Co-Occurrence and Levels of Mycotoxins in Fish Feeds in Kenya
Abstract
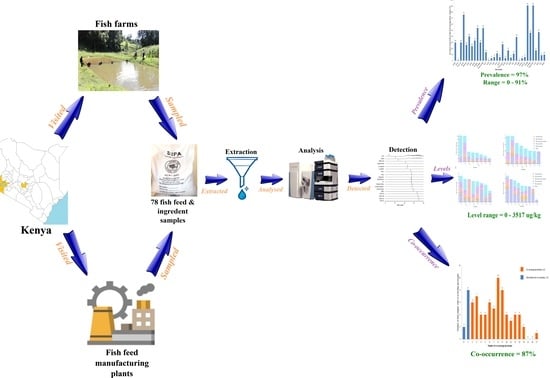
:1. Introduction
2. Results
2.1. Samples Composition
2.2. Mycotoxin Prevalence
2.3. Mycotoxin Levels
2.4. Mycotoxins Co-Occurrence
2.5. Mycotoxin Dietary Exposure Estimates for Fish
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Samples
5.2. Reagents and Solutions
5.2.1. Reagents
5.2.2. Solutions Prepared
5.3. Sample Preparation
5.4. Analysis
5.5. Method Evaluation and Quality Control
5.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mazumder, P.M.; Mazumder, R.; Mazumder, A.; Sasmal, D.S. Antimicrobial activity of the mycotoxin citrinin obtained from the fungus Penicillium citrinum. Anc. Sci. Life 2002, 21, 191–197. [Google Scholar]
- Darwish, A.M.G. Fungal mycotoxins and natural antioxidants: Two sides of the same coin and significance in food safety. Microb. Biosyst. J. 2019, 4, 1–16. [Google Scholar]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [Green Version]
- Reverberi, M.; Ricelli, A.; Zjalic, S.; Fabbri, A.A.; Fanelli, C. Natural functions of mycotoxins and control of their biosynthesis in fungi. Appl. Microbiol. Biotechnol. 2010, 87, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Alshannaq, A.; Yu, J.H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freire, L.; Sant’Ana, A.S. Modified mycotoxins: An updated review on their formation, detection, occurrence, and toxic effects. Food Chem. Toxicol. 2018, 111, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Boonen, J.; Malysheva, S.V.; Taevernier, L.; di Mavungu, J.D.; de Saeger, S.; de Spiegeleer, B. Human skin penetration of selected model mycotoxins. Toxicology 2012, 301, 21–32. [Google Scholar] [CrossRef]
- Wilson, B.J. Hazards of mycotoxins to public health. J. Food Prot. 1978, 41, 375–384. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Improving Public Health through Mycotoxin Control; Pitt, J.I., Wild, C.P., Baan, R.A., Gelderblom, W.C.A., Miller, J.D., Riley, R.T., Wu, F., Eds.; International Agency for Research on Cancer: Lyon, France, 2012; ISBN 978-92-832-2214-9. [Google Scholar]
- Peraica, M.; Radić, B.; Lucić, A.; Pavlović, M. Toxic effects of mycotoxins in humans. Bull. World Health Organ. 1999, 77, 754–766. [Google Scholar]
- Marroquín-Cardona, A.G.; Johnson, N.M.; Phillips, T.D.; Hayes, A.W. Mycotoxins in a Changing Global Environment—A Review. Food Chem. Toxicol. 2014, 69, 220–230. [Google Scholar]
- Anater, A.; Manyes, L.; Meca, G.; Ferrer, E.; Luciano, F.B.; Pimpão, C.T.; Font, G. Mycotoxins and their consequences in aquaculture: A review. Aquaculture 2016, 451, 1–10. [Google Scholar] [CrossRef]
- Murugesan, G.R.; Ledoux, D.R.; Naehrer, K.; Berthiller, F.; Applegate, T.J.; Grenier, B.; Phillips, T.D.; Schatzmayr, G. Prevalence and effects of mycotoxins on poultry health and performance, and recent development in mycotoxin counteracting strategies. Poult. Sci. 2015, 94, 1298–1315. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.A.; Rodrigues, I.; Starkl, V.; Naehrer, K.; Hofstetter, U. Mycotoxins in aquaculture: Occurrence in feeds components and impact on animal performance. In Proceedings of the Avances en Nutricion Acuicola X—Memorias del Decimo Simposio Internacinal de Nutricion Acuiola; Cruz-Suarez, L.E., Ricque Marie, D., Tapia-Salazar, M., Nieto-López, M.G., Villarreal-Cavazos, D.A., Gamboa-Delgado, J., Eds.; Universidad Autónoma de Nuevo León: Monterrey, Mexico, 2010; pp. 502–513. [Google Scholar]
- Lumlertdacha, S.; Lovell, R.T.; Shelby, R.A.; Lenz, S.D.; Kemppainen, B.W. Growth, hematology, and histopathology of channel catfish, Ictalurus punctatus, fed toxins from Fusarium moniliforme. Aquaculture 1995, 130, 201–218. [Google Scholar] [CrossRef]
- Santacroce, M.P.; Conversano, M.C.; Casalino, E.; Lai, O.; Zizzadoro, C.; Centoducati, G.; Crescenzo, G. Aflatoxins in aquatic species: Metabolism, toxicity and perspectives. Rev. Fish Biol. Fish. 2008, 18, 99–130. [Google Scholar] [CrossRef]
- Manning, B.B. Nutrition and Fish Health. In Nutrition and Feeding of Fish; Lim, C., Webster, C.D., Eds.; Food Products Press: Binghamton, NY, USA, 1998; pp. 115–122. ISBN 1-56022-887-3. [Google Scholar]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Multi-mycotoxin screening of feed and feed raw materials from Africa. World Mycotoxin J. 2018, 11, 369–383. [Google Scholar] [CrossRef]
- Muthomi, J.W.; Ndung’u, J.K.; Gathumbi, J.K.; Mutitu, E.W.; Wagacha, J.M. The occurrence of Fusarium species and mycotoxins in Kenyan wheat. Crop Prot. 2008, 27, 1215–1219. [Google Scholar] [CrossRef]
- Smith, M.C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [Green Version]
