Monitoring of Canopy Stress Symptoms in New Zealand Kauri Trees Analysed with AISA Hyperspectral Data
Abstract
:1. Introduction
1.1. Kauri and Kauri Dieback Disease
1.2. Remote Sensing for Stress Monitoring
1.3. Approach and Objectives
- (1).
- Identify the best band and index combinations to detect stress symptoms in kauri crowns for both the full spectral range (VIS–SWIR) and the VNIR1 spectral range. The selected band-combinations should not exceed six wavelengths, to be suitable for a multispectral platform.
- (2).
- Test the performance of a pre-defined band combination for stress detection, which was defined in Meiforth et al. (2019) [11] to locate kauri trees.
- (3).
- Test the performance of the model and indices-selections that was developed on mean crown values in a pixel-based approach by calculating the model on indices raster.
2. Materials and Methods
2.1. Study Area
2.2. Remote Sensing Data and Preparation
2.3. Reference Crowns
2.4. Field Attributes and Stress Assessment
2.5. Calculation and Selection of Crown Based Attributes
2.6. Selection and Parameterisation of the Algorithms
2.7. Test of Pixel-Based Application
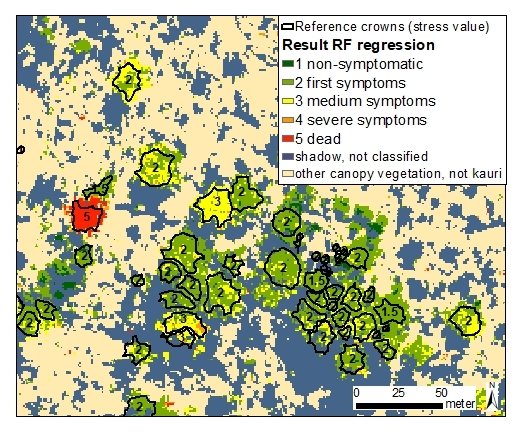
3. Results
3.1. Inter- Versus within Crown-Variability
3.2. Spectral Characteristics of Kauri for Different Stress Symptom and Size Classes
3.3. Objective 1: Best Correlating Band and Index Combinations with Canopy Stress Symptoms
3.3.1. Indices for Stress Detection in the Full Spectral Range (VIS–SWIR2)
3.3.2. Indices for Stress Detection in the VIS-NIR1 Spectral Range
3.4. Objective 2: Test Performance of Pre-Selected Index Combinations
3.5. Objective 3: Test Pixel-Based Application
4. Discussion
4.1. Discussion: Crown Variability and Crown Spectra in Different Stress Levels
4.2. Discussion Objective 1: Index Combinations for Stress Detection
4.3. Discussion Objective 2: Performance of Band Selection for Kauri Detection
4.4. Discussion Objective 3: Pixel-Based Application
4.5. Discussion: Application and Further Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Photo Illustration of the Kauri Canopy Score Scheme Used by Auckland Council 2015

Appendix B. Assessment Scheme for Stress Symptoms in Kauri Crowns Based on RGB Aerial Images
| Symptom Class | Description | Size Small 1 | Size Medium 1 | Size Large 1 |
|---|---|---|---|---|
| Value 1—No Symptoms | Leaves: green, green/blue; Canopy density: dense, no to small gaps; Bare branches: <1% |  |  |  |
| Value 2—First Symptoms/Open Crowns | Leaf colour: green to yellowish; Canopy density: small gaps; Bare branches: 1 to 5% (small branches) |  |  |  |
| Value 3—Medium Symptoms | Leaves: green with yellow or brown; Canopy density: small to medium gaps visible; Bare branches: 5%–30% |  |  |  |
| Value 4—Severe Symptoms | Leaves: yellow-green to brown, Canopy density: sparse, many gaps, understory partly visible Bare branches: >=30% visible as linear structures Epiphytes and climbers possible |  |  |  |
| Value 5—Dead Trees | Leaves: dead, brown leaves possible Epiphytes and climbers possible Canopy density: Gaps and understory visible, Bare branches: 100% dead branches, visible as linear structures |  |  |  |
Appendix C. Vegetation Indices Overview Table
| Index | Equation 1 | Literature | Name, Association | VIS-SWIR 2 | VIS-NIR 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbr. 1 | all | S | M | L | all | S | M | L | |||
| LIC2 | = 440/690 | [109] | Lichtenthaler Index (light use efficiency) | x | |||||||
| BGI | = 450/550 | [110] | Blue Green Pigment Index (leaf pigments) | x | |||||||
| PRI | = (531 − 570)/(531 + 570) | [137] | Photochemical Reflectance Index (light use efficiency) | x | x | ||||||
| MLO | = 531/645 | [111] | M. Locherer Chlorophyll Index (light use efficiency) | x | |||||||
| mRGI | = 685/550 (original: 690) | [110] | Red-Green Pigment Index (leaf pigments) | x | x | ||||||
| NDVI-H | = (800 − 670)/(800 + 670) | [138] | Normalized Difference VI–Haboudane (chlorophyll, LAI) | x | x | x | |||||
| SR670_800 | = 800/670 | [139] | Simple Ratio 800/670 Ratio VI (chlorophyll, LAI) | x | |||||||
| mSRa 675,700 | = 675/700 (original 672, 708) | [112] | Simple Ratio (chlorophyll, carotenoids) | x | |||||||
| mNDVI-A | = (900 − 685)/(900 + 685) (original: 680) | [120] | Normalized Difference VI–Aparicio (Broadband Greenness) | x | x | x | |||||
| GM2 | = 750/700 | [122] | Gitelson and Merzlyak Index (chlorophyll content) | x | |||||||
| mRVI | = 750/680 (original: 745, 645) | [108] | Ratio VI (chlorophyll content) | x | |||||||
| MSI | = 1600/820 | [57] | Moisture Stress Index (canopy water) | x | |||||||
| WBI | = 900/970 | [59] | Water Band Index (canopy water) | x | x | x | x | ||||
| LWVI2 | = (1094 − 1205)/(1094 + 1205) | [121] | Leaf Water VI (leaf/canopy water) | x | x | ||||||
