Effect of Poly-l-Lysine Polycation on the Glucose Oxidase/Ferricyanide Composite-Based Second-Generation Blood Glucose Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
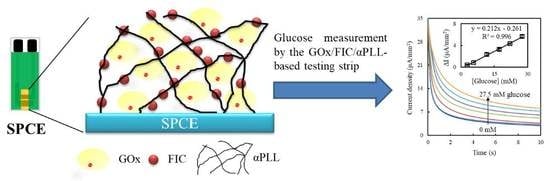
2.2. Preparation of GOx/FIC/αPLL-Modified SPCEs
2.3. Electrochemical Measurement
2.4. UV–Visible Spectroscopy
2.5. Stability of GOx/FIC/αPLL-Modified SPCE Strips
3. Results and Discussion
3.1. Effect of αPLL on FIC
3.2. Effect of FIC Concentration
3.3. Effect of Other Anions
3.4. Sensing Properties of GOx/FIC/αPLL-Modified SPCEs
3.5. Testing Strips for Real Samples
3.6. Long-Term Stability
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhu, Z.; Garcia-Gancedo, L.; Flewitt, A.J.; Xie, H.; Moussy, F.; Milne, W.I. A critical review of glucose biosensors based on carbon nanomaterials: Carbon nanotubes and graphene. Sensors 2012, 12, 5996–6022. [Google Scholar] [CrossRef]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Shi, G.J.; Shi, G.R.; Zhou, J.Y.; Zhang, W.J.; Gao, C.Y.; Jiang, Y.P.; Zi, Z.G.; Zhao, H.H.; Yang, Y.; Yu, J.Q. Involvement of growth factors in diabetes mellitus and its complications: A general review. Biomed. Pharmacother. 2018, 101, 510–527. [Google Scholar] [CrossRef]
- La Spada, L. Metasurfaces for Advanced Sensing and Diagnostics. Sensors 2019, 19, 355. [Google Scholar] [CrossRef] [PubMed]
- Klonoff, D.C.; Facp, M.D. Overview of Fluorescence Glucose Sensing: A Technology with a Bright Future. J. Diabetes Sci. Technol. 2012, 6, 1242–1250. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Ji, Z.; Du, Y.; Chen, S. In vivo noninvasive blood glucose detection using near-infrared spectrum based on the PSO-2ANN model. Technol. Health Care 2018, 26, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.; Feldman, B. Electrochemical Glucose Sensors and Their Applications in Diabetes Management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, T.; Zhang, J.; Lu, Y. Transforming the blood glucose meter into a general healthcare meter for in vitro diagnostics in mobile health. Biotechnol. Adv. 2016, 34, 331–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metkar, S.K.; Girigoswami, K. Diagnostic biosensors in medicine—A review. Diagn. Biosens. Med. 2019, 17, 271–283. [Google Scholar] [CrossRef]
- Pappa, A.M.; Parlak, O.; Scheiblin, G.; Mailley, P.; Salleo, A.; Owens, R.M. Organic Electronics for Point-of-Care Metabolite Monitoring. Trends Biotechnol. 2018, 36, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Zheng, D.; Al-Rubeaan, K.; Luong, J.H.; Sheu, F.S. Technology behind commercial devices for blood glucose monitoring in diabetes management: A review. Anal. Chim. Acta 2011, 703, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, K.; Minami, Y.; Taira, S.; Katano, H. Promotion and Suppression Effects of Cationic Polymer ε-Poly-l-lysine on the Glucose Oxidase Reaction with Ferrocene Derivatives as Oxidants with Different Charges. Anal. Sci. 2014, 30, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.T.; Hsiao, H.C.; Fang, M.Y.; Zen, J.M. Superior long-term stability of a glucose biosensor based on inserted barrel plating gold electrodes. Biosens. Bioelectron. 2009, 25, 383–387. [Google Scholar] [CrossRef]
