-Alvarado YC, Cruz-Aguilar M, Velazquez-Soto H, Villalobos-Gonzalez E, Ornelas-Hall G, Perez-Tapia SM, Jimenez-Martinez MC. Evaluation of the Abdala Vaccine: Antibody and Cellular Response to the RBD Domain of SARS-CoV-2. Vaccines. 2023; 11(12):1787. https://doi.org/10.3390/vaccines11121787
Chicago/Turabian StyleIslas-Vazquez, Lorenzo, Yan Carlos Alvarado-Alvarado, Marisa Cruz-Aguilar, Henry Velazquez-Soto, Eduardo Villalobos-Gonzalez, Gloria Ornelas-Hall, Sonia Mayra Perez-Tapia, and Maria C. Jimenez-Martinez. 2023. "Evaluation of the Abdala Vaccine: Antibody and Cellular Response to the RBD Domain of SARS-CoV-2" Vaccines 11, no. 12: 1787. https://doi.org/10.3390/vaccines11121787