| NDNI | = (1510 − 1680)/(1510 + 1680) | [69] | Normalized Difference Nitrogen Index (leaf nitrogen) | x | x | x | |||||
| mSWIRVI | = 37.27 × (2210 + 2090) + 26.27 × (2210 − 2090) − 0.57 (original: 2208) | [125] | Shortwave Infrared Green VI (cellulose/dry matter) | x | x | x | |||||
Appendix D. Separabilities between Small and Large Crown Spectra
| Wavelenghts | Jeffries Matusita Separability | Transformed Divergense | Amplitude D | Shape ϴ | |
|---|---|---|---|---|---|
| VIS | 437–700 nm | 1.401 | 1.460 | 1.54 | 0.002 |
| NIR1 | 700–970 nm | 1.475 | 1.529 | 1.28 | 0.013 |
| NIR2 | 970–1327 nm | 1.419 | 1.524 | 1.13 | 0.013 |
| SWIR1 | 1467–1771 nm | 1.369 | 1.456 | 1.51 | 0.006 |
| SWIR2 | 1994–2435 nm | 1.373 | 1.461 | 1.55 | 0.003 |
Appendix E. Confusion matrix for a Random Forest Classification to Identify Dead and Dying Trees
| Class 123 | Class 45 | Total | Users Accuracy | |
|---|---|---|---|---|
| Class 123 | 1043 | 13 | 1056 | 98.8 |
| Class 45 | 25 | 177 | 202 | 87.6 |
| Total | 1068 | 190 | 1258 | |
| Producers Accuracy | 97.7 | 93.2 | 97.0 |
References
- Wyse, S.V.; Burns, B.R.; Wright, S.D. Distinctive vegetation communities are associated with the long-lived conifer Agathis australis (New Zealand kauri, Araucariaceae) in New Zealand rainforests. Austral Ecol. 2014, 39, 388–400. [Google Scholar] [CrossRef]
- Shortland, T.; Wood, W. Kia Toitu He Kauri, Kauri Dieback Tangata Whenua Roopu Cultural Impact Assessment; Repo Consultancy Limited: Whangarei, New Zealand, 2011. [Google Scholar]
- Beever, R.E.; Waipara, N.W.; Ramsfield, T.D.; Dick, M.A.; Horner, I.J. Kauri (Agathis australis) under threat from Phytophthora. Phytophthoras For. Nat. Ecosyst. 2009, 74. [Google Scholar]
- Sanson, J. Independent Review of the State of Kauri Dieback Knowledge; Lincoln University: Lincoln, New Zealand, 2016. [Google Scholar]
- MPI. kauri Dieback Distribution. A Map Published on the Kauri Dieback Website. Ministry for Primary Industries, Biosecurity New Zealand, Wellington. 2020. Available online: https://www.kauridieback.co.nz/media/2037/kauri-dieback-distribution_20190930_350dpi.jpg (accessed on 1 February 2020).
- Alastair Jamieson, L.H.; Nick, W.; Jack, C. Survey of Kauri Dieback in the Hunua Ranges; Pseudomonas & Phytophthora; New Zealand Plant Protection Society: Auckland, New Zealand, 2011; pp. 60–65. [Google Scholar]
- Waipara, N.; Hill, S.; Hill, L.; Hough, E.; Horner, I. Surveillance methods to determine tree health, distribution of kauri dieback disease and associated pathogens. N. Z. Plant Prot. 2013, 66, 235–241. [Google Scholar] [CrossRef] [Green Version]
- MPI. Kia toitu he kauri–Keep kauri standing. New Zealands strategy for managing kauri dieback disease. Minist. Prim. Ind. 2014, 2014, 24. [Google Scholar]
- Ecroyd, C. Biological flora of New Zealand 8. Agathis australis (D. Don) Lindl.(Araucariaceae) Kauri. N. Z. J. Bot. 1982, 20, 17–36. [Google Scholar] [CrossRef]
- Steward, G.A.; Beveridge, A.E. A review of New Zealand kauri (Agathis australis (D. Don) Lindl.): Its ecology, history, growth and potential for management for timber. N. Z. J. For. Sci. 2010, 40, 33–59. [Google Scholar]
- Meiforth, J.J.; Buddenbaum, H.; Hill, J.; Shepherd, J.; Norton, D.A. Detection of New Zealand Kauri Trees with AISA Aerial Hyperspectral Data for Use in Multispectral Monitoring. Remote Sens. 2019, 11, 2865. [Google Scholar] [CrossRef] [Green Version]
- Meiforth, J.J. Photos, Waitakere Ranges Photo Staken During Fieldwork in January to March 2016; Waitakere Ranges: Auckland, New Zealand, 2016. [Google Scholar]
- Auckland Council. Helicopter Survey for Kauri Dieback in the Waitakere Ranges; Unpublished aerial photos; Auckland Council: Auckland, New Zealand, 2016. [Google Scholar]
- Scott, P.; Williams, N. Phytophthora diseases in New Zealand forests. N. Z. J. For. 2014, 59, 14–21. [Google Scholar]
- Hall, R.; Castilla, G.; White, J.; Cooke, B.; Skakun, R.J. Remote sensing of forest pest damage: A review and lessons learned from a Canadian perspective. Can. Entomol. 2016, 148, S296–S356. [Google Scholar] [CrossRef]
- Senf, C.; Seidl, R.; Hostert, P. Remote sensing of forest insect disturbances: Current state and future directions. Int. J. Appl. Earth Obs. Geoinf. 2017, 60, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, C.; Mohammed, C. Application of remote sensing technologies for assessing planted forests damaged by insect pests and fungal pathogens: A review. Curr. For. Rep. 2017, 3, 75–92. [Google Scholar] [CrossRef]
- Wulder, M.A.; Dymond, C.C.; White, J.C.; Leckie, D.G.; Carroll, A.L. Surveying mountain pine beetle damage of forests: A review of remote sensing opportunities. For. Ecol. Manag. 2006, 221, 27–41. [Google Scholar] [CrossRef]
