Soil Organic Carbon Mapping from Remote Sensing: The Effect of Crop Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Sample Collection
2.2. Remote Sensing Data
2.2.1. Airborne Images
2.2.2. Spaceborne Images
2.3. Spectral Indices
2.4. Weather Data
2.5. Soil Organic Carbon Predictive Models
2.5.1. Spectral Pre-Processing
2.5.2. PLSR Model
2.5.3. CAI Thresholding
2.6. Linking CAI and NBR2
2.7. Image Classification for Crop Residue Cover Quantification
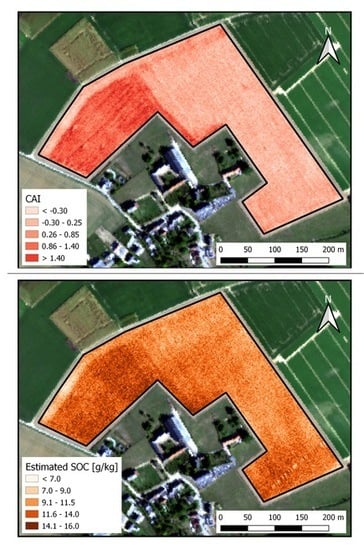
3. Results
3.1. Quality of the APEX Spectra
3.2. SOC Prediction Models
3.2.1. Without CAI Threshold
3.2.2. With CAI Threshold
3.3. Crop Residue Detection Using Sentinel-2
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Treatment | LV | RMSE * | R2 | RPD |
|---|---|---|---|---|
| Reflectance | 7 | 2.40 | 0.38 | 1.24 |
| Absorbance | 13 | 2.14 | 0.49 | 1.39 |
| SG-R | 7 | 2.42 | 0.36 | 1.23 |
| SG-A | 9 | 2.33 | 0.40 | 1.28 |
| FD | 7 | 2.26 | 0.44 | 1.32 |
| SG-FD | 12 | 2.36 | 0.39 | 1.26 |
| SD | 9 | 2.80 | 0.24 | 1.06 |
| SG-SD | 12 | 2.31 | 0.41 | 1.28 |
| SNV | 5 | 2.45 | 0.33 | 1.21 |
| SNV detrend | 4 | 2.40 | 0.36 | 1.24 |
| CR | 6 | 2.29 | 0.42 | 1.29 |
| CAI | 0.00 | 0.25 | 0.50 | 0.75 | 1.00 | 1.25 | 1.50 | 1.75 | 2.00 | 2.25 | 2.50 | 2.75 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | - | |||||||||||
| 0.25 | 0.532 | - | ||||||||||
| 0.50 | 0.312 | 0.598 | - | |||||||||
| 0.75 | 0.253 | 0.491 | 0.871 | - | ||||||||
| 1.00 | 0.195 | 0.383 | 0.726 | 0.852 | - | |||||||
| 1.25 | 0.114 | 0.219 | 0.465 | 0.565 | 0.69 | - | ||||||
| 1.50 | 0.059 | 0.106 | 0.248 | 0.313 | 0.396 | 0.65 | - | |||||
| 1.75 | 0.063 | 0.115 | 0.270 | 0.341 | 0.430 | 0.696 | 0.946 | - | ||||
| 2.00 | 0.057 | 0.107 | 0.260 | 0.330 | 0.420 | 0.689 | 0.948 | 0.997 | - | |||
| 2.25 | 0.057 | 0.107 | 0.260 | 0.330 | 0.420 | 0.689 | 0.948 | 0.997 | 1.000 | - | ||
| 2.50 | 0.057 | 0.107 | 0.260 | 0.330 | 0.420 | 0.689 | 0.948 | 0.997 | 1.000 | 1.000 | - | |
| 2.75 | 0.049 | 0.092 | 0.230 | 0.294 | 0.377 | 0.634 | 0.994 | 0.938 | 0.940 | 0.940 | 0.940 | - |
| 3.00 | 0.046 | 0.087 | 0.223 | 0.287 | 0.369 | 0.627 | 0.99 | 0.934 | 0.936 | 0.936 | 0.936 | 0.997 |
References
- Stockmann, U.; Padarian, J.; McBratney, A.; Minasny, B.; de Brogniez, D.; Montanarella, L.; Hong, S.Y.; Rawlins, B.G.; Field, D.J. Global soil organic carbon assessment. Glob. Food Secur. 2015, 6, 9–16. [Google Scholar] [CrossRef]
- Le Quéré, C.; Peters, G.P.; Andres, R.J.; Andrew, R.M.; Boden, T.A.; Ciais, P.; Friedlingstein, P.; Houghton, R.A.; Marland, G.; Moriarty, R.; et al. Global carbon budget 2013. Earth Syst. Sci. Data 2014, 6, 235–263. [Google Scholar] [CrossRef] [Green Version]