- Kedera, C.J.; Plattner, R.D.; Desjardins, A.E. Incidence of Fusarium spp. and levels of fumonisin B1 in maize in Western Kenya. Appl. Environ. Microbiol. 1999, 65, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Lanyasunya, T.P.; Wamae, L.W.; Musa, H.H.; Olowofeso, O.; Lokwaleput, I.K. The risk of mycotoxins contamination of dairy feed and milk on small holder dairy farms in Kenya. Pakistan J. Nutr. 2005, 4, 162–169. [Google Scholar]
- Wagacha, J.M.; Muthomi, J.W. Mycotoxin problem in Africa: Current status, implications to food safety and health and possible management strategies. Int. J. Food Microbiol. 2008, 124, 1–12. [Google Scholar] [CrossRef]
- Mwihia, J.T.; Straetmans, M.; Ibrahim, A.; Njau, J.; Muhenje, O.; Guracha, A.; Gikundi, S.; Mutonga, D.; Tetteh, C.; Likimani, S.; et al. Aflatoxin levels in locally grown maize from Makueni District, Kenya. East Afr. Med. J. 2008, 85, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mwihia, E.W.; Mbuthia, P.G.; Eriksen, G.S.; Gathumbi, J.K.; Maina, J.G.; Mutoloki, S.; Waruiru, R.M.; Mulei, I.R.; Lyche, J.L. Occurrence and levels of aflatoxins in fish feeds and their potential effects on fish in Nyeri, Kenya. Toxins 2018, 10, 543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alakonya, A.E.; Monda, E.O.; Ajanga, S. Fumonisin B1and aflatoxin B1 levels in Kenyan maize. J. Plant Pathol. 2009, 91, 459–464. [Google Scholar] [CrossRef]
- Kang’ethe, E.K.; Lang’a, K.A. Aflatoxin B1 and M1 contamination of animal feeds and milk from urban centers in Kenya. Afr. Health Sci. 2009, 9, 218–226. [Google Scholar]
- Rodrigues, I.; Handl, J.; Binder, E.M. Mycotoxin occurrence in commodities, feeds and feed ingredients sourced in the middle East and Africa. Food Addit. Contam. Part B Surveill. 2011, 4, 168–179. [Google Scholar] [CrossRef] [Green Version]
- Sirma, A.J.; Ouko, E.O.; Murithi, G.; Mburugu, C.; Mapenay, I.; Ombui, J.; Kang’ethe, E.; Korhonen, H. Prevalence of aflatoxin contamination in cereals from Nandi county, Kenya. Int. J. Agric. Sci. Vet. Med. 2015, 3, 55–63. [Google Scholar]
- Makau, C.M.; Matofari, J.W.; Muliro, P.S.; Bebe, B.O. Aflatoxin B1 and Deoxynivalenol contamination of dairy feeds and presence of Aflatoxin M1 contamination in milk from smallholder dairy systems in Nakuru, Kenya. Int. J. Food Contam. 2016, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Senerwa, D.M.; Sirma, A.J.; Mtimet, N.; Kang’ethe, E.K.; Grace, D.; Lindahl, J.F. Prevalence of aflatoxin in feeds and cow milk from five counties in Kenya. Afr. J. Food Agric. Nutr. Dev. 2016, 16, 11004–11021. [Google Scholar] [CrossRef]
- Marijani, E.; Wainaina, J.M.; Charo-Karisa, H.; Nzayisenga, L.; Munguti, J.; Gnonlonfin, G.J.B.; Kigadye, E.; Okoth, S. Mycoflora and mycotoxins in finished fish feed and feed ingredients from smallholder farms in East Africa. Egypt. J. Aquat. Res. 2017, 43, 169–176. [Google Scholar] [CrossRef]
- Marijani, E.; Kigadye, E.; Okoth, S. Occurrence of Fungi and Mycotoxins in Fish Feeds and Their Impact on Fish Health. Int. J. Microbiol. 2019, 2019, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Nyikal, J.; Misore, A.; Nzioka, C.; Njuguna, C.; Muchiri, E.; Njau, J.; Maingi, S.; Njoroge, J.; Mutiso, J.; Onteri, J.; et al. Outbreak of aflatoxin poisoning—Eastern and central provinces, Kenya, January-July 2004. Morb. Mortal. Wkly. Rep. 2004, 53, 790–793. [Google Scholar]
- Azziz-Baumgartner, E.; Lindblade, K.; Gieseker, K.; Rogers, H.S.; Kieszak, S.; Njapau, H.; Schleicher, R.; McCoy, L.F.; Misore, A.; DeCock, K.; et al. Case-control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environ. Health Perspect. 2005, 113, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- Kenya Bureau of Standards (KEBS). KS 2289-1:2016 Standard on Compounded tilapia feeds—Specification. Part 1: Complete Feeds, 1st ed.; Kenya Bureau of Standards (KEBS): Nairobi, Kenya, 2015.
- European Commission COMMISSION REGULATION (EU) No 574/2011 of 16 June 2011 amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council as regards maximum levels for nitrite, melamine, Ambrosia spp. and carry-over of certain coccidiostats and histomonostats and consolidating Annexes I and II thereto. Off. J. Eur. Union 2011, L159/7, 7–24. [CrossRef]
- European Commission Commission recomendations of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals a nd cereal products (2013/165/EU). Off. J. Eur. Union 2013, L19, 12–15. [CrossRef]
- European Commission Commission Recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins inproducts intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–9.