- Silva, C.R.; Olthoff, A.; de la Mata, J.A.D.; Alonso, A.P. Remote monitoring of forest insect defoliation. A review. For. Syst. 2013, 22, 377–391. [Google Scholar]
- Lausch, A.; Erasmi, S.; King, D.; Magdon, P.; Heurich, M. Understanding forest health with remote sensing-part I—A review of spectral traits, processes and remote-sensing characteristics. Remote Sens. 2016, 8, 1029. [Google Scholar] [CrossRef] [Green Version]
- Lausch, A.; Erasmi, S.; King, D.; Magdon, P.; Heurich, M. Understanding forest health with remote sensing-part II—A review of approaches and data models. Remote Sens. 2017, 9, 129. [Google Scholar] [CrossRef] [Green Version]
- Coops, N.; Stanford, M.; Old, K.; Dudzinski, M.; Culvenor, D.; Stone, C. Assessment of Dothistroma needle blight of Pinus radiata using airborne hyperspectral imagery. Phytopathology 2003, 93, 1524–1532. [Google Scholar] [CrossRef] [Green Version]
- Misurec, J.; Kopacková, V.; Lhotakova, Z.; Albrechtova, J.; Hanus, J.; Weyermann, J.; Entcheva-Campbell, P. Utilization of hyperspectral image optical indices to assess the Norway spruce forest health status. J. Appl. Remote Sens. 2012, 6, 063545. [Google Scholar]
- Abdel-Rahman, E.M.; Mutanga, O.; Adam, E.; Ismail, R. Detecting Sirex noctilio grey-attacked and lightning-struck pine trees using airborne hyperspectral data, random forest and support vector machines classifiers. ISPRS J. Photogramm. Remote Sens. 2014, 88, 48–59. [Google Scholar] [CrossRef]
- Fassnacht, F.E.; Latifi, H.; Ghosh, A.; Joshi, P.K.; Koch, B. Assessing the potential of hyperspectral imagery to map bark beetle-induced tree mortality. Remote Sens. Environ. 2014, 140, 533–548. [Google Scholar] [CrossRef]
- Näsi, R.; Honkavaara, E.; Blomqvist, M.; Lyytikäinen-Saarenmaa, P.; Hakala, T.; Viljanen, N.; Kantola, T.; Holopainen, M. Remote sensing of bark beetle damage in urban forests at individual tree level using a novel hyperspectral camera from UAV and aircraft. Urban For. Urban Green. 2018, 30, 72–83. [Google Scholar] [CrossRef]
- Sandino, J.; Pegg, G.; Gonzalez, F.; Smith, G. Aerial mapping of forests affected by pathogens using UAVs, hyperspectral sensors, and artificial intelligence. Sensors 2018, 18, 944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontius, J.; Martin, M.; Plourde, L.; Hallett, R. Ash decline assessment in emerald ash borer-infested regions: A test of tree-level, hyperspectral technologies. Remote Sens. Environ. 2008, 112, 2665–2676. [Google Scholar] [CrossRef]
- Pu, R.; Kelly, M.; Anderson, G.L.; Gong, P.J.P.E.; Sensing, R. Using CASI hyperspectral imagery to detect mortality and vegetation stress associated with a new hardwood forest disease. Photogramm. Eng. Remote Sens. 2008, 74, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Michez, A.; Piégay, H.; Lisein, J.; Claessens, H.; Lejeune, P. Classification of riparian forest species and health condition using multi-temporal and hyperspatial imagery from unmanned aerial system. Environ. Monit. Assess. 2016, 188, 146. [Google Scholar] [CrossRef] [Green Version]
- Thenkabail, P.S.; Lyon, J.G.; Huete, A. Hyperspectral Remote Sensing of Vegetation; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Lawrence, R.; Labus, M. Early detection of Douglas-fir beetle infestation with subcanopy resolution hyperspectral imagery. West. J. Appl. For. 2003, 18, 202–206. [Google Scholar] [CrossRef] [Green Version]
- López-López, M.; Calderón, R.; González-Dugo, V.; Zarco-Tejada, P.; Fereres, E. Early detection and quantification of almond red leaf blotch using high-resolution hyperspectral and thermal imagery. Remote Sens. 2016, 8, 276. [Google Scholar] [CrossRef] [Green Version]
- Lowe, A.; Harrison, N.; French, A.P. Hyperspectral image analysis techniques for the detection and classification of the early onset of plant disease and stress. Plant Methods 2017, 13, 80. [Google Scholar] [CrossRef]
- Dotzler, S.; Hill, J.; Buddenbaum, H.; Stoffels, J. The Potential of EnMAP and Sentinel-2 Data for Detecting Drought Stress Phenomena in Deciduous Forest Communities. Remote Sens. 2015, 7, 14227–14258. [Google Scholar] [CrossRef] [Green Version]
- Garrity, S.R.; Allen, C.D.; Brumby, S.P.; Gangodagamage, C.; McDowell, N.G.; Cai, D.M. Quantifying tree mortality in a mixed species woodland using multitemporal high spatial resolution satellite imagery. Remote Sens. Environ. 2013, 129, 54–65. [Google Scholar] [CrossRef]
- Meng, J.; Li, S.; Wang, W.; Liu, Q.; Xie, S.; Ma, W. Mapping forest health using spectral and textural information extracted from spot-5 satellite images. Remote Sens. 2016, 8, 719. [Google Scholar] [CrossRef] [Green Version]
- Townsend, P.A.; Singh, A.; Foster, J.R.; Rehberg, N.J.; Kingdon, C.C.; Eshleman, K.N.; Seagle, S.W. A general Landsat model to predict canopy defoliation in broadleaf deciduous forests. Remote Sens. Environ. 2012, 119, 255–265. [Google Scholar] [CrossRef]
