Ecological Monitoring with Spy Satellite Images—The Case of Red Wood Ants in Romania
Abstract
:1. Introduction
- assess the effectiveness and efficiency of using old and recent satellite images for ecological monitoring of forest ants (i.e., to estimate potential habitat for ants); and
- identify the major changes in RWA distribution after 60 years and their causes.
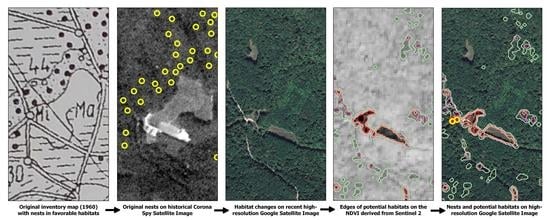
2. Materials and Methods
2.1. Study Area
2.2. Nest Inventory and Statistical Analysis
2.3. Habitat Assessment
2.3.1. Initial Habitat
- those adjacent to permanent forest edges (e.g., to road, agricultural areas or grassland);
- those adjacent to temporary forest edges (between e.g., young/short and old/tall forest). This also includes the case of open borderlines between stands. Even if such lines were narrow, enough radiation could penetrate to the ground at least at young ages, since trees with smaller heights cannot overshadow such lines. If trees forming the edge have less dense crowns (e.g., oaks, pines, larch), conditions will remain open longer;
- those inside or adjacent to stands with more open canopies. In such cases, the habitat would be permanent if the canopy is more open due to species (e.g., oaks), or temporary if it is due to recent silvicultural works (e.g., thinning) or age of stand (i.e., before reaching canopy closure).
2.3.2. Recent Habitat
- −1 < NDVI < 0.5336 = open area, far away from edge;
- 0.5336 < NDVI < 0.6115 = shaded open area near edge, outside the stand;
- 0.6115 < NDVI < 0.6375 = the edge of the forest;
- 0.6375 < NDVI < 0.6634 = well-lit area near edge, inside the stand;
- 0.6634 < NDVI < 1 = closed, dense canopy, inside stand, far from edges.
3. Results
3.1. RWA Distribution Pattern
3.2. RWA Distribution and Habitats
4. Discussion
4.1. Old Map and Satellite Image
4.2. Solar Radiation Regime
4.3. Changes in Habitat Suitability
- an area formerly open in the 1960s, with clear edge and presence of nests, inside the nearby stand, which is now covered by a tall and dense stand (which has closed the edge previously offering favorable conditions) (Figure 7A);
- an area freshly planted with conifers in the 1960s, with open canopy and with evident edges towards surrounding stands (offering good conditions inside and around the edge). The area is now covered by a dense stand (dark canopy, no edges). Below, a previously closed dense stand (in the 1960s) now provides better conditions (access to more radiation) being opened by regeneration cuttings (Figure 7B).
4.4. Changes in Ant Species
4.5. Classification of Stands
4.6. Satellite Images and Derived Products
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A



| Inventory Year | 1960 | 2018 |
|---|---|---|
| Observed Mean Distance (m) | 72.8 | 18.8 |
| Expected Mean Distance (m) | 123.0 | 114.7 |
| Nearest Neighbor Ratio | 0.5919 | 0.1639 |
| z-score | −16.178 * | −36.207 * |
| number of nests | 429 | 512 |
| Forest Stand Categories | Forest Stand Sub-Categories | Area (ha) | No. of Nests | Density (Nests/100 ha) |
|---|---|---|---|---|
| F. sylvatica and diverse hardwood species | Fagus sylvatica & Carpinus betulus mixed stands | 229.50 | 45 | 20 |
| TOTAL | 229.50 | 45 | 20 | |
| Pure Q. robur forests | Pure Q. robur stands on plateaus of medium productivity | 23.76 | 8 | 34 |
| TOTAL | 23.76 | 8 | 34 | |