- Oldfield, E.E.; Bradford, M.A.; Wood, S.A. Global meta-analysis of the relationship between soil organic matter and crop yields. SOIL 2019, 5, 15–32. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Chevallier, F.; Gomez, C.; Guanter, L.; Hicke, J.A.; Huete, A.R.; Ichii, K.; Ni, W.; Pang, Y.; Rahman, A.F.; et al. Remote sensing of the terrestrial carbon cycle: A review of advances over 50 years. Remote Sens. Environ. 2019, 233, 111383. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Žižala, D.; Saberioon, M.; Borůvka, L. Soil organic carbon and texture retrieving and mapping using proximal, airborne and Sentinel-2 spectral imaging. Remote Sens. Environ. 2018, 218, 89–103. [Google Scholar] [CrossRef]
- Castaldi, F.; Palombo, A.; Santini, F.; Pascucci, S.; Pignatti, S.; Casa, R. Evaluation of the potential of the current and forthcoming multispectral and hyperspectral imagers to estimate soil texture and organic carbon. Remote Sens. Environ. 2016, 179, 54–65. [Google Scholar] [CrossRef]
- Castaldi, F.; Chabrillat, S.; Jones, A.; Vreys, K.; Bomans, B.; van Wesemael, B. Soil Organic Carbon Estimation in Croplands by Hyperspectral Remote APEX Data Using the LUCAS Topsoil Database. Remote Sens. 2018, 10, 153. [Google Scholar] [CrossRef] [Green Version]
- Castaldi, F.; Hueni, A.; Chabrillat, S.; Ward, K.; Buttafuoco, G.; Bomans, B.; Vreys, K.; Brell, M.; van Wesemael, B. Evaluating the capability of the Sentinel 2 data for soil organic carbon prediction in croplands. ISPRS J. Photogramm. Remote Sens. 2019, 147, 267–282. [Google Scholar] [CrossRef]
- Moura-Bueno, J.M.; Dalmolin, R.S.D.; ten Caten, A.; Dotto, A.C.; Demattê, J.A.M. Stratification of a local VIS-NIR-SWIR spectral library by homogeneity criteria yields more accurate soil organic carbon predictions. Geoderma 2019, 337, 565–581. [Google Scholar] [CrossRef]
- Vaudour, E.; Gilliot, J.M.; Bel, L.; Lefevre, J.; Chehdi, K. Regional prediction of soil organic carbon content over temperate croplands using visible near-infrared airborne hyperspectral imagery and synchronous field spectra. Int. J. Appl. Earth Obs. Geoinf. 2016, 49, 24–38. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, H.; Shi, T.; Chen, Y.; Jiang, Q.; Linderman, M. Prediction of soil organic carbon stock by laboratory spectral data and airborne hyperspectral images. Geoderma 2019, 337, 32–41. [Google Scholar] [CrossRef]
- Chabrillat, S.; Ben-Dor, E.; Cierniewski, J.; Gomez, C.; Schmid, T.; van Wesemael, B. Imaging Spectroscopy for Soil Mapping and Monitoring. Surv. Geophys. 2019, 40, 361–399. [Google Scholar] [CrossRef] [Green Version]
- Loizzo, R.; Guarini, R.; Longo, F.; Scopa, T.; Formaro, R.; Facchinetti, C.; Varacalli, G. Prisma: The Italian Hyperspectral Mission. In Proceedings of the IGARSS 2018–2018 IEEE International Geoscience and Remote Sensing Symposium, Valencia, Spain, 22–27 July 2018; pp. 175–178. [Google Scholar]
- Guanter, L.; Kaufmann, H.; Segl, K.; Foerster, S.; Rogass, C.; Chabrillat, S.; Kuester, T.; Hollstein, A.; Rossner, G.; Chlebek, C.; et al. The EnMAP Spaceborne Imaging Spectroscopy Mission for Earth Observation. Remote. Sens. 2015, 7, 8830–8857. [Google Scholar] [CrossRef] [Green Version]