- Wang, J.; Sammis, T.W.; Gutschick, V.P.; Gebremichael, M.; Dennis, S.O.; Harrison, R.E. Review of satellite remote sensing use in forest health studies. Open Geogr. J. 2010, 3, 28–42. [Google Scholar] [CrossRef]
- Wang, H.; Pu, R.; Zhang, Z. Mapping Robinia Pseudoacacia Forest Health Conditions by Using Combined Spectral, Spatial and Textureal Information Extracted from Ikonos Imagery. Int. Arch. Photogramm. Remote Sens. Spatial Inf. Sci. 2016, XLI-B8, 1425–1429. [Google Scholar] [CrossRef] [Green Version]
- Waser, L.T.; Küchler, M.; Jütte, K.; Stampfer, T. Evaluating the potential of WorldView-2 data to classify tree species and different levels of ash mortality. Remote Sens. 2014, 6, 4515–4545. [Google Scholar] [CrossRef] [Green Version]
- White, J.C.; Wulder, M.A.; Brooks, D.; Reich, R.; Wheate, R.D. Detection of red attack stage mountain pine beetle infestation with high spatial resolution satellite imagery. Remote Sens. Environ. 2005, 96, 340–351. [Google Scholar] [CrossRef]
- Aasen, H.; Honkavaara, E.; Lucieer, A.; Zarco-Tejada, P.J. Quantitative remote sensing at ultra-high resolution with UAV spectroscopy: A review of sensor technology, measurement procedures, and data correction workflows. Remote Sens. 2018, 10, 1091. [Google Scholar] [CrossRef] [Green Version]
- Coops, N.C.; Goodwin, N.; Stone, C.; Sims, N. Application of narrow-band digital camera imagery to plantation canopy condition assessment. Can. J. Remote Sens. 2006, 32, 19–32. [Google Scholar] [CrossRef]
- Pietrzykowski, E.; Sims, N.; Stone, C.; Pinkard, L.; Mohammed, C. Predicting Mycosphaerella leaf disease severity in a Eucalyptus globulus plantation using digital multi-spectral imagery. South. Hemisph. For. J. 2007, 69, 175–182. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berni, J.A.; Suárez, L.; Sepulcre-Cantó, G.; Morales, F.; Miller, J.R. Imaging chlorophyll fluorescence with an airborne narrow-band multispectral camera for vegetation stress detection. Remote Sens. Environ. 2009, 113, 1262–1275. [Google Scholar] [CrossRef]
- Meddens, A.J.; Hicke, J.A.; Vierling, L.A. Evaluating the potential of multispectral imagery to map multiple stages of tree mortality. Remote Sens. Environ. 2011, 115, 1632–1642. [Google Scholar] [CrossRef]
- Asner, G.P. Hyperspectral remote sensing of canopy chemistry, physiology, and biodiversity in tropical rainforests. In Hyperspectral Remote Sensing of Tropical and Sub-Tropical Forests; Kalacsca, M., Sances, G.A., Eds.; Azofeita CRC Press: Boca Raton, FL, USA, 2008; pp. 261–296. [Google Scholar]
- Jensen, J.R. Introductory Digital Image Processing: A Remote Sensing Perspective, 4th ed.; Pearson Education, Inc.: Glenview, IL, USA, 2016. [Google Scholar]
- Kaufmann, H.E.A. Hyperspectral Algorithms: Report in the Frame of EnMAP Preparation Activities; Deutsches GeoForschungsZentrum GFZ: Potsdam, Germany, 2010. [Google Scholar]
- Berni, J.; Zarco-Tejada, P.; Sepulcre-Cantó, G.; Fereres, E.; Villalobos, F. Mapping canopy conductance and CWSI in olive orchards using high resolution thermal remote sensing imagery. Remote Sens. Environ. 2009, 113, 2380–2388. [Google Scholar] [CrossRef]
- Calderón, R.; Navas-Cortés, J.; Zarco-Tejada, P. Early detection and quantification of Verticillium wilt in olive using hyperspectral and thermal imagery over large areas. Remote Sensing. 2015, 7, 5584–5610. [Google Scholar] [CrossRef] [Green Version]
- Strasser, R.J.; Srivastava, A. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.; Noland, T.L.; Sampson, P. Vegetation stress detection through chlorophyll a+ b estimation and fluorescence effects on hyperspectral imagery. J. Environ. Qual. 2002, 31, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, G.A. Hyperspectral remote sensing of plant pigments. J. Exp. Bot. 2006, 58, 855–867. [Google Scholar] [CrossRef] [Green Version]
- Suárez, L.; Zarco-Tejada, P.J.; Sepulcre-Cantó, G.; Pérez-Priego, O.; Miller, J.; Jiménez-Muñoz, J.; Sobrino, J. Assessing canopy PRI for water stress detection with diurnal airborne imagery. Remote Sens. Environ. 2008, 112, 560–575. [Google Scholar] [CrossRef]
- Hunt Jr, E.R.; Rock, B.N. Detection of changes in leaf water content using near-and middle-infrared reflectances. Remote Sens. Environ. 1989, 30, 43–54. [Google Scholar] [CrossRef]
- Gao, B.-C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Save, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Hendry, G.A.; Houghton, J.D.; Brown, S.B. The degradation of chlorophyll-a biological enigma. New Phytol. 1987, 1987, 255–302. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Woolley, J.T. Reflectance and transmittance of light by leaves. Plant Physiol. 1971, 47, 656–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef] [Green Version]