- Koge, J.; Opola, F.; Obwanga, B.; Kilelu, C.; Rurangwa, E. A Comparative study of aquaculture sector development in Egypt, Ghana and Nigeria: Insight and Lessons for Kenya. In Proceedings of the 3R Kenya Workshop, Nairobi, Kenya, 5–7 September 2018; pp. 1–18. [Google Scholar]
- Lattice Consulting. Lattice Consulting Market Study of the Aquaculture Market in Kenya; Lattice Consulting: Nairobi, Kenya, 2016. [Google Scholar]
- New, M.B. Feed and Feeding of Fish and Shrimp; ADCP/REP; FAO and UNEP: Rome, Italy, 1987. [Google Scholar]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, 4718. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J. 2017, 15, 4851. [Google Scholar] [CrossRef] [Green Version]
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cottrill, B.; Cravedi, J.-P.; di Domenico, A.; Doerge, D.; Dogliotti, E.; Edler, L.; et al. Scientific Opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 2011, 9, 2481. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for animal health related to the presence of fumonisins, their modified forms and hidden forms in feed. EFSA J. 2018, 16, 5242. [Google Scholar] [CrossRef]
- Benford, D.; Ceccatelli, S.; Cottrill, B.; DiNovi, M.; Dogliotti, E.; Edler, L.; Farmer, P.; Fürst, P.; Hoogenboom, L. (Ron); Knutsen, H.K.; et al. Scientific Opinion on risks for animal and public health related to the presence of nivalenol in food and feed. EFSA J. 2013, 11, 3262. [Google Scholar] [CrossRef]
- Benford, D.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Dogliotti, E.; Edler, L.; Farmer, P.; Fürst, P.; Hoogenboom, L. (Ron); Knutsen, H.K.; et al. Scientific Opinion on the risks to human and animal health related to the presence of beauvericin and enniatins in food and feed. EFSA J. 2014, 12, 3802. [Google Scholar] [CrossRef]
- Benford, D.; Ceccatelli, S.; Cottrill, B.; DiNovi, M.; Dogliotti, E.; Edler, L.; Farmer, P.; Fürst, P.; Hoogenboom, L. (Ron); Helle Katrine Knutsen, A.-K.L.H.; et al. Scientific Opinion on the risk for public and animal health related to the presence of sterigmatocystin in food and feed. EFSA J. 2013, 11, 3254. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Risks to human and animal health related to the presence of moniliformin in food and feed. EFSA J. 2018, 16, 5082. [Google Scholar] [CrossRef]
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cottrill, B.; Cravedi, J.-P.; Di Domenico, A.; Doerge, D.; Dogliotti, E.; Edler, L.; et al. Scientific Opinion on Ergot alkaloids in food and feed. EFSA J. 2012, 10, 2798. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Contaminants in Food Chain on a request from the Commission related to ergot as undesirable substance in animal feed. EFSA J. 2005, 225, 1–27. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; et al. Risk assessment of aflatoxins in food. EFSA J. 2020, 18, 6040. [Google Scholar] [CrossRef]
- Benford, D.; Ceccatelli, S.; Cottrill, B.; DiNovi, M.; Dogliotti, E.; Edler, L.; Farmer, P.; Fürst, P.; Hoogenboom, L. (Ron); Knutsen, H.K.; et al. Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA J. 2014, 12, 3916. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, L.; Sahlstrom, S.; Rud, I.; Uhlig, S.; Fæste, C.K.; Eriksen, G.S.; Divon, H.H. Effect of primary processing on the distribution of free and modified Fusarium mycotoxins in naturally contaminated oats. World Mycotoxin J. 2017, 10, 73–88. [Google Scholar] [CrossRef]
- Uhlig, S.; Eriksen, G.S.; Hofgaard, I.S.; Krska, R.; Beltrán, E.; Sulyok, M. Faces of a changing climate: Semi-quantitative multi-mycotoxin analysis of grain grown in exceptional climatic conditions in Norway. Toxins 2013, 5, 1682–1697. [Google Scholar] [CrossRef]
- Johny, A.; Fæste, C.K.; Bogevik, A.S.; Berge, G.M.; Fernandes, J.M.O.; Ivanova, L. Development and validation of a liquid chromatography high-resolution mass spectrometry method for the simultaneous determination of mycotoxins and phytoestrogens in plant-based fish feed and exposed fish. Toxins 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.H. Limit of Detection and Its Establishment in Analytical Chemistry. Heal. Environ. J. 2011, 2, 38–43. [Google Scholar]
- Saadati, N.; Abdullah, M.P.; Zakaria, Z.; Sany, S.B.T.; Rezayi, M.; Hassonizadeh, H. Limit of detection and limit of quantification development procedures for organochlorine pesticides analysis in water and sediment matrices. Chem. Cent. J. 2013, 7, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streit, E.; Schwab, C.; Sulyok, M.; Naehrer, K.; Krska, R.; Schatzmayr, G. Multi-mycotoxin screening reveals the occurrence of 139 different secondary metabolites in feed and feed ingredients. Toxins 2013, 5, 504–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, M.; Pardo, A.; Pose, G. Mycotoxigenic fungi and natural co-occurrence of mycotoxins in rainbow trout (Oncorhynchus mykiss) feeds. Toxins 2015, 7, 4595–4609. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D. Mycotoxins in small grains and maize: Old problems, new challenges. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 219–230. [Google Scholar] [CrossRef]
- Namulawa, V.T.; Mutiga, S.; Musimbi, F.; Akello, S.; Ngángá, F.; Kago, L.; Kyallo, M.; Harvey, J.; Ghimire, S. Assessment of fungal contamination in fish feed from the Lake Victoria Basin, Uganda. Toxins 2020, 12, 233. [Google Scholar] [CrossRef] [Green Version]
- Pietsch, C.; Kersten, S.; Burkhardt-Holm, P.; Valenta, H.; Dänicke, S. Occurrence of deoxynivalenol and zearalenone in commercial fish feed: An initial study. Toxins 2013, 5, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current situation of mycotoxin contamination and co-occurrence in animal feed focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [Green Version]
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Bandyopadhyay, R.; Müller, J.; Vanlauwe, B. Mycotoxins in Sub-Saharan Africa: Present situation, socio-economic impact, awareness, and outlook. Food Control 2017, 72, 110–122. [Google Scholar] [CrossRef]
- Juan, C.; Raiola, A.; Mañes, J.; Ritieni, A. Presence of mycotoxin in commercial infant formulas and baby foods from Italian market. Food Control 2014, 39, 227–236. [Google Scholar] [CrossRef]
- Mutiga, S.K.; Morales, L.; Angwenyi, S.; Wainaina, J.; Harvey, J.; Das, B.; Nelson, R.J. Association between agronomic traits and aflatoxin accumulation in diverse maize lines grown under two soil nitrogen levels in Eastern Kenya. Field Crop. Res. 2017, 205, 124–134. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Hofstetter, U.; Schatzmayr, D.; Jenkins, T. Mycotoxins in Southeast Asian aquaculture: Plant-based meals and finished feeds. World Mycotoxin J. 2018, 11, 265–275. [Google Scholar] [CrossRef]
- Bhat, R.; Reddy, K.R.N. Challenges and issues concerning mycotoxins contamination in oil seeds and their edible oils: Updates from last decade. Food Chem. 2017, 215, 425–437. [Google Scholar] [CrossRef]
- European Commision. Directive 2002/32/EC of the European Parliament and of the Council; European Commision: Brussels, Belgium, 2002; Volume L0032, pp. 1–30. [Google Scholar]
- Williams, D.E. The rainbow trout liver cancer model: Response to environmental chemicals and studies on promotion and chemoprevention. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 155, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooft, J.M.; Elmor, A.E.H.I.; Encarnação, P.; Bureau, D.P. Rainbow trout (Oncorhynchus mykiss) is extremely sensitive to the feed-borne Fusarium mycotoxin deoxynivalenol (DON). Aquaculture 2011, 311, 224–232. [Google Scholar] [CrossRef]
- Hooft, J.M.; Wu, P.; Powell, C.D.; Lou, Y.; Squires, E.J.; Cant, J.P.; Sulyok, M.; Krska, R.; Bureau, D.P. A comparative investigation of the effects of feed-borne deoxynivalenol (DON) on growth performance, nutrient utilization and metabolism of detoxification in rainbow trout (Oncorhynchus mykiss) and Nile tilapia (Oreochromis niloticus) fed di. Aquaculture 2019, 505, 306–318. [Google Scholar] [CrossRef]
- Anh-Tuan, N.; Grizzle, J.M.; Lovell, R.T.; Manning, B.B.; Rottinghaus, G.E. Growth and hepatic lesions of Nile tilapia (Oreochromis niloticus) fed diets containing aflatoxin B1. Aquaculture 2002, 212, 311–319. [Google Scholar] [CrossRef]
- García-Herranz, V.; Valdehita, A.; Navas, J.M.; Fernández-Cruz, M.L. Cytotoxicity against fish and mammalian cell lines and endocrine activity of the mycotoxins beauvericin, deoxynivalenol and ochratoxin-A. Food Chem. Toxicol. 2019, 127, 288–297. [Google Scholar] [CrossRef]
- FAO and IFIF Good practices for the feed industry—Implementing the codex alimentarius code of practice on good animal feeding. FAO Anim. Prod. Heal. Man. 2010, No.9. 1–79.