- Feingersh, T.; Dor, E.B. SHALOM—A Commercial Hyperspectral Space Mission. In Optical Payloads for Space Missions; Wiley: Hoboken, NJ, USA, 2015; pp. 247–263. [Google Scholar]
- Vaudour, E.; Gomez, C.; Loiseau, T.; Baghdadi, N.; Loubet, B.; Arrouays, D.; Ali, L.; Lagacherie, P. The Impact of Acquisition Date on the Prediction Performance of Topsoil Organic Carbon from Sentinel-2 for Croplands. Remote Sens. 2019, 11, 2143. [Google Scholar] [CrossRef] [Green Version]
- Angelopoulou, T.; Tziolas, N.; Balafoutis, A.; Zalidis, G.; Bochtis, D. Remote Sensing Techniques for Soil Organic Carbon Estimation: A Review. Remote Sens. 2019, 11, 676. [Google Scholar] [CrossRef] [Green Version]
- Rodionov, A.; Pätzold, S.; Welp, G.; Pallares, R.C.; Damerow, L.; Amelung, W. Sensing of Soil Organic Carbon Using Visible and Near-Infrared Spectroscopy at Variable Moisture and Surface Roughness. Soil Sci. Soc. Am. J. 2014, 78, 949–957. [Google Scholar] [CrossRef]
- Nocita, M.; Stevens, A.; Noon, C.; van Wesemael, B. Prediction of soil organic carbon for different levels of soil moisture using Vis-NIR spectroscopy. Geoderma 2013, 199, 37–42. [Google Scholar] [CrossRef]
- Bartholomeus, H.; Kooistra, L.; Stevens, A.; van Leeuwen, M.; van Wesemael, B.; Ben-Dor, E.; Tychon, B. Soil Organic Carbon mapping of partially vegetated agricultural fields with imaging spectroscopy. Int. J. Appl. Earth Obs. Geoinf. 2011, 13, 81–88. [Google Scholar] [CrossRef]
- Rodionov, A.; Pätzold, S.; Welp, G.; Pude, R.; Amelung, W. Proximal field Vis-NIR spectroscopy of soil organic carbon: A solution to clear obstacles related to vegetation and straw cover. Soil Tillage Res. 2016, 163, 89–98. [Google Scholar] [CrossRef]
- Daughtry, C.; McMurtrey, J.E.; Chapelle, E.W.; Hunter, W.J.; Steiner, J.L. Measuring crop residue cover using remote sensing. Theor. Appl. Clim. 1996, 54, 17–26. [Google Scholar] [CrossRef]
- Nagler, P.L.; Inoue, Y.; Glenn, E.P.; Russ, A.L.; Daughtry, C.S.T. Cellulose absorption index (CAI) to quantify mixed soil–plant litter scenes. Remote Sens. Environ. 2003, 87, 310–325. [Google Scholar] [CrossRef]
- Ben-Dor, E. The reflectance spectra of the organic matter in the visible near infrared and the short wave infrared region during the controlled decomposition process. Remote Sens. Environ. 1997, 61, 1–15. [Google Scholar] [CrossRef]
- Demattê, J.A.M.; Fongaro, C.T.; Rizzo, R.; Safanelli, J.L. Geospatial Soil Sensing System (GEOS3): A powerful data mining procedure to retrieve soil spectral reflectance from satellite images. Remote Sens. Environ. 2018, 212, 161–175. [Google Scholar] [CrossRef]
- Castaldi, F.; Chabrillat, S.; Don, A.; van Wesemael, B. Soil Organic Carbon Mapping Using LUCAS Topsoil Database and Sentinel-2 Data: An Approach to Reduce Soil Moisture and Crop Residue Effects. Remote Sens. 2019, 11, 2121. [Google Scholar] [CrossRef] [Green Version]
- Shi, P.; Castaldi, F.; van Wesemael, B.; Van Oost, K. Vis-NIR spectroscopic assessment of soil aggregate stability and aggregate size distribution in the Belgian Loam Belt. Geoderma 2020, 357, 113958. [Google Scholar] [CrossRef]