- Glenn, E.; Huete, A.; Nagler, P.; Nelson, S. Relationship between remotely-sensed vegetation indices, canopy attributes and plant physiological processes: What vegetation indices can and cannot tell us about the landscape. Sensors 2008, 8, 2136–2160. [Google Scholar] [CrossRef] [Green Version]
- Jago, R.A.; Cutler, M.E.; Curran, P.J. Estimating canopy chlorophyll concentration from field and airborne spectra. Remote Sens. Environ. 1999, 68, 217–224. [Google Scholar] [CrossRef]
- Eitel, J.U.; Vierling, L.A.; Litvak, M.E.; Long, D.S.; Schulthess, U.; Ager, A.A.; Krofcheck, D.J.; Stoscheck, L. Broadband, red-edge information from satellites improves early stress detection in a New Mexico conifer woodland. Remote Sens. Environ. 2011, 115, 3640–3646. [Google Scholar] [CrossRef]
- Kim, Y.; Glenn, D.M.; Park, J.; Ngugi, H.K.; Lehman, B.L. Hyperspectral image analysis for water stress detection of apple trees. Comput. Electron. Agric. 2011, 77, 155–160. [Google Scholar] [CrossRef]
- Penuelas, J.; Baret, F.; Filella, I. Semi-empirical indices to assess carotenoids/chlorophyll a ratio from leaf spectral reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Serrano, L.; Penuelas, J.; Ustin, S.L. Remote sensing of nitrogen and lignin in Mediterranean vegetation from AVIRIS data: Decomposing biochemical from structural signals. Remote Sens. Environ. 2002, 81, 355–364. [Google Scholar] [CrossRef]
- Rautiainen, M.; Lukeš, P.; Homolova, L.; Hovi, A.; Pisek, J.; Mottus, M. Spectral properties of coniferous forests: A review of in situ and laboratory measurements. Remote Sens. 2018, 10, 207. [Google Scholar] [CrossRef] [Green Version]
- Zagolski, F.; Pinel, V.; Romier, J.; Alcayde, D.; Fontanari, J.; Gastellu-Etchegorry, J.; Giordano, G.; Marty, G.; Mougin, E.; Joffre, R. Forest canopy chemistry with high spectral resolution remote sensing. Int. J. Remote Sens. 1996, 17, 1107–1128. [Google Scholar] [CrossRef]
- Gong, P.; Pu, R.; Biging, G.S.; Larrieu, M.R. Estimation of forest leaf area index using vegetation indices derived from Hyperion hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2003, 41, 1355–1362. [Google Scholar] [CrossRef] [Green Version]
- Barry, K.M.; Stone, C.; Mohammed, C. Crown-scale evaluation of spectral indices for defoliated and discoloured eucalypts. Int. J. Remote Sens. 2008, 29, 47–69. [Google Scholar] [CrossRef] [Green Version]
- Schlerf, M.; Atzberger, C.; Hill, J. Remote sensing of forest biophysical variables using HyMap imaging spectrometer data. Remote Sens. Environ. 2005, 95, 177–194. [Google Scholar] [CrossRef] [Green Version]
- Asner, G.P. Biophysical and biochemical sources of variability in canopy reflectance. Remote Sens. Environ. 1998, 64, 234–253. [Google Scholar] [CrossRef]
- Ollinger, S.V. Sources of variability in canopy reflectance and the convergent properties of plants. New Phytol. 2011, 189, 375–394. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Morales, A.; Berjón, A.; Agüera, J. Hyperspectral indices and model simulation for chlorophyll estimation in open-canopy tree crops. Remote Sens. Environ. 2004, 90, 463–476. [Google Scholar] [CrossRef]
- Hernández-Clemente, R.; Navarro-Cerrillo, R.M.; Suárez, L.; Morales, F.; Zarco-Tejada, P.J. Assessing structural effects on PRI for stress detection in conifer forests. Remote Sens. Environ. 2011, 115, 2360–2375. [Google Scholar] [CrossRef]
- Baret, F.; Vanderbilt, V.C.; Steven, M.D.; Jacquemoud, S. Use of spectral analogy to evaluate canopy reflectance sensitivity to leaf optical properties. Remote Sens. Environ. 1994, 48, 253–260. [Google Scholar] [CrossRef]
- Kupiec, J.; Curran, P. Decoupling effects of the canopy and foliar biochemicals in AVIRIS spectra. Int. J. Remote Sens. 1995, 16, 1731–1739. [Google Scholar] [CrossRef]
- Bellgard, S.E.; Weir, B.S.; Pennycook, S.R.; Paderes, E.P.; Winks, C.; Beever, R.E. Specialist Phytophthora Research: Biology. Pathology Ecology and Detection of PTA. Mpi Contract 2013, 2013, 11927. [Google Scholar]
- Chappell, P.R. The climate and weather of Auckland. Niwa Sci. Technol. Ser. Auckl. 2012, 2013, 60. [Google Scholar]
- ESRI. World Topographic Map-WMTS service. Sources: Esri, HERE, Garmin, Intermap, INCREMENT P, GEBCO, USGS, FAO, NPS, NRCAN, GeoBase, IGN, Kadaster NL, Ordnance Survey, Esri Japan, METI, Esri China (Hong Kong), © OpenStreetMap contributors, GIS User Community; ESRI: Redlands, CA, USA, 2020. [Google Scholar]
- LINZ. Auckland 0.5m Rural Aerial Photos (2010–2012). National Imagery. WMS Service. Available online: https://data.linz.govt.nz/layer/51769-auckland-05m-rural-aerial-photos-2010–2012/ (accessed on 15 October 2019).
- Schlaepfer, D. PARGE-Parametric Geocoding & Orthorectification for Airborne Optical Scanner Data. Available online: http://www.rese.ch/products/parge/ (accessed on 11 June 2019).