- Luo, Y.; Liu, X.; Li, J. Updating techniques on controlling mycotoxins—A review. Food Control 2018, 89, 123–132. [Google Scholar] [CrossRef]
- Moturi, W.K.N. Factors likely to enhance mycotoxin introduction into the human diet through maize in Kenya. Afr. J. Food Agric. Nutr. Dev. 2008, 8, 265–277. [Google Scholar] [CrossRef]
- Barbosa, T.S.; Pereyra, C.M.; Soleiro, C.A.; Dias, E.O.; Oliveira, A.A.; Keller, K.M.; Silva, P.P.O.; Cavaglieri, L.R.; Rosa, C.A.R. Mycobiota and mycotoxins present in finished fish feeds from farms in the Rio de Janeiro State, Brazil. Int. Aquat. Res. 2013, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Alinezhad, S.; Faridi, M.; Falahatkar, B.; Nabizadeh, R.; Davoodi, D. Effects of nanostructured zeolite and aflatoxin B1 in growth performance, immune parameters and pathological conditions of rainbow trout Oncorhynchus mykiss. Fish Shellfish Immunol. 2017, 70, 648–655. [Google Scholar] [CrossRef]
- Fallah, A.A.; Pirali-Kheirabadi, E.; Rahnama, M.; Saei-Dehkordi, S.S.; Pirali-Kheirabadi, K. Mycoflora, aflatoxigenic strains of Aspergillus section Flavi and aflatoxins in fish feed. Qual. Assur. Saf. Crop. Foods 2014, 6, 419–424. [Google Scholar] [CrossRef]
- El-Sayed, Y.S.; Khalil, R.H. Toxicity, biochemical effects and residue of aflatoxin B1 in marine water-reared sea bass (Dicentrarchus labrax L.). Food Chem. Toxicol. 2009, 47, 1606–1609. [Google Scholar] [CrossRef]
- Bailey, G.S.; Williams, D.E.; Wilcox, J.; Loveland, P.M.; Coulombe, R.A.; Hendricks, J.D. Aflatoxin B1 carcinogenesis and its relation to DNA adduction formation and adduct persistence in sensitive and resistant Salmonid fish. Carcinogenesis 1988, 9, 1919–1926. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Deoxynivalenol in food and feed: Occurrence and exposure. EFSA J. 2013, 11, 3379. [Google Scholar] [CrossRef]
- Council for Agricultural Science and Technology (CAST). Mycotoxicoses of Animals. In Mycotoxins: Risks in Plant, Animal, and Human Systems; Council for Agricultural Science and Technology: Ames, IA, USA, 2002; p. 65. ISBN 1-887383-22-0. [Google Scholar]
- He, C.H.; Fan, Y.H.; Wang, Y.; Huang, C.Y.; Wang, X.C.; Zhang, H. Bin The individual and combined effects of deoxynivalenol and aflatoxin B1 on primary hepatocytes of Cyprinus carpio. Int. J. Mol. Sci. 2010, 11, 3760–3768. [Google Scholar] [CrossRef] [Green Version]
- Matejova, I.; Modra, H.; Blahova, J.; Franc, A.; Fictum, P.; Sevcikova, M.; Svobodova, Z. The effect of mycotoxin deoxynivalenol on haematological and biochemical indicators and histopathological changes in rainbow trout (Oncorhynchus mykiss). Biomed. Res. Int. 2014, 2014, 310680. [Google Scholar] [CrossRef] [Green Version]
- Ran, R.; Wang, C.; Han, Z.; Wu, A.; Zhang, D.; Shi, J. Determination of deoxynivalenol (DON) and its derivatives: Current status of analytical methods. Food Control 2013, 34, 138–148. [Google Scholar] [CrossRef]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; de Saeger, S.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Poppenberger, B.; Berthiller, F.; Lucyshyn, D.; Sieberer, T.; Schuhmacher, R.; Krska, R.; Kuchler, K.; Glössl, J.; Luschnig, C.; Adam, G. Detoxification of the Fusarium Mycotoxin Deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 47905–47914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovando-Martínez, M.; Ozsisli, B.; Anderson, J.; Whitney, K.; Ohm, J.B.; Simsek, S. Analysis of deoxynivalenol and deoxynivalenol-3-glucoside in hard red spring wheat inoculated with Fusarium graminearum. Toxins 2013, 5, 2522–2532. [Google Scholar] [CrossRef] [Green Version]
- Nagl, V.; Schwartz, H.; Krska, R.; Moll, W.-D.; Knasmüller, S.; Ritzmann, M.; Adam, G.; Berthiller, F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in rats. Toxicol. Lett. 2012, 213, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Nagl, V.; Woechtl, B.; Schwartz-Zimmermann, H.E.; Hennig-Pauka, I.; Moll, W.D.; Adam, G.; Berthiller, F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in pigs. Toxicol. Lett. 2014, 229, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef]
- Pinton, P.; Tsybulskyy, D.; Lucioli, J.; Laffitte, J.; Callu, P.; Lyazhri, F.; Grosjean, F.; Bracarense, A.P.; Kolf-clauw, M.; Oswald, I.P. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: Differential effects on morphology, barrier function, tight junction proteins, and mitogen-activated protein kinases. Toxicol. Sci. 2012, 130, 180–190. [Google Scholar] [CrossRef]
- Mahdjoubi, C.K.; Arroyo-manzanares, N.; Hamini-kadar, N. Multi-mycotoxin occurrence and exposure. Toxins 2020, 12, 194. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Iwahashi, Y. Phytotoxicity evaluation of type B trichothecenes using a Chlamydomonas reinhardtii model system. Toxins 2014, 6, 453–463. [Google Scholar] [CrossRef]