- TermehYousefi, A.; Bagheri, S.; Kadri, N.A.; Mahmood, M.R.; Ikeda, S. Constant Glucose Biosensor Based on Vertically Aligned Carbon Nanotube Composites. Int. J. Electrochem. Sci. 2015, 10, 4183–4192. [Google Scholar]
- Uematsu, K.; Yamasaki, M.; Hibi, T.; Katano, H. Promotion Effect by ε-Poly-L-lysine on the Enzymatic Reaction of Glucose Oxidase with Ferricyanide Ion as an Oxidant. Anal. Sci. 2012, 28, 657–660. [Google Scholar] [CrossRef]
- Hu, J. The evolution of commercialized glucose sensors in China. Biosens. Bioelectron. 2009, 24, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jia, J.; Dong, S. Organically Modified Sol-Gel/Chitosan Composite Based Glucose Biosensor. Electroanalysis 2003, 15, 608–612. [Google Scholar] [CrossRef]
- Dervisevic, M.; Cevik, E.; Senel, M. Development of glucose biosensor based on reconstitution of glucose oxidase onto polymeric redox mediator coated pencil graphite electrodes. Enzyme Microb. Technol. 2014, 68, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Fatoni, A.; Numnuam, A.; Kanatharana, P.; Limbut, W.; Thammakhet, C.; Thavarungkul, P. A highly stable oxygen-independent glucose biosensor based on a chitosan-albumin cryogel incorporated with carbon nanotubes and ferrocene. Sens. Actuators B Chem. 2013, 185, 725–734. [Google Scholar] [CrossRef]
- Ghica, M.E.; Brett, C.M.A. A glucose biosensor using methyl viologen redox mediator on carbon film electrodes. Anal. Chim. Acta 2005, 532, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.R.; Lee, Y.T.; Sawada, K.; Takao, H.; Ishida, M. Development of a disposable glucose biosensor using electroless-plated Au/Ni/copper low electrical resistance electrodes. Biosens. Bioelectron. 2008, 24, 410–414. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Li, L.; Du, Z.; Xu, S.; Zhang, M.; Yin, X.; Wang, T. A novel amperometric biosensor based on NiO hollow nanospheres for biosensing glucose. Talanta 2008, 77, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Nagata, R.; Yokoyama, K.; Clark, S.A.; Karube, I. A glucose sensor fabricated by the screen printing technique. Biosens. Bioelectron. 1995, 10, 261–267. [Google Scholar] [CrossRef]
- Nien, P.-C.; Tung, T.-S.; Ho, K.-C. Amperometric Glucose Biosensor Based on Entrapment of Glucose Oxidase in a Poly(3,4-ethylenedioxythiophene) Film. Electroanalysis 2006, 18, 1408–1415. [Google Scholar] [CrossRef]
- Xu, H.; Li, G.; Wu, J.; Wang, Y.; Liu, J. A glucose oxidase sensor based on screen-printed carbon electrodes modified by polypyrrole. Proc. IEEE 2005, 1917–1920. [Google Scholar]
- Lee, S.-H.; Fang, H.-Y.; Chen, W.-C. Amperometric glucose biosensor based on screen-printed carbon electrodes mediated with hexacyanoferrate–chitosan oligomers mixture. Sens. Actuators B Chem. 2006, 117, 236–243. [Google Scholar] [CrossRef]
- Katano, H.; Tatsumi, H.; Hibi, T.; Ikdat, T.; Tsukatani, T. Application of Polyammonium Cations to Enzyme-immobilized Electrode: Application as Enzyme Stabilizer for Bilirubin Oxidase. Anal. Sci. 2008, 24, 1421–1424. [Google Scholar] [CrossRef] [Green Version]