- Richter, R.; Schläpfer, D. ATCOR-4 User Guide, Version 7.3.0, April 2019. Atmospheric/Topographic Correction for Airborne Imagery; ReSe Applications LLC: Wil, Switzerland, 2019. [Google Scholar]
- Khosravipour, A.; Skidmore, A.K.; Isenburg, M. Generating spike-free digital surface models using LiDAR raw point clouds: A new approach for forestry applications. Int. J. Appl. Earth Obs. Geoinf. 2016, 52, 104–114. [Google Scholar] [CrossRef]
- rapidlasso-GmbH. LAStools. Software Suite for LiDAR Processing. Developed by Martin Insenburg. Available online: https://rapidlasso.com/lastools/ (accessed on 1 October 2019).
- MPI. Airbone LiDAR and RGB aerial images in the Waitakere Ranges. In Flown by AAM-New Zealand for the Ministry for Primary Industries on the 30 January 2016; MPI: Wellington, New Zealand, 2016. [Google Scholar]
- Auckland Council, A. Auckland 0.075m Urban Aerial Photos (2017), RGB, Waitakere Ranges. Available online: https://data.linz.govt.nz/layer/95497-auckland-0075m-urban-aerial-photos-2017/ (accessed on 12 April 2019).
- Singers, N.; Osborne, B.; Lovegrove, T.; Jamieson, A.; Boow, J.; Sawyer, J.; Webb, C. Indigenous Terrestrial and Wetland Ecosystems of Auckland; Auckland Council: Auckland, New Zealand, 2017; Available online: http://www.knowledgeauckland.org.nz (accessed on 20 July 2019).
- Hurst, J.; Allen, R. A Permanent Plot Method for Monitoring Indigenous Forests-Expanded Manual, Version 4; Landcare Research Contract report LC0708/028; Landcare Research New Zealand Limited: Lincoln, New Zealand, 2007. [Google Scholar]
- DOC. The Foliar Browse Index field manual. In An Update of a Method for Monitoring Possum (Trichosurus Vulpecula) Damage to Forest Communities; Department of Conservation: Christchurch, New Zealand, 2014. [Google Scholar]
- Trimble. eCognition® Developer 9.3 User Guide; Trimble Germany GmbH: Munich, Germany, 2018; p. 274. [Google Scholar]
- Harris-Geospatial. Vegetation Indices in Envi. Available online: http://www.harrisgeospatial.com/docs/VegetationIndices.html (accessed on 10 October 2018).
- van der Linden, S.; Rabe, A.; Held, M.; Jakimow, B.; Leitão, P.; Okujeni, A.; Schwieder, M.; Suess, S.; Hostert, P. The EnMAP-Box—A toolbox and application programming interface for EnMAP data processing. Remote Sens. 2015, 7, 11249–11266. [Google Scholar] [CrossRef] [Green Version]
- Witten, I.H.; Frank, E.; Hall, M.A.; Pal, C.J. Data Mining: Practical Machine Learning Tools and Techniques; Morgan Kaufmann: San Francisco, CA, USA, 2016. [Google Scholar]
- Lazaridis, D.C.; Verbesselt, J.; Robinson, A.P. Penalized regression techniques for prediction: A case study for predicting tree mortality using remotely sensed vegetation indices. Can. J. For. Res. 2010, 41, 24–34. [Google Scholar] [CrossRef]
- Belgiu, M.; Drăguţ, L. Random forest in remote sensing: A review of applications and future directions. ISPRS J. Photogramm. Remote Sens. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- Pal, M. Random forest classifier for remote sensing classification. Int. J. Remote Sens. 2005, 26, 217–222. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Gislason, P.O.; Benediktsson, J.A.; Sveinsson, J.R. Random forests for land cover classification. Pattern Recog. Lett. 2006, 27, 294–300. [Google Scholar] [CrossRef]
- Breiman, L. Statistical modeling: The two cultures. Qual. Control Appl. Stat. 2003, 48, 81–82. [Google Scholar]
- Quinlan, J.R. Learning with continuous classes. In Proceedings of the 5th Australian Joint Conference on Artificial Intelligence, Hobart, Australia, 16–18 November 1992; pp. 343–348. [Google Scholar]
- Dalponte, M.; Ørka, H.O.; Ene, L.T.; Gobakken, T.; Næsset, E. Tree crown delineation and tree species classification in boreal forests using hyperspectral and ALS data. Remote Sens. Environ. 2014, 140, 306–317. [Google Scholar] [CrossRef]
- Kaartinen, H.; Hyyppä, J.; Yu, X.; Vastaranta, M.; Hyyppä, H.; Kukko, A.; Holopainen, M.; Heipke, C.; Hirschmugl, M.; Morsdorf, F. An international comparison of individual tree detection and extraction using airborne laser scanning. Remote Sens. 2012, 4, 950–974. [Google Scholar] [CrossRef] [Green Version]
- Zhen, Z.; Quackenbush, L.J.; Zhang, L. Trends in automatic individual tree crown detection and delineation—Evolution of LiDAR data. Remote Sens. 2016, 8, 333. [Google Scholar] [CrossRef] [Green Version]
- Birth, G.S.; McVey, G.R. Measuring the Color of Growing Turf with a Reflectance Spectrophotometer 1. Agron. J. 1968, 60, 640–643. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Vegetation stress: An introduction to the stress concept in plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berjón, A.; López-Lozano, R.; Miller, J.R.; Martín, P.; Cachorro, V.; González, M.; De Frutos, A. Assessing vineyard condition with hyperspectral indices: Leaf and canopy reflectance simulation in a row-structured discontinuous canopy. Remote Sens. Environ. 2005, 99, 271–287. [Google Scholar] [CrossRef]
- Locherer, M. Capacity of the Hyperspectral Satellite Mission EnMAP for the Multiseasonal Monitoring of Biophysical and Biochemical Land Surface Parameters in Agriculture by Transferring an Analysis Method for Airborne Image Spectroscopy to the Spaceborne Scale; lmu: Munic, Germany, 2014. [Google Scholar]
- Datt, B. Remote sensing of chlorophyll a, chlorophyll b, chlorophyll a+ b, and total carotenoid content in eucalyptus leaves. Remote Sens. Environ. 1998, 66, 111–121. [Google Scholar] [CrossRef]