- Aupanun, S.; Poapolathep, S.; Giorgi, M.; Imsilp, K.; Poapolathep, A. An overview of the toxicology and toxicokinetics of fusarenon-X, a type B trichothecene mycotoxin. J. Vet. Med. Sci. 2017, 79, 6–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, Y.; Sato, N.; Ishii, K.; Sakai, K.; Tsunoda, H. Biological and chemical detection of trichothecene mycotoxins of Fusarium species. Appl. Microbiol. 1973, 25, 699–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuhmacher-Wolz, U.; Heine, K.; Schneider, K. Report on Toxicity Data on Trichothecene Mycotoxins HT-2 and T-2 Toxins (CT/EFSA/CONTAM/2010/03); Forschungs- und Beratungsinstitut Gefahrstoffe GmbH (FoBiG): Freiburg, Germany, 2010. [Google Scholar]
- European Food Safety Authority (EFSA). Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- Agag, B.I. Mycotoxins in Foods and Feeds 3-Zearalenone. Ass. Univ. Bull. Environ. Res. 2004, 7, 159–176. [Google Scholar]
- Bravin, F.; Duca, R.C.; Balaguer, P.; Delaforge, M. In vitro cytochrome P450 formation of a mono-hydroxylated metabolite of zearalenone exhibiting estrogenic activities: Possible occurrence of this metabolite in vivo. Int. J. Mol. Sci. 2009, 10, 1824–1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woźny, M.; Brzuzan, P.; Wolińska, L.; Góra, M.; Łuczyński, M.K. Differential gene expression in rainbow trout (Oncorhynchus mykiss) liver and ovary after exposure to zearalenone. Comp. Biochem. Physiol. Part C 2012, 156, 221–228. [Google Scholar] [CrossRef]
- Rheeder, J.P.; Marasas, W.F.O.; Vismer, H.F. Production of fumonisin analogs by Fusarium species. Appl. Environ. Microbiol. 2002, 68, 2101–2105. [Google Scholar] [CrossRef] [Green Version]
- Agag, B.I. Mycotoxins in foods and feeds 4-Fumonisins. Ass. Univ. Bull. Environ. Res. 2005, 8, 115–140. [Google Scholar]
- Scott, P.M. Ergot alkaloids: Extent of human and animal exposure. World Mycotoxin J. 2009, 2, 141–149. [Google Scholar] [CrossRef]
- Matejova, I.; Svobodova, Z.; Vakula, J.; Mares, J.; Modra, H. Impact of mycotoxins on aquaculture fish species: A review. J. World Aquac. Soc. 2017, 48, 186–200. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging mycotoxins: Beyond traditionally determined food contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, A.; Berrada, H.; Ruiz, M.J.; Caloni, F.; Coccini, T.; Spicer, L.J.; Perego, M.C.; Lafranconi, A. A review of the mycotoxin enniatin B. Front. Public Heal. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Belisario, A.; Tafuri, A.; Ritieni, A.; Corazza, L.; Logrieco, A. Production of beauvericin by different races of Fusarium oxysporum f. sp. melonis, the fusarium wilt agent of muskmelon. Eur. J. Plant Pathol. 2002, 108, 661–666. [Google Scholar] [CrossRef]
- Solhaug, A.; Eriksen, G.S.; Holme, J.A. Mechanisms of action and toxicity of the mycotoxin alternariol: A review. Basic Clin. Pharmacol. Toxicol. 2016, 119, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Dellafiora, L.; Oswald, I.P.; Dorne, J.L.; Galaverna, G.; Battilani, P.; Dall’Asta, C. An in silico structural approach to characterize human and rainbow trout estrogenicity of mycotoxins: Proof of concept study using zearalenone and alternariol. Food Chem. 2020, 312, 126088. [Google Scholar] [CrossRef]
- Kovalsky, P.; Kos, G.; Nährer, K.; Schwab, C.; Jenkins, T.; Schatzmayr, G.; Sulyok, M.; Krska, R. Co-occurrence of regulated, masked and emerging mycotoxins and secondary metabolites in finished feed and maize–An extensive survey. Toxins 2016, 8, 363. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Wahhab, M.A.; Hasan, A.M.; Aly, S.E.; Mahrous, K.F. Adsorption of sterigmatocystin by montmorillonite and inhibition of its genotoxicity in the Nile tilapia fish (Oreachromis nilaticus). Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2005, 582, 20–27. [Google Scholar] [CrossRef]
- Keller, N.P.; Nesbitt, C.; Sarr, B.; Phillips, T.D.; Burow, G.B. pH regulation of sterigmatocystin and aflatoxin biosynthesis in Aspergillus spp. Phytopathology 1997, 87, 643–648. [Google Scholar] [CrossRef] [Green Version]
- Bertero, A.; Moretti, A.; Spicer, L.J.; Caloni, F. Fusarium molds and mycotoxins: Potential species-specific effects. Toxins 2018, 10, 244. [Google Scholar] [CrossRef] [Green Version]
- Weber, J.; Vaclavikova, M.; Wiesenberger, G.; Haider, M.; Hametner, C.; Fröhlich, J.; Berthiller, F.; Adam, G.; Mikula, H.; Fruhmann, P. Chemical synthesis of culmorin metabolites and their biologic role in culmorin and acetyl-culmorin treated wheat cells. Org. Biomol. Chem. 2018, 16, 2043–2048. [Google Scholar] [CrossRef] [Green Version]
- McCormick, S.P.; Alexander, N.J.; Harris, L.J. CLM1 of Fusarium graminearum encodes a longiborneol synthase required for culmorin production. Appl. Environ. Microbiol. 2010, 76, 136–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenglein, S.A. Fusarium poae: A pathogen that needs more attention. J. Plant Pathol. 2009, 91, 25–36. [Google Scholar] [CrossRef]