- Katano, H.; Uematsu, K.; Hibi, T.; Ikeda, T.; Tsukatani, T. application of Poly[oxyethylene(dimethylimino)propyl-(dimethylimino)ethylene] as enzyme stabilizer for bilirubin oxidase immobilized electrode. Anal. Sci. 2009, 25, 1077–1081. [Google Scholar] [CrossRef]
- Katano, H.; Sugimoto, Y.; Uematsu, K.; Hibi, T. Kinetic Study of the Thermal Inactivation of Glucose Oxidase in the Presence of Denaturant and Stabilizer by Means of Bioelectrocatalysis Method. Anal. Sci. 2011, 27, 979–983. [Google Scholar] [CrossRef]
- Uematsu, K.; Ueno, T.; Katano, H. Effect of ε-Poly-L-lysine on a Glucose Sensor Based on Glucose Oxidase and Ferricyanide Ion. Jpn. Soc. Anal. Chem. 2018, 34, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, L. Facile direct electron transfer in glucose oxidase modified electrodes. Electrochim. Acta 2009, 54, 4316–4320. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Mani, V.; Chen, S.-M.; Dinesh, B.; Huang, S.-T. The Immobilization of Glucose Oxidase at Manganese Dioxide Particles-Decorated Reduced Graphene Oxide Sheets for the Fabrication of a Glucose Biosensor. Ind. Eng. Chem. Res. 2014, 53, 15582–15589. [Google Scholar] [CrossRef]
- Sudhakara Prasad, K.; Muthuraman, G.; Zen, J.M. The role of oxygen functionalities and edge plane sites on screen-printed carbon electrodes for simultaneous determination of dopamine, uric acid and ascorbic acid. Electrochem. Commun. 2008, 10, 559–563. [Google Scholar] [CrossRef]
- Chiu, M.H.; Wei, W.C.; Zen, J.M. The role of oxygen functionalities at carbon electrode to the electrogenerated chemiluminescence of Ru(bpy)32+. Electrochem. Commun. 2011, 13, 605–607. [Google Scholar] [CrossRef]
- Nicholson, R.S.; Shain, I. Theory of Stationary Electrode Polarography: Single Scan and Cyclic Methods Applied to Reversible, Irreversible, and Kinetic Systems. Anal. Chem. 1964, 36, 706–723. [Google Scholar] [CrossRef]
- Katano, H.; Tatsumi, H.; Hibi, T.; Ikdat, T.; Tsukatani, T. Application of Polyammonium Cations to Enzyme-immobilized Electrode: Voltammetric Behavior of Polycation-hexacyanoferrate Anion Complexes and Applicability as Electron-Transfer Mediator. Anal. Sci. 2008, 24, 1415–1419. [Google Scholar] [CrossRef]
- Che, X.; Yuan, R.; Chai, Y.; Li, J.; Song, Z.; Li, W.; Zhong, X. A glucose biosensor based on chitosan-Prussian blue-multiwall carbon nanotubes-hollow PtCo nanochains formed by one-step electrodeposition. Colloids Surf. B Biointerfaces 2011, 84, 454–461. [Google Scholar] [CrossRef]
- Pauliukaite, R.; Ghica, M.E.; Fatibello-Filho, O.; Brett, C.M.A. Electrochemical impedance studies of chitosan-modified electrodes for application in electrochemical sensors and biosensors. Electrochim. Acta 2010, 55, 6239–6247. [Google Scholar] [CrossRef]
- Kuzuya, T.; Nakagawa, S.; Satoh, J.; Kanazawa, Y.; Iwamoto, Y.; Kobayashi, M.; Nanjo, K.; Sasaki, A.; Seino, Y.; Ito, C.; et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res. Clin. Pract. 2002, 55, 65–85. [Google Scholar] [Green Version]
- Şenel, M.; Nergiz, C.; Çevik, E. Novel reagentless glucose biosensor based on ferrocene cored asymmetric PAMAM dendrimers. Sens. Actuators B Chem. 2013, 176, 299–306. [Google Scholar] [CrossRef]