| Mixed forests with Q. petraea & F. sylvatica of medium respectively high productivity | Mixed hardwood stands (F. sylvatica & Q. petraea most abundant) of high productivity | 112.44 | 15 | 13 |
| Mixed hardwood stands (F. sylvatica & Q. petraea most abundant) of medium productivity | 1320.03 | 214 | 16 | |
| TOTAL | 1432.47 | 229 | 16 | |
| Mixed forests with Q. robur & Q. petraea & F. sylvatica of medium productivity | Mixed hardwood stands (Q.robur & Q. petraea most abundant) of medium productivity | 26.88 | 8 | 30 |
| Mixed hardwood stands dominated by Q. petraea of medium productivity | 50.76 | 38 | 75 | |
| Mixed hardwood stands dominated by Q. robur of medium productivity | 34.45 | 26 | 76 | |
| TOTAL | 112.09 | 72 | 64 | |
| Artificial and semi-artificial standsof conifers with some hardwoods | Plantations of conifers (Larix decidua, Pinus sylvestris & Picea abies) and Q. petraea | 9.00 | 25 | 278 |
| Plantations of Picea abies, Pinus sylvestris and diverse hardwood species | 9.02 | 36 | 400 | |
| Pure Picea abies plantations | 2.54 | 7 | 276 | |
| Young hardwood plantations (<10 year.) | 50.08 | 7 | 14 | |
| TOTAL | 70.64 | 75 | 106 | |
| GRAND TOTAL | 1868.46 | 429 | 23 | |
| Type of Canopy | Area (ha) | Proportion of Area (%) | Number of Nests | Proportion of Nests (%) |
|---|---|---|---|---|
| Open grounds | 25.7 | 1.3 | 5 | 1.0 |
| Light canopy | 95.1 | 4.7 | 12 | 2.3 |
| Mixed canopy | 472.5 | 23.1 | 26 | 5.1 |
| Shade canopy | 1446.3 | 70.9 | 469 | 91.6 |
| Total | 2039.6 | 100.0 | 512 | 100 |
| Canopy Cover (%) | Proportion of Nests (%) | Total (%) | ||
|---|---|---|---|---|
| Number of Forestry Works | ||||
| 0 | 1 | ≥2 | ||
| 0 | 1.0 * | 0.0 | 0.0 | 1.0 |
| 20 | 0.0 | 0.0 | 0.2 | 0.2 |
| 60 | 0.0 | 0.0 | 4.4 | 4.4 |
| 70 | 0.0 | 0.4 | 8.0 | 8.4 |
| 80 | 0.2 | 13.1 | 32.0 | 45.3 |
| 90 | 9.3 | 1.8 | 27.8 | 38.9 |
| 100 | 0.0 | 0.0 | 1.8 | 1.8 |
| Total | 10.5 | 15.3 | 74.2 | 100.0 |
References
- Heinrichs, J.A.; Bender, D.J.; Schumaker, N.H. Habitat degradation and loss as key drivers of regional population extinction. Ecol. Modell. 2016, 335, 64–73. [Google Scholar] [CrossRef]
- Fischer, J.; Lindenmayer, D.B. Landscape modification and habitat fragmentation: A synthesis. Glob. Ecol. Biogeogr. 2007, 16, 265–280. [Google Scholar] [CrossRef]
- Watt, A.S. Pattern and process in the plant community. J. Ecol. 1947, 35, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Kimmins, J.P. Forest Ecology: A Foundation for Sustainable Forest Management and Environmental Ethics in Forestry, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2004; ISBN 9780130662583. [Google Scholar]
- Agosti, D.; Majer, J.; Alonso, L.E.; Schultz, T. Ants: Standard Methods for Measuring and Monitoring Biodiversity; Agosti, D., Majer, J., Alonso, L.E., Schultz, T., Eds.; Smithsonian Institution Press: Washington, DC, USA; London, UK, 2000. [Google Scholar]
- Underwood, E.C.; Fisher, B.L. The role of ants in conservation monitoring: If, when, and how. Biol. Conserv. 2006, 132, 166–182. [Google Scholar] [CrossRef]
- Ellison, A.M. Out of Oz: Opportunities and Challenges for Using Ants (Hymenoptera: Formicidae) as Biological Indicators in North-Temperate Cold Biomes. Myrmecological News. 2012. Available online: https://dash.harvard.edu/bitstream/handle/1/8519155/Ellison_2012_OutofOz.pdf (accessed on 28 January 2021).