- Roujean, J.-L.; Breon, F.-M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Rouse, J.W., Jr.; Haas, R.H.; Schell, J.; Deering, D. Monitoring the vernal advancement and retrogradation (green wave effect) of natural vegetation. In NASA Gsfct Type Report; Texas A&M University: College Station, TX, USA, 1973. [Google Scholar]
- Ustin, S.L.; Roberts, D.A.; Gardner, M.; Dennison, P. Evaluation of the potential of Hyperion data to estimate wildfire hazard in the Santa Ynez Front Range, Santa Barbara, California. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium, Toronto, ON, Canada, 24–28 June 2002; pp. 796–798. [Google Scholar]
- Curran, P.J.; Dungan, J.L.; Macler, B.A.; Plummer, S.E.; Peterson, D.L. Reflectance spectroscopy of fresh whole leaves for the estimation of chemical concentration. Remote Sens. Environ. 1992, 39, 153–166. [Google Scholar] [CrossRef]
- Nagler, P.L.; Inoue, Y.; Glenn, E.; Russ, A.; Daughtry, C. Cellulose absorption index (CAI) to quantify mixed soil–plant litter scenes. Remote Sens. Environ. 2003, 87, 310–325. [Google Scholar] [CrossRef]
- Asner, G.P.; Knapp, D.E.; Cooper, A.N.; Bustamante, M.M.; Olander, L.P. Ecosystem structure throughout the Brazilian Amazon from Landsat observations and automated spectral unmixing. Earth Interact. 2005, 9, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Baret, F.; Clevers, J.; Steven, M. The robustness of canopy gap fraction estimates from red and near-infrared reflectances: A comparison of approaches. Remote Sens. Environ. 1995, 54, 141–151. [Google Scholar] [CrossRef]
- Aparicio, N.; Villegas, D.; Araus, J.; Casadesus, J.; Royo, C. Relationship between growth traits and spectral vegetation indices in durum wheat. Crop Sci. 2002, 42, 1547–1555. [Google Scholar] [CrossRef]
- Galvao, L.S.; Formaggio, A.R.; Tisot, D.A. Discrimination of sugarcane varieties in Southeastern Brazil with EO-1 Hyperion data. Remote Sens. Environ. 2005, 94, 523–534. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Fourty, T.; Baret, F.; Jacquemoud, S.; Schmuck, G.; Verdebout, J. Leaf optical properties with explicit description of its biochemical composition: Direct and inverse problems. Remote Sens. Environ. 1996, 56, 104–117. [Google Scholar] [CrossRef]
- Clark, R.N.; King, T.V.; Ager, C.; Swayze, G.A. Initial Vegetation Species and Senescience/Stress Indicator Mapping in the San Luis Valley, Colorado Using Imaging Spectrometer Data; NASA: Washington, DC, USA, 1995.
- Lobell, D.B.; Asner, G.P.; Law, B.E.; Treuhaft, R.N. Subpixel canopy cover estimation of coniferous forests in Oregon using SWIR imaging spectrometry. J. Geophys. Res. Atmos. 2001, 106, 5151–5160. [Google Scholar] [CrossRef] [Green Version]
- Beauchamp, A.J. The Detection of Phytophthora Taxon “Agathis” in the Second Round of Surveillance Sampling-With Discussion of the Implications for Kauri Dieback Management of All Surveillance Activity; Department of Conservation: Auckland, New Zealand, 2013.
- Wulder, M.A.; White, J.C.; Coops, N.C.; Butson, C.R. Multi-temporal analysis of high spatial resolution imagery for disturbance monitoring. Remote Sens. Environ. 2008, 112, 2729–2740. [Google Scholar] [CrossRef]
- Verbesselt, J.; Robinson, A.; Stone, C.; Culvenor, D. Forecasting tree mortality using change metrics derived from MODIS satellite data. For. Ecol. Manage. 2009, 258, 1166–1173. [Google Scholar] [CrossRef]
- Verbesselt, J.; Hyndman, R.; Newnham, G.; Culvenor, D. Detecting trend and seasonal changes in satellite image time series. Remote Sens. Environ. 2010, 114, 106–115. [Google Scholar] [CrossRef]
- Meigs, G.W.; Kennedy, R.E.; Gray, A.N.; Gregory, M.J. Spatiotemporal dynamics of recent mountain pine beetle and western spruce budworm outbreaks across the Pacific Northwest Region, USA. For. Ecol. Manag. 2015, 339, 71–86. [Google Scholar] [CrossRef] [Green Version]
- Solberg, S.; Næsset, E.; Hanssen, K.H.; Christiansen, E. Mapping defoliation during a severe insect attack on Scots pine using airborne laser scanning. Remote Sens. Environ. 2006, 102, 364–376. [Google Scholar] [CrossRef]
- Kantola, T.; Vastaranta, M.; Yu, X.; Lyytikainen-Saarenmaa, P.; Holopainen, M.; Talvitie, M.; Kaasalainen, S.; Solberg, S.; Hyyppa, J. Classification of defoliated trees using tree-level airborne laser scanning data combined with aerial images. Remote Sens. 2010, 2, 2665–2679. [Google Scholar] [CrossRef] [Green Version]
- Vastaranta, M.; Kantola, T.; Lyytikäinen-Saarenmaa, P.; Holopainen, M.; Kankare, V.; Wulder, M.; Hyyppä, J.; Hyyppä, H. Area-based mapping of defoliation of Scots pine stands using airborne scanning LiDAR. Remote Sens. 2013, 5, 1220–1234. [Google Scholar] [CrossRef] [Green Version]
- Shendryk, I.; Broich, M.; Tulbure, M.G.; McGrath, A.; Keith, D.; Alexandrov, S.V. Mapping individual tree health using full-waveform airborne laser scans and imaging spectroscopy: A case study for a floodplain eucalypt forest. Remote Sens. Environ. 2016, 187, 202–217. [Google Scholar] [CrossRef]
- Hakala, T.; Nevalainen, O.; Kaasalainen, S.; Mäkipää, R. Multispectral Lidar Time Series of Pine Canopy Chlorophyll Content. Biogeosciences 2015, 12, 1629–1634. [Google Scholar] [CrossRef] [Green Version]
- Hill, L.; Waipara, N. Canopy Score Illustration for Kauri Dieback Field Survey conducted for Auckland Council; Biosecurity Department: Auckland, New Zealand, 2010.