- Frisvad, F.J.C.; Thrane, U.; Samson, R.A.; Pitt, J.I. Important mycotoxins and the fungi which produce them. In Advances in Experimental Medicine and Biology; Back, N., Cohen, I.R., Kritchevsky, D., Lajtha, A., Paoletti, R., Eds.; Spinger Science: New York, NY, USA, 2006; Volume 571, pp. 3–32. ISBN 9780387283852. [Google Scholar]
- Kumar, V.; Basu, M.S.; Rajendran, T.P. Mycotoxin research and mycoflora in some commercially important agricultural commodities. Crop Prot. 2008, 27, 891–905. [Google Scholar] [CrossRef]
- Brera, C.; De Santis, B.; Debegnach, F.; Miraglia, M. Mycotoxins. In Comprehensive Analytical Chemistry: Food Contaminants and Residue Analysis; Picó, Y., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2008; Volume 51, pp. 363–427. ISBN 9780444530196. [Google Scholar]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.L. Some major mycotoxins and their mycotoxicoses-An overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef]
- Mo, H.G.J.; Pietri, A.; MacDonald, S.J.; Anagnostopoulos, C.; Spanjere, M. Survey on sterigmatocystin in food. EFSA Support. Publ. 2015, EN-774, 1–56. [Google Scholar] [CrossRef] [Green Version]
- Huff, W.E.; Kubena, L.F.; Harvey, R.B.; Doerr, J.A. Mycotoxin interactions in poultry and swine. J. Anim. Sci. 1988, 66, 2351–2355. [Google Scholar] [CrossRef] [Green Version]
- Grenier, B.; Oswald, I.P. Mycotoxin co-contamination of food and feed: Meta-analysis of publications describing toxicological interactions. World Mycotoxin J. 2011, 4, 285–313. [Google Scholar] [CrossRef]
- Juan, C.; Mañes, J.; Raiola, A. Evaluation of beauvericin and enniatins in Italian cereal products and multicereal food by liquid chromatography coupled to triple quadrupole mass spectrometry. Food Chem. 2013, 140, 755–762. [Google Scholar] [CrossRef]
- Prosperini, A.; Font, G.; Ruiz, M.J. Interaction effects of Fusarium enniatins (A, A1, B and B1) combinations on in vitro cytotoxicity of Caco-2 cells. Toxicol. Vitr. 2014, 28, 88–94. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Kolf-Clauw, M.; Gauthier, T.; Abrami, R.; Abiola, F.A.; Oswald, I.P.; Puel, O. New insights into mycotoxin mixtures: The toxicity of low doses of Type B trichothecenes on intestinal epithelial cells is synergistic. Toxicol. Appl. Pharmacol. 2013, 272, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Puel, O.; Oswald, I.P. Toxicological interactions between the mycotoxins deoxynivalenol, nivalenol and their acetylated derivatives in intestinal epithelial cells. Arch. Toxicol. 2015, 89, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.N.; Wang, J.Q.; Li, S.L.; Zhang, Y.D.; Zheng, N. Aflatoxin M1 cytotoxicity against human intestinal Caco-2 cells is enhanced in the presence of other mycotoxins. Food Chem. Toxicol. 2016, 96, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Gheux, A.; Coton, M.; Madec, S.; Hymery, N.; Coton, E. In vitro co-culture models to evaluate acute cytotoxicity of individual and combined mycotoxin exposures on Caco-2, THP-1 and HepaRG human cell lines. Chem. Biol. Interact. 2018, 281, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Fernández-Franzón, M.; Font, G.; Ruiz, M.J. Toxicity evaluation of individual and mixed enniatins using an in vitro method with CHO-K1 cells. Toxicol. Vitr. 2013, 27, 672–680. [Google Scholar] [CrossRef]
- Zhou, H.; George, S.; Li, C.; Gurusamy, S.; Sun, X.; Gong, Z.; Qian, H. Combined toxicity of prevalent mycotoxins studied in fish cell line and zebrafish larvae revealed that type of interactions is dose-dependent. Aquat. Toxicol. 2017, 193, 60–71. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Faria, M.A.; Cunha, S.C.; Ferreira, I.M.P.L.V.O. Toxicological interactions between mycotoxins from ubiquitous fungi: Impact on hepatic and intestinal human epithelial cells. Chemosphere 2018, 202, 538–548. [Google Scholar] [CrossRef]
- Oh, S.Y.; Cedergreen, N.; Yiannikouris, A.; Swamy, H.V.L.N.; Karrow, N.A. Assessing interactions of binary mixtures of Penicillium mycotoxins (PMs) by using a bovine macrophage cell line (BoMacs). Toxicol. Appl. Pharmacol. 2017, 318, 33–40. [Google Scholar] [CrossRef]
- Ruiz, M.J.; Macáková, P.; Juan-García, A.; Font, G. Cytotoxic effects of mycotoxin combinations in mammalian kidney cells. Food Chem. Toxicol. 2011, 49, 2718–2724. [Google Scholar] [CrossRef]
- Mulei, I.R.; Nyaga, P.N.; Mbuthia, P.G.; Waruiru, R.M.; Njagi, L.W.; Mwihia, E.W.; Gamil, A.A.A.; Evensen, Ø.; Mutoloki, S. Infectious pancreatic necrosis virus isolated from farmed rainbow trout and tilapia in Kenya is identical to European isolates. J. Fish Dis. 2018, 1–10. [Google Scholar] [CrossRef]
- Kenya Bureau of Standards (KEBS). KS ISO 6497:2002 Standard on Animal Feeding Stuffs—Sampling; Kenya Bureau of Standards (KEBS): Nairobi, Kenya, 2002.
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chronicles Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- International Programme on Chemical Safety (IPCS). Risk assesment and its role in risk analysis. In Principles and Methods for the Risk Assessment of Chemicals in Food; World Health Organization: Geneva, Switzerland, 2009; pp. 1–16. ISBN 978-92-4-157240-8. [Google Scholar]