- Ribas, C.R.; Campos, R.B.F.; Schmidt, F.A.; Solar, R.R.C. Ants as indicators in Brazil: A review with suggestions to improve the use of ants in environmental monitoring programs. Psyche 2012, 2012, 636749. [Google Scholar] [CrossRef] [Green Version]
- Pașcovici, V. Contribuții la problema combaterii biologice în păduri cu ajutorul furnicilor—Inițierea unor cercetări în masivul păduros Poieni-Iași. Rev. Pădurilor 1961, 76, 295–299. (In Romanian) [Google Scholar]
- Pașcovici, V.; Simionescu, A.; Podariu, M.; Pentiuc, V.; Caraman, V. Cercetări Privind Furnicile de Pădure din R.S. România și Folosirea lor în Combaterea Dăunătorilor Forestieri; Editura Silvica: Ilfov, Romania, 1968. (In Romanian) [Google Scholar]
- Pașcovici, V. Combatere Biologică cu Ajutorul Furnicilor din Genul Formica, L. (Hymenop. Formicidae) și Necesitatea Utilizării ei în R.P.R.: Inițierea unor Cercetări în Masivul Păduros Poieni-Iași; Ministerul Agriculturii, Ed.; Editura Agro-Silvică: Bucharest, Romania, 1961. (In Romanian) [Google Scholar]
- Sorvari, J.; Hakkarainen, H. Forest clearing and sex ratio in forest-dwelling wood ant Formica aquilonia. Naturwissenschaften 2007, 94, 392–395. [Google Scholar] [CrossRef]
- Grevé, M.E.; Hager, J.; Weisser, W.W.; Schall, P.; Gossner, M.M.; Feldhaar, H. Effect of forest management on temperate ant communities. Ecosphere 2018, 9, e02303. [Google Scholar] [CrossRef]
- Wellenstein, G. Zur Frage der Standortansprüche. hügelbauender Waldameisen (Formica rufa-Gruppe). Z. Angew. Zool. 1967, 54, 139–166. [Google Scholar]
- Procter, D.S.; Cottrell, J.; Watts, K.; Robinson, E.J.H. Do non-native conifer plantations provide benefits for a native forest specialist, the wood ant Formica lugubris? For. Ecol. Manag. 2015, 357, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Sudd, J.H.; Douglas, J.M.; Gaynard, T.; Murray, D.M.; Stockdale, J.M. The distribution of wood-ants (Formica lugubris Zetterstedt) in a Northern English forest. Ecol. Entomol. 1977, 2, 301–313. [Google Scholar] [CrossRef]
- Kilpeläinen, J.; Punttila, P.; Finér, L.; Niemelä, P.; Domisch, T.; Jurgensen, M.F.; Neuvonen, S.; Ohashi, M.; Risch, A.C.; Sundström, L. Distribution of ant species and mounds (Formica) in different-aged managed spruce stands in eastern Finland. J. Appl. Entomol. 2008, 132, 315–325. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Robinson, E.J.H. The relationship between canopy cover and colony size of the wood ant Formica lugubris—Implications for the thermal effects on a keystone ant species. PLoS ONE 2014, 9, e116113. [Google Scholar] [CrossRef] [PubMed]
- Kadochová, Š.; Frouz, J. Red wood ants Formica polyctena switch off active thermoregulation of the nest in autumn. Insectes Soc. 2014, 61, 297–306. [Google Scholar] [CrossRef]
- Punttila, P.; Kilpeläinen, J. Distribution of Mound-Building Ant Species (Formica spp., Hymenoptera) in Finland: Preliminary results of a national survey. Ann. Zool. Fenn. 2009, 46, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Reymond, A.; Purcell, J.; Cherix, D.; Guisan, A.; Pellissier, L. Functional diversity decreases with temperature in high elevation ant fauna. Ecol. Entomol. 2013, 38, 364–373. [Google Scholar] [CrossRef]
- Del Toro, I.; Berberich, G.M.; Ribbons, R.R.; Berberich, M.B.; Sanders, N.J.; Ellison, A.M. Nests of red wood ants (Formica rufa-group) are positively associated with tectonic faults: A double-blind test. PeerJ 2017, 2017, e3903. [Google Scholar] [CrossRef] [Green Version]
- Niemelä, J.; Haila, Y.; Punttila, P. The importance of small-scale heterogeneity in boreal forests: Variation in diversity in forest-floor invertebrates across the succession gradient. Ecography 1996, 19, 352–368. [Google Scholar] [CrossRef]