- Sherrod, L.A.; Dunn, G.; Peterson, G.; Kolberg, R. Inorganic Carbon Analysis by Modified Pressure-Calcimeter Method. Soil Sci. Soc. Am. J. 2002, 66, 299. [Google Scholar] [CrossRef]
- VITO. APEX 2018-Hesbania Campaign Data Delivery Report; APX-VTO-HESBANIA2018; VITO: Mol, Belgium, 2019. [Google Scholar]
- Gege, P.; Fries, J.; Haschberger, P.; Schötz, P.; Schwarzer, H.; Strobl, P.; Suhr, B.; Ulbrich, G.; Jan Vreeling, W. Calibration facility for airborne imaging spectrometers. ISPRS J. Photogramm. Remote Sens. 2009, 64, 387–397. [Google Scholar] [CrossRef]
- Vreys, K.; Iordache, M.-D.; Biesemans, J.; Meuleman, K. Geometric correction of APEX hyperspectral data. Misc. Geogr. 2016, 20, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Schaepman, M.E.; Jehle, M.; Hueni, A.; D’Odorico, P.; Damm, A.; Weyermann, J.; Schneider, F.D.; Laurent, V.; Popp, C.; Seidel, F.C.; et al. Advanced radiometry measurements and Earth science applications with the Airborne Prism Experiment (APEX). Remote Sens. Environ. 2015, 158, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P.; et al. Sentinel-2: ESA’s Optical High-Resolution Mission for GMES Operational Services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- Daughtry, C.S.T. Discriminating Crop Residues from Soil by Shortwave Infrared Reflectance. Agron. J. 2001, 93, 125–131. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Scheel, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. In Proceedings of the 3rd Earth Resource Technology Satellite (ERTS) Symposium, Washington, DC, USA, 10–14 December 1973; pp. 48–62. [Google Scholar]
- Van Deventer, A.P.; Ward, A.D.; Gowda, P.H.; Lyon, J.G. Using Thematic Mapper Data to Identify Contrasting Soil Plains and Tillage Practices. Am. Soc. Photogramm. Remote Sens. 1997, 63, 87–93. [Google Scholar]
- Stevens, A.; Udelhoven, T.; Denis, A.; Tychon, B.; Lioy, R.; Hoffmann, L.; van Wesemael, B. Measuring soil organic carbon in croplands at regional scale using airborne imaging spectroscopy. Geoderma 2010, 158, 32–45. [Google Scholar] [CrossRef]
- Norris, K.H.; Williams, P.C. Optimization of mathematical treatments of raw near-infrared signal in the measurement of protein in hard red spring wheat. I. Influence of particle size. Cereal Chem. 1984, 61, 158–165. [Google Scholar]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard Normal Variate Transformation and De-Trending of Near-Infrared Diffuse Reflectance Spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Clark, R.N.; Roush, T.L. Reflectance spectroscopy: Quantitative analysis techniques for remote sensing applications. J. Geophys. Res. Solid Earth 1984, 89, 6329–6340. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Nocita, M.; Stevens, A.; Toth, G.; Panagos, P.; van Wesemael, B.; Montanarella, L. Prediction of soil organic carbon content by diffuse reflectance spectroscopy using a local partial least square regression approach. Soil Biol. Biochem. 2014, 68, 337–347. [Google Scholar] [CrossRef]