| Mycotoxins | Prevalence % (x) | Range µg/kg | Mean ± S µg/kg | 10th Percentile µg/kg | 25th Percentile µg/kg | Median µg/kg | 75th Percentile µg/kg | 90th Percentile µg/kg |
|---|---|---|---|---|---|---|---|---|
| Aflatoxins | ||||||||
| AFB1 | 29 (23) | <14.7–43.6 | 10.8 ± 8.4 | <14.7 | <14.7 | <14.7 | <14.7 | 17.2 |
| AFG1 | 1 (1) | <155.8 | <155.8 | <155.8 | <155.8 | <155.8 | <155.8 | <155.8 |
| AF | 29 (23) | <14.7–93.6 | 14.2 ± 19.2 | <14.7 | <14.7 | <14.7 | <14.7 | 21.7 |
| Deoxynivalenol and its metabolites | ||||||||
| DON | 76 (59) | <40.4–819.9 | 168.9 ± 202.0 | <40.4 | <40.4 | 66.9 | 263.2 | 456.3 |
| DON3G | 26 (20) | <46.8–97.5 | 31.7 ± 20.9 | <46.8 | <46.8 | <46.8 | <46.8 | 66.0 |
| Zearalenone and its metabolites | ||||||||
| ZEN | 40 (31) | <38.0–757.9 | 136.0 ± 170.7 | <38.0 | <38.0 | 58.8 | 191.1 | 367.8 |
| αZEL | 24 (19) | <22.2–288.4 | 61.6 ± 76.1 | <22.2 | <22.2 | 26.7 | 79.4 | 161.6 |
| βZEL | 33 (26) | <16.0–79.8 | 31.3 ± 23.5 | <16.0 | <16.0 | 28.4 | 48.2 | 64.7 |
| Fumonisins | ||||||||
| FUMB1 | 54 (42) | <63.0–1427.4 | 247.6 ± 331.9 | <63.0 | <63.0 | 116.8 | 302 | 622 |
| FUMB2 | 29 (23) | <68.9–649.2 | 120.0 ± 158.4 | <68.9 | <68.9 | <68.9 | 146.7 | 230.6 |
| FUMB | 54 (42) | <63.0–2076.6 | 313.3 ± 455.0 | <63.0 | <63.0 | 160.7 | 336.5 | 785.7 |
| Ergot alkaloids | ||||||||
| ECO | 14 (11) | 37.6–64.3 | 47.5 ± 9.8 | 38.8 | 39.4 | 42.3 | 55.3 | 59.5 |
| ECR | 1 (1) | <24.9 | <24.9 | <24.9 | <24.9 | <24.9 | <24.9 | <24.9 |
| ENV | 4 (3) | <21.9 | <21.9 | <21.9 | <21.9 | <21.9 | <21.9 | <21.9 |
| ESN | 6 (5) | <38.4–144.2 | 48.1 ± 54.4 | <38.4 | <38.4 | <38.4 | 38.5 | 101.9 |
| ETA | 12 (9) | <29.3–1895.6 | 301.5 ± 602.5 | 28.9 | 58.5 | 87.2 | 166.6 | 585.1 |
| αECP | 6 (5) | <41.0–81.3 | 32.7 ± 27.2 | <41.0 | <41.0 | <41.0 | <41.0 | 57.0 |
| ERG | 27 (21) | <20.7–2055.3 | 175.5 ± 437.2 | <20.7 | 38.9 | 58.5 | 111.7 | 206.3 |
| Other trichothecenes | ||||||||
| FUSX | 4 (3) | <56.0 | <56.0 | <56.0 | <56.0 | <56.0 | <56.0 | <56.0 |
| HT2 | 17 (13) | <41.6–411.8 | 60.7 ± 108.7 | <41.6 | <41.6 | <41.6 | <41.6 | 101.3 |
| NEO | 5 (4) | <177.7 | <177.7 | <177.7 | <177.7 | <177.7 | <177.7 | <177.7 |
| NIV | 12 (9) | <40.3–76.0 | 53.0 ± 24.8 | <40.3 | <40.3 | 66.3 | 69.8 | 72.2 |
| Alternariol and its metabolites | ||||||||
| AOH | 38 (30) | <36.2–43.3 | 18.9 ± 4.6 | <36.2 | <36.2 | <36.2 | <36.2 | <36.2 |
| AME | 1 (1) | 94.5 | 94.5 | 94.5 | 94.5 | 94.5 | 94.5 | 94.5 |
| Enniatins | ||||||||
| ENNA | 4 (3) | <26.1 | <26.1 | <26.1 | <26.1 | <26.1 | <26.1 | <26.1 |
| ENNA1 | 6 (5) | <13.5–23.8 | 11.8 ± 7.6 | <13.5 | <13.5 | <13.5 | 14.7 | 20.2 |
| ENNB | 91 (71) | <38.8–150.0 | 41.9 ± 36.5 | <38.8 | <38.8 | <38.8 | 55.3 | 118.4 |
| ENNB1 | 46 (36) | <12.9–43.5 | 23.0 ± 8.5 | 14.8 | 16.6 | 23.2 | 27 | 35.3 |
| ENN | 91 (71) | 19.4–186.7 | 54.9 ± 44.2 | 19.4 | 19.4 | 36.8 | 73.3 | 121.9 |
| Other mycotoxins | ||||||||
| CUL | 17 (13) | <42.3–288.7 | 136.9 ± 73.5 | 59.9 | 84.1 | 141.6 | 185.4 | 216.1 |
| BEA | 47 (37) | <15.9–841.8 | 84.4 ± 148.3 | <15.9 | <15.9 | 34.4 | 87.9 | 216.7 |
| STC | 9 (7) | <30.5–3517.1 | 591.3 ± 1298.0 | <30.5 | <30.5 | <30.5 | 280.4 | 1645.8 |
| MON | 10 (8) | <218.9–2583.4 | 839.3 ± 818.5 | 213.2 | 286.6 | 530.4 | 1192.3 | 1633.2 |
| Regulated Mycotoxin | Feed Characteristic | ML/IL/GV | Level/Value (µg/kg) | Samples > ML/IL/GV % (x) | Ref. |
|---|---|---|---|---|---|
| AFB1 | Complete tilapia feed | ML | 5 | 43 (10) † | [37] |
| Animal feed materials | ML | 20 | 0 | [38] | |
| Complementary and complete animal feed | ML | 10 | 13 (3) ‡ | [38] | |
| AF | Complete tilapia feed | ML | 10 | 13 (3) ‡ | [37] |
| OTA | Animal feed materials—cereals and cereal products | GV | 250 | 0 | [40] |
| DON | Animal feed materials—Cereals and cereal products and maize by-products | GV | 8000–12,000 | 0 | [40] |
| Complementary and complete animal feedstuffs | GV | 5000 | 0 | [40] | |
| Total FUMB | Animal feed materials—maize and maize products | GV | 60,000 | 0 | [40] |
| Complementary and complete feedstuffs for fish | GV | 10,000 | 0 | [40] | |
| ZEN | Animal feed materials—Cereals and cereal products and maize by-products | GV | 2000–3000 | 0 | [40] |
| T2 + HT2 | Cereal products for animal feed | IL | 500–2000 | 0 | [39] |
| Compound animal feed | IL | 250 | 1 (1) π |
| Regulated Mycotoxins | AFB1 % (x) | AFG1 % (x) | DON % (x) | ZEN % (x) | FUMB1 % (x) | FUMB2 % (x) | HT2 % (x) | Total AF % (x) | Total FUMB % (x) |
|---|---|---|---|---|---|---|---|---|---|
| AFB1 | 30 (23) | ||||||||
| AFG1 | <1 (1) | <1 (1) | |||||||
| DON | 28 (22) | <1 (1) | 76 (59) | ||||||
| ZEN | 15 (12) | <1 (1) | 35 (27) | 40 (31) | |||||
| FUMB1 | 24 (19) | <1 (1) | 49 (38) | 36 (28) | 54 (42) | ||||
| FUMB2 | 13 (10) | <1 (1) | 26 (20) | 26 (20) | 30 (23) | 30 (23) | |||