- Véle, A.; Holuša, J.; Frouz, J. Ecological requirements of some ant species of the genus Formica (Hymenoptera, Formicidae) in spruce forests. J. For. Sci. 2009, 55, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Kasimova, R.G.; Tishin, D.; Obnosov, Y.V.; Dlussky, G.M.; Baksht, F.B.; Kacimov, A.R. Ant mound as an optimal shape in constructal design: Solar irradiation and circadian brood/fungi-warming sorties. J. Theor. Biol. 2014, 355, 21–32. [Google Scholar] [CrossRef]
- Niță, M.D.; Munteanu, C.; Gutman, G.; Abrudan, I.V.; Radeloff, V.C. Widespread forest cutting in the aftermath of World War II captured by broad-scale historical Corona spy satellite photography. Remote Sens. Environ. 2018, 204, 322–332. [Google Scholar] [CrossRef]
- Munteanu, C.; Kamp, J.; Nita, M.D.; Klein, N.; Kraemer, B.M.; Müller, D.; Koshkina, A.; Prishchepov, A.V.; Kuemmerle, T. Cold War spy satellite images reveal long-term declines of a philopatric keystone species in response to cropland expansion. Proc. Biol. Sci. 2020, 287, 20192897. [Google Scholar] [CrossRef] [PubMed]
- NASA Jet Propulsation Laboratory. Shuttle Radar Topography Mission Global 1 Arc Second [Data Set]. Available online: https://www2.jpl.nasa.gov/srtm/ (accessed on 28 January 2021).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Gösswald, K. Rassenstudien an der roten Waldameise Formica rufa L. auf systematischer, ökologischer, physiologischer und biologischer Grundlage. Z. Angew. Entomol. 1941, 28, 62–124. [Google Scholar] [CrossRef]
- Betrem, J.G. Enkele opmerking ontrent de sorten van de Formica rufa-Groep (Hym.). Entom. Ber. 1953, 14, 322–326. [Google Scholar]
- Yarrow, I.H.H. The British ants allied to Formica rufa L.(Hym., Formicidae). Trans. Soc. Br. Entomol. 1955, 12, 1–48. [Google Scholar]
- U.S. Geological Survey. Declassified Intelligence Satellite Photographs: U.S. Geological Survey Fact Sheet 2008–3054; U.S. Geological Survey Earth Resources Observation and Science (EROS) Center: Sioux Falls, SD, USA, 2008. [Google Scholar]
- Kaim, D.; Kozak, J.; Kolecka, N.; Ziółkowska, E.; Ostafin, K.; Ostapowicz, K.; Gimmi, U.; Munteanu, C.; Radeloff, V.C. Broad scale forest cover reconstruction from historical topographic maps. Appl. Geogr. 2016, 67, 39–48. [Google Scholar] [CrossRef]
- Google Maps Poieni, Iasi Area. Available online: https://www.google.com/maps/@47.0309215,27.6454192,14z (accessed on 18 March 2020).
- Berberich, G.M.; Dormann, C.F.; Klimetzek, D.; Berberich, M.B.; Sanders, N.J.; Ellison, A.M. Detection probabilities for sessile organisms. Ecosphere 2016, 7, e01546. [Google Scholar] [CrossRef]
- Seifert, B. The Ants of Central and North Europe; Lutra: Tauer, Germany, 2018. [Google Scholar]
- Roquer-Beni, L.; Rodrigo, A.; Arnan, X.; Klein, A.M.; Fornoff, F.; Boreux, V.; Bosch, J. A novel method to measure hairiness in bees and other insect pollinators. Ecol. Evol. 2020, 10, 2979–2990. [Google Scholar] [CrossRef] [Green Version]
- Environmental Systems Research Institute (ESRI). ARCGIS Desktop, Version 10.6; Environmental Systems Research Institute: Redlands, CA, USA, 2016. [Google Scholar]
- QGIS Geographic Information System. OSGeo QGIS 3.4. Available online: http://qgis.osgeo.org2019 (accessed on 28 January 2021).
- The MathWorks Inc. Matlab2017 (R2010a); The MathWorks Inc.: Natick, MS, USA, 2017. [Google Scholar]
- R Core Team. R. A Language and Environment for Statistical Computin; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed on 18 March 2020).