- Kuek Lawrence, C.S.; Tan, S.N.; Floresca, C.Z. A “green” cellulose paper based glucose amperometric biosensor. Sens. Actuators B Chem. 2014, 193, 536–541. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, H.; Sun, C. Synthesis of Ferrocene-Branched Chitosan Derivatives: Redox Polysaccharides and their Application to Reagentless Enzyme-Based Biosensors. Macromol. Rapid Commun. 2007, 28, 265–270. [Google Scholar] [CrossRef]
- Merchant, S.A.; Glatzhofer, D.T.; Schmidtke, D.W. Effects of Electrolyte and pH on the Behavior of Cross-Linked Films of Ferrocene-Modified Poly(ethylenimine). Langmuir 2007, 23, 11295–11302. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Wang, D.; Lin, Y.; Yang, L.; Yu, P.; Jiang, W.; Mao, L. Strong interaction between imidazolium-based polycationic polymer and ferricyanide: Toward redox potential regulation for selective in vivo electrochemical measurements. Anal. Chem. 2012, 84, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Chieffo, L.R.; Brenckle, M.A.; Siebert, S.M.; Liu, M.; Strikwerda, A.C.; Fan, K.; Kaplan, D.L.; Zhang, X.; Averitt, R.D.; et al. Metamaterials on paper as a sensing platform. Adv. Mater. 2011, 23, 3197–3201. [Google Scholar] [CrossRef]
- Yoo, E.H.; Lee, S.Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef] [PubMed]









| SPCEs | Ret (Ω) | Zw (Ω) | CPE (µF) | α (×1000) | Rs (Ω) |
|---|---|---|---|---|---|
| Preoxidized | 318.73 ± 4.47 | 505.25 ± 1.48 | 1.34 ± 0.01 | 659.75 ± 4.32 | 445.75 ± 1.64 |
| 0.5 mM αPLL | 237.38 ± 1.98 | 476.25 ± 3.34 | 1.57 ± 0.01 | 570.75 ± 8.20 | 457.25 ± 0.43 |
| 5 mM αPLL | 201.93 ± 3.47 | 875.25 ± 3.27 | 6.39 ± 0.03 | 436.75 ± 1.92 | 486.50 ± 0.50 |
| Modification of GOx/FIC/αPLL (mM) | Sensitivity (nA/mM mm2) | Linear Range (mM) | kcat (1/s) | Km (mM) | kcat/Km |
|---|---|---|---|---|---|
| 0.5/9.5/0.5 | 60.20 ± 5.94 | 1~6 | 2.06 | 12.44 | 0.166 |
| 0.5/99.5/0.5 | 117.40 ± 20.22 | 1~7 | 8.10 | 30.25 | 0.268 |
| 0.5/99.5/5 | 128.73 ± 7.46 | 2~7 | 16.96 | 59.64 | 0.284 |
| Electrodes | Sensitivity (nA/mM mm2) | Linear Range (mM) | LOD (mM) | Ref. |
|---|---|---|---|---|
| PAMAM-Fc/GOx/GCEs 1 | 65.4 | 1.0–22.0 | 0.48 | [41] |
| Fc–COOH/GOx /Cellulose/SPCEs | - | 1.0–5.0 | 0.18 | [42] |
| Fc/Chi/GOx/GCEs 2 | 8.6 | 1.0–6.0 | - | [43] |
| PVP-Os/GOx/SPCEs 3 | 120.0 | - | - | [44] |
| GOx/FIC/Pim/MWCNT/GCEs 4 | 0.4 | 0.3–1.5 | - | [45] |
| GOx/FIC/PLL/SPCEs | 212.1 | 2.8–27.5 | 2.32 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.-J.; Wu, C.-C.; Chang, K.-S. Effect of Poly-l-Lysine Polycation on the Glucose Oxidase/Ferricyanide Composite-Based Second-Generation Blood Glucose Sensors. Sensors 2019, 19, 1448. https://doi.org/10.3390/s19061448
Lin M-J, Wu C-C, Chang K-S. Effect of Poly-l-Lysine Polycation on the Glucose Oxidase/Ferricyanide Composite-Based Second-Generation Blood Glucose Sensors. Sensors. 2019; 19(6):1448. https://doi.org/10.3390/s19061448
Chicago/Turabian StyleLin, Ming-Jie, Ching-Chou Wu, and Ko-Shing Chang. 2019. "Effect of Poly-l-Lysine Polycation on the Glucose Oxidase/Ferricyanide Composite-Based Second-Generation Blood Glucose Sensors" Sensors 19, no. 6: 1448. https://doi.org/10.3390/s19061448