| HT2 | 6 (5) | <1 (1) | 17 (13) | 5 (4) | 9.0 (7) | - | 17 (13) | ||
| Total AF | 30 (23) | <1 (1) | 28 (22) | 15 (12) | 24 (19) | 5 (4) | 6 (5) | 30 (23) | |
| Total FUMB | 24 (19) | <1 (1) | 49 (38) | 40 (28) | 54 (42) | 10 (8) | 9 (7) | 24 (19) | 54 (42) |
| Mycotoxin 1 | Mycotoxin 2 | Prevalence | Correlation Coefficient | |
|---|---|---|---|---|
| % (x) | rs | p-Value | ||
| ENNB1 | ENNB | 46 (36) | 0.77 | <0.0001 |
| FUMB1 | FUMB2 | 30 (23) | 0.60 | 0.0026 |
| Total ZEL | ZEN | 28 (22) | 0.88 | <0.0001 |
| ZEN | αZEL | 22 (17) | 0.72 | 0.0012 |
| ZEN | βZEL | 21 (16) | 0.72 | 0.0016 |
| ENN | CUL | 17 (13) | 0.58 | 0.0368 |
| Mycotoxin | Levels in Feed (µg/kg) | NOAEL | LOAEL | PDI (µg/kg bw/day) | NOAEL | LOAEL | Toxic Endpoint Reported by EFSA | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | Median | (µg/kg Feed) | Range | Median | (µg/kg bw/day) | |||||
| AFB1 | <14.7–43.6 | <14.7 | <0.18–0.54 | <0.18 | ||||||
| AFG1 | <155.8 | <155.8 | <1.92 | <1.92 | ||||||
| AF | <14.7–93.6 | <14.7 | <0.18–1.15 | <0.18 | ||||||
| DON | <40.4–819.9 | 66.9 | 600–800 ‡ | <0.50–10.11 | 0.83 | Decreased feed intake, body weight gain, growth rate, feed and efficiency, retained nitrogen, recovered energy, energy retention efficiency and nitrogen retention | [44] | |||
| DON3G | <46.8–97.5 | <46.8 | n.s. | n.s. | <0.58–1.20 | <0.58 | n.s. | n.s. | [44] | |
| ZEN | <38.0–757.9 | 58.8 | 300β | <0.47–9.35 | 0.73 | 9 β | Decreased number of monocytes, increased number of granulocytes and increased lipid peroxidation in liver and gill and altered the carbohydrate metabolism. | [45] | ||
| αZEL | <22.2–288.4 | 26.7 | n.s. | n.s. | <0.27–3.56 | 0.33 | n.s. | n.s. | [45] | |
| βZEL | <16.0–79.8 | 28.4 | n.s. | n.s. | <0.20–0.98 | 0.35 | n.s. | n.s. | [45] | |
| FUMB1 | <63.0–1427.4 | 116.8 | 10,000 α–20,000 β | 10,000π | <0.78–17.6 | 1.44 | 400 α | 500 π | Reduced weight gain in Nile tilapia Pathological alterations in liver, pancreas, kidney, heart and brain, changes in haematological parameters and reduced body weight gain in carp Reduced body weight gain and microscopic liver lesions in catfish | [47] |
| FUMB2 | <68.9–649.2 | <68.9 | <0.85–8.01 | <0.85 | ||||||
| FUMB | <63.0–2076.6 | 160.7 | 10,000α–20,000β | 10,000π | <0.78–25.61 | 1.98 | 400 α | 500 π | Reduced weight gain in Nile tilapia Pathological alterations in liver, pancreas, kidney, heart and brain, changes in haematological parameters and reduced body weight gain in carp Reduced body weight gain and microscopic liver lesions in catfish | [47] |
| ECO | 37.6–64.3 | 42.3 | 0.46–0.79 | 0.52 | ||||||
| ECR | <24.9 | <24.9 | <0.31 | <0.31 | ||||||
| ENV | <21.9 | <21.9 | <0.27 | <0.27 | ||||||
| ESN | <38.4–144.2 | <38.4 | <0.47–1.78 | <0.47 | ||||||
| ETA | <29.3–1895.6 | 87.2 | <0.36–23.38 | 1.08 | ||||||
| αECP | <41.0–81.3 | <41.0 | <0.51–1.00 | <0.51 | ||||||
| ERG | <20.7–2055.3 | 58.5 | <0.26–25.35 | <0.72 | ||||||
| FUSX | <56.0 | <56.0 | <0.69 | <0.69 | ||||||
| HT2 | <41.6–411.8 | <41.6 | <0.51–5.08 | <0.51 | 13 †,π | Reduced feed intake, growth and haematocrit values as well as increased mortality | [46,55] | |||
| NEO | <177.7 | <177.7 | <2.19 | <2.19 | ||||||
| NIV | <40.3–76.0 | 66.3 | n.s. | n.s. | <0.50–0.94 | 0.82 | n.s. | n.s. | [48,55] | |
| AOH | <36.2–43.3 | <36.2 | <0.45–0.53 | <0.45 | ||||||
| AME | 94.5 | 94.5 | 1.17 | 1.17 | ||||||
| ENNA | <26.1 | <26.1 | n.s. | n.s. | <0.32 | <0.32 | n.s. | n.s. | [49] | |
| ENNA1 | <13.5–23.8 | <13.5 | n.s. | n.s. | <0.17–0.29 | <0.17 | n.s. | n.s. | [49] | |
| ENNB | <38.8–150.0 | <38.8 | n.s. | n.s. | <0.48–1.85 | <0.48 | n.s. | n.s. | [49] | |
| ENNB1 | <12.9–43.5 | 23.2 | n.s. | n.s. | <0.16–0.54 | 0.29 | n.s. | n.s. | [49] | |
| ENN | 19.4–186.7 | 36.8 | n.s. | n.s. | 0.24–2.30 | 0.45 | n.s. | n.s. | [49] | |
| CUL | <42.3–288.7 | 141.6 | <0.52–3.56 | 1.75 | ||||||
| BEA | <15.9–841.8 | 34.4 | n.s. | n.s. | <0.20–10.38 | 0.42 | n.s. | n.s. | [49] | |
| STC | <30.5–3517.1 | <30.5 | <0.38–43.38 | <0.38 | ||||||
| MON | <218.9–2583.4 | 530.4 | n.s. | n.s. | <2.70–31.86 | 6.54 | n.s. | n.s. | [51] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwihia, E.W.; Lyche, J.L.; Mbuthia, P.G.; Ivanova, L.; Uhlig, S.; Gathumbi, J.K.; Maina, J.G.; Eshitera, E.E.; Eriksen, G.S. Co-Occurrence and Levels of Mycotoxins in Fish Feeds in Kenya. Toxins 2020, 12, 627. https://doi.org/10.3390/toxins12100627
Mwihia EW, Lyche JL, Mbuthia PG, Ivanova L, Uhlig S, Gathumbi JK, Maina JG, Eshitera EE, Eriksen GS. Co-Occurrence and Levels of Mycotoxins in Fish Feeds in Kenya. Toxins. 2020; 12(10):627. https://doi.org/10.3390/toxins12100627
Chicago/Turabian StyleMwihia, Evalyn Wanjiru, Jan Ludvig Lyche, Paul Gichohi Mbuthia, Lada Ivanova, Silvio Uhlig, James K. Gathumbi, Joyce G. Maina, Eric Emali Eshitera, and Gunnar Sundstøl Eriksen. 2020. "Co-Occurrence and Levels of Mycotoxins in Fish Feeds in Kenya" Toxins 12, no. 10: 627. https://doi.org/10.3390/toxins12100627