- Chang, C.-W.; Laird, D.A.; Mausbach, M.J.; Hurburgh, C.R. Near-Infrared Reflectance Spectroscopy–Principal Components Regression Analyses of Soil Properties Journal Paper no. J-18766 of the Iowa Agric. and Home Econ. Exp. Stn., Ames, IA. Soil Sci. Soc. Am. J. 2001, 65, 480–490. [Google Scholar] [CrossRef] [Green Version]
- Minasny, B. Why Calculating RPD is Redundant; The Newsletter of the Pedometrics Commission of the International Union of Soil Sciences: Vienna, Austria, 2013. [Google Scholar]
- Vašát, R.; Kodešová, R.; Klement, A.; Borůvka, L. Simple but efficient signal pre-processing in soil organic carbon spectroscopic estimation. Geoderma 2017, 298, 46–53. [Google Scholar] [CrossRef]
- Gomez, C.; Lagacherie, P.; Coulouma, G. Continuum removal versus PLSR method for clay and calcium carbonate content estimation from laboratory and airborne hyperspectral measurements. Geoderma 2008, 148, 141–148. [Google Scholar] [CrossRef]
- Webster, R.; Margaret, A.O. Geostatistics for Environmental Scientists, 2nd ed.; John Wiley & Sons Ldt: Chichester, UK, 2007. [Google Scholar]
- Shi, P.; Castaldi, F.; van Wesemael, B.; Van Oost, K. Large-Scale, High-Resolution Mapping of Soil Aggregate Stability in Croplands Using APEX Hyperspectral Imagery. Remote. Sens. 2020, 12, 666. [Google Scholar] [CrossRef] [Green Version]
- Debaene, G.; Niedźwiecki, J.; Pecio, A.; Żurek, A. Effect of the number of calibration samples on the prediction of several soil properties at the farm-scale. Geoderma 2014, 214, 114–125. [Google Scholar] [CrossRef]
- Musick, H.B.; Pelletier, R.E. Response to soil moisture of spectral indexes derived from bidirectional reflectance in thematic mapper wavebands. Remote Sens. Environ. 1988, 25, 167–184. [Google Scholar] [CrossRef]
- Quemada, M.; Daughtry, C.S.T. Spectral Indices to Improve Crop Residue Cover Estimation under Varying Moisture Conditions. Remote Sens. 2016, 8, 660. [Google Scholar] [CrossRef] [Green Version]
- Haubrock, S.N.; Chabrillat, S.; Lemmnitz, C.; Kaufmann, H. Surface soil moisture quantification models from reflectance data under field conditions. Int. J. Remote Sens. 2010, 29, 3–29. [Google Scholar] [CrossRef]
- Yue, J.; Tian, Q.; Dong, X.; Xu, N. Using broadband crop residue angle index to estimate the fractional cover of vegetation, crop residue, and bare soil in cropland systems. Remote Sens. Environ. 2020, 237, 111538. [Google Scholar] [CrossRef]
- Cierniewski, J.; Ceglarek, J. Annual dynamics of shortwave radiation of bare arable lands on a global scale incorporating their roughness. Environ. Earth Sci. 2018, 77, 777. [Google Scholar] [CrossRef] [Green Version]
- Ward, K.J.; Chabrillat, S.; Neumann, C.; Foerster, S. A remote sensing adapted approach for soil organic carbon prediction based on the spectrally clustered LUCAS soil database. Geoderma 2019, 353, 297–307. [Google Scholar] [CrossRef]





