- Baddeley, A.; Rubak, E.; Turner, R. Spatial Point Patterns: Methodology and Applications with R, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781482210200. [Google Scholar]
- Ord, J.K.; Getis, A. Local spatial autocorrelation statistics: Distributional issues and an application. Geogr. Anal. 1995, 27, 286–306. [Google Scholar] [CrossRef]
- Getis, A.; Ord, J.K. The analysis of spatial association by use of distance statistics. In Advances in Spatial Science; Springer: Cham, Switzerland, 2010; Volume 61, pp. 127–145. [Google Scholar]
- Gamon, J.A.; Field, C.B.; Goulden, M.L.; Griffin, K.L.; Hartley, A.E.; Joel, G.; Penuelas, J.; Valentini, R. Relationships between NDVI, canopy structure, and photosynthesis in three Californian vegetation types. Ecol. Appl. 1995, 5, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Jenks, G.F. The data model concept in statistical mapping. Int. Yearb. Cartogr. 1967, 7, 186–190. [Google Scholar]
- Wellenstein, G. Die Beeinflussung der forstlichen Arthropodenfauna durch Waldameisen (Formica rufa-Gruppe). Z. Angew. Entomol. 1957, 41, 368–385. [Google Scholar] [CrossRef]
- Wellenstein, G. Waldbewohnende Ameisen, ihre Bedeutung, ihre Biologie, ihre Hege und ihr Schutz, 2nd ed.; Allgäuer Zeitungsverlag GmbH: Kempten, Germany, 1990. [Google Scholar]
- Gordon, D.M. The development of an ant colony’s foraging range. Anim. Behav. 1995, 49, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Palladini, J.D.; Jones, M.G.; Sanders, N.J.; Jules, E.S. The recovery of ant communities in regenerating temperate conifer forests. For. Ecol. Manag. 2007, 242, 619–624. [Google Scholar] [CrossRef]
- Tăușan, I.; Dauber, J.; Trică, M.R.; Marko, B. Succession in ant communities (Hymenoptera: Formicidae) in deciduous forest clear-cuts—An Eastern European case study. Eur. J. Entomol. 2017, 114, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Ellis, S.; Franks, D.W.; Robinson, E.J.H. Ecological consequences of colony structure in dynamic ant nest networks. Ecol. Evol. 2017, 7, 1170–1180. [Google Scholar] [CrossRef] [Green Version]
- Duma, I. The impact of red wood ants Formica rufa on the distribution of invertebrate fauna from the forest’s floor. Ann. West Univ. Timis. Ser. Biol. 2003, 5–6, 121–130. [Google Scholar]
- Berberich, G.M.; Klimetzek, D.; Paraschiv, M.; Stancioiu, P.T.; Grumpe, A. Biogeostatistics confirm: Even a low total number of red wood ant nests provide new information on tectonics in the East Carpathian Orogen (Romania). Ecol. Indic. 2019, 101, 486–500. [Google Scholar] [CrossRef]
- Perron, M.; Glanzmann, I.; Freitag, A. Habitatselektion von zwei Waldameisenarten (Formica rufa und F. polyctena). Schweiz. Z. Forstwes. 2019, 170, 32–39. [Google Scholar] [CrossRef]
- Klimetzek, D. Die Variabilität der Standortansprüche hügelbauender Waldameisen der Formica rufa-Gruppe (Hymenoptera: Formicidae). Mitt. Badischen Landesver. Naturkd. Nat. N.F. 1973, 11, 9–25. [Google Scholar]
- Klimetzek, D.; Kaiser, M. Zur Ökologie der Formica rufa-Gruppe. Waldhygiene 1995, 20, 243–254. [Google Scholar]
- Klimetzek, D. Population studies on hill building wood-ants of the Formica rufa-group. Oecologia 1981, 48, 418–421. [Google Scholar] [CrossRef] [PubMed]







| RWA Habitat Category | Area (ha) | Proportion of Area (%) | Number of Nests | Proportion of Nests (%) |
|---|---|---|---|---|
| (1) open area, far away from edge | 6.5 | 0.3 | - | - |
| (2) shaded open area near edge, outside the stand | 15.5 | 0.8 | 16 | 3 |
| (3) the edge of the forest | 19.7 | 1.0 | 31 | 6 |
| (4) well-lit area near edge, inside the stand | 97.2 | 4.8 | 66 | 13 |
| (5) closed, dense canopy, inside stand, far from edges | 1900.7 | 93.1 | 399 | 78 |
| Total | 2039.6 | 100.0 | 512 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimetzek, D.; Stăncioiu, P.T.; Paraschiv, M.; Niță, M.D. Ecological Monitoring with Spy Satellite Images—The Case of Red Wood Ants in Romania. Remote Sens. 2021, 13, 520. https://doi.org/10.3390/rs13030520
Klimetzek D, Stăncioiu PT, Paraschiv M, Niță MD. Ecological Monitoring with Spy Satellite Images—The Case of Red Wood Ants in Romania. Remote Sensing. 2021; 13(3):520. https://doi.org/10.3390/rs13030520
Chicago/Turabian StyleKlimetzek, Dietrich, Petru Tudor Stăncioiu, Marius Paraschiv, and Mihai Daniel Niță. 2021. "Ecological Monitoring with Spy Satellite Images—The Case of Red Wood Ants in Romania" Remote Sensing 13, no. 3: 520. https://doi.org/10.3390/rs13030520