| MSI | APEX | |
|---|---|---|
| Altitude (km) | 786 | 3.6 |
| Sensor type | multispectral | hyperspectral |
| Spectral range (nm) | 443–2190 | 413–2431 |
| Spectral bands | 13 | 285 |
| Resolution | ||
| Spatial (m) | 10–20–60 | 2 |
| Temporal (day) | 5 | — |
| Spectral (nm) | 15–180 | 2–13 |
| Noisy bands (nm) | — | 413–440, 1310–1555, 1750–2000 |
| Signal to noise (SNR) | ||
| VNIR | 89:1 to 168:1 | 50:1 to 700:1 [8] |
| SWIR | 50:1 to 100:1 | 40:1 to 600:1 * [8] |
| Index | Equation | MSI Bands | APEX Bands | Reference |
|---|---|---|---|---|
| NDVI | Rred: 665 nm (B4) RNIR: 842 nm (B8) | Rred: 664 nm RNIR: 842 nm | [35] | |
| CAI | R2.0: 2026 nm R2.1: 2100 nm R2.2: 2214 nm | [34] | ||
| NBR2 | RSWIR1: 1610 nm (B11) RSWIR2: 2190 nm (B12) | [36] |
| Descriptive Statistics | Tenfold-Cross-Validation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CAI Threshold | n | Min * | Max * | Mean * | Std * | CV (%) | LV | RMSE * | R2 | RPD |
| 0.00 | 40 | 7.5 | 16.5 | 10.9 | 2.1 | 19.5 | 3 | 1.98 | 0.14 | 1.07 |
| 0.25 | 58 | 7.5 | 16.5 | 11.2 | 2.2 | 19.8 | 4 | 1.84 | 0.32 | 1.20 |
| 0.50 | 75 | 7.5 | 19.9 | 11.5 | 2.6 | 22.4 | 6 | 1.87 | 0.48 | 1.38 |
| 0.75 | 80 | 7.5 | 19.9 | 11.6 | 2.8 | 23.0 | 13 | 1.75 | 0.59 | 1.51 |
| 1.00 | 90 | 7.5 | 19.9 | 11.7 | 2.8 | 23.4 | 4 | 2.11 | 0.40 | 1.29 |
| 1.25 | 95 | 7.5 | 20.0 | 11.8 | 3.0 | 24.0 | 4 | 2.21 | 0.39 | 1.29 |
| 1.50 | 99 | 7.5 | 20.2 | 12.0 | 3.0 | 25.0 | 13 | 2.31 | 0.45 | 1.30 |
| 1.75 | 100 | 7.5 | 20.2 | 12.0 | 3.0 | 24.9 | 5 | 2.33 | 0.40 | 1.28 |
| 2.00–2.50 | 102 | 7.5 | 20.2 | 12.0 | 3.0 | 24.7 | 12 | 2.26 | 0.45 | 1.32 |
| 2.75 | 103 | 7.5 | 20.2 | 12.1 | 3.0 | 24.7 | 14 | 2.25 | 0.47 | 1.32 |
| 3.00 | 104 | 7.5 | 20.2 | 12.1 | 3.0 | 24.5 | 13 | 2.13 | 0.49 | 1.39 |
| Model | Sill (g2/kg2) | Range (m) |
|---|---|---|
| Nugget effect | 0.023 | |
| Exponential * | 0.109 | 335 |
| Coefficients | R2 | n | ||
|---|---|---|---|---|
| CAI (APEX)~NBR2 (APEX) | Intercept | −1.403 *** | 0.83 | 188 |
| Slope | 25.955 *** | |||
| NBR2 (APEX)~NBR2 (S-2) | Intercept | 0.003 *** | 0.43 | 188 |
| Slope | 0.642 *** | |||
| CAI (APEX)~NBR2 (S-2) | Intercept | 0.039 *** | 0.47 | 188 |
| Slope | 0.023 *** | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvorakova, K.; Shi, P.; Limbourg, Q.; van Wesemael, B. Soil Organic Carbon Mapping from Remote Sensing: The Effect of Crop Residues. Remote Sens. 2020, 12, 1913. https://doi.org/10.3390/rs12121913
Dvorakova K, Shi P, Limbourg Q, van Wesemael B. Soil Organic Carbon Mapping from Remote Sensing: The Effect of Crop Residues. Remote Sensing. 2020; 12(12):1913. https://doi.org/10.3390/rs12121913
Chicago/Turabian StyleDvorakova, Klara, Pu Shi, Quentin Limbourg, and Bas van Wesemael. 2020. "Soil Organic Carbon Mapping from Remote Sensing: The Effect of Crop Residues" Remote Sensing 12, no. 12: 1913. https://doi.org/10.3390/rs12121913