- Gamon, J.; Penuelas, J.; Field, C. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Pearson, R.L.; Miller, L.D. Remote mapping of standing crop biomass for estimation of the productivity of the shortgrass prairie. In Proceedings of the Eighth International Symposium on Remote Sensing of Environment, Ann Arbor, MI, USA, 2–6 October 1972; Volume VIII, p. 1355. [Google Scholar]
- Jeffreys, H. An invariant form for the prior probability in estimation problems. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 1946, 186, 453–461. [Google Scholar]
- Richards, J.A. Remote Sensing Digital Image Analysis; Springer: Berlin, Germany, 1999; Volume 3. [Google Scholar]
- Price, J.C. How unique are spectral signatures? Remote Sens. Env. 1994, 49, 181–186. [Google Scholar] [CrossRef]










| Spectral Range | Electromagnetic Wavelengths |
|---|---|
| Visible (VIS) | 437–700 nm 1 |
| 1st near-infrared (NIR1) | 700–ca. 970 nm 2 |
| 2nd near-infrared (NIR2) | 970–1327 nm |
| 1st shortwave infrared (SWIR1) | 1467–1771 nm 3 |
| 2nd shortwave infrared (SWIR2) | 1994–2435 nm 1,3 |
| Stress Symptom Class 3 | Total | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Crown Size Class 1 | large | 34 2 | 249 | 17 | 19 | 12 | 331 |
| medium | 23 2 | 374 | 44 | 42 | 66 | 549 | |
| small | 61 | 176 | 35 | 48 | 58 | 378 | |
| total | 84 | 833 | 96 | 109 | 136 | 1258 | |
| Crown / | # of | Centre Wavelengths of Selected Bands in nm 2 | Indices | RF | M5P | LR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stand Sizes | Bands | VIS | NIR1 | NIR2 | SWIR1 | SWIR2 | corr 4 (stdev) | MAE 3 | RMSE | corr 4 (stdev) | MAE 3 | RMSE | corr 4 (stdev) | MAE 3 | RMSE | |
| Best Index Combinations for All Crown Sizes | ||||||||||||||||
| All Crown Sizes | 10 | 450, 550, 670 | 800 | 1094, 1205 | 1510, 1680 | 2090, 2210 | NDVI-H, LWVI2, mSWIRVI, NDNI, BGI, SR(670,800) | 0.938 (0.01) | 0.25 | 0.4 | 0.93 (0.01) | 0.28 | 0.43 | 0.91 (0.01) | 0.36 | 0.49 |
| 6 1 | 450, 550, 670 | 800 | 1094, 1205 | NDVI-H, LWVI2, BGI, SR(670,800) | 0.932 (0.01) | 0.27 | 0.42 | 0.93 (0.01) | 0.28 | 0.43 | 0.90 (0.01) | 0.37 | 0.5 | |||
| 4 | 670 | 800 | 1094, 1205 | NDVIHa, LWVI2, SR(670,800) | 0.926 (0.01) | 0.28 | 0.44 | 0.93 (0.01) | 0.28 | 0.44 | 0.90 (0.01) | 0.38 | 0.51 | |||
| Baseline Index Combination (6 Bands, 4 Indices) Applied to Different Crown Sizes in Individual Models | ||||||||||||||||
| Small | 6 | 450, 550, 670 | 800 | 1094, 1205 | NDVI-H, LWVI2, BGI, SR(670,800) | 0.919 (0.02) | 0.39 | 0.55 | 0.91 (0.02) | 0.43 | 0.58 | 0.90 (0.01) | 0.47 | 0.6 | ||
| Medium | 6 | 450, 550, 670 | 800 | 1094, 1205 | NDVI-H, LWVI2, BGI, SR(670,800) | 0.945 (0.01) | 0.25 | 0.38 | 0.93 (0.01) | 0.3 | 0.42 | 0.91 (0.01) | 0.36 | 0.48 | ||
| Large | 6 | 450, 550, 670 | 800 | 1094, 1205 | NDVI-H, LWVI2, BGI, SR(670,800) | 0.887 (0.05) | 0.18 | 0.34 | 0.86 (0.05) | 0.26 | 0.39 | 0.86 (0.05) | 0.26 | 0.39 | ||
| 4-Band Index Combination Applied to Different Crown Sizes in Individual Models | ||||||||||||||||
| Small | 6 | 670 | 800 | 1094, 1205 | NDVI-H, LWVI2, SR(670,800) | 0.915 (0.02) | 0.39 | 0.56 | 0.91 (0.02) | 0.43 | 0.58 | 0.90 (0.01) | 0.47 | 0.6 | ||
| Medium | 6 | 670 | 800 | 1094, 1205 | NDVI-H, LWVI2, SR(670,800) | 0.936 (0.01) | 0.26 | 0.41 | 0.93 (0.01) | 0.3 | 0.43 | 0.91 (0.01) | 0.36 | 0.49 | ||
| Large | 6 | 670 | 800 | 1094, 1205 | NDVI-H, LWVI2, SR(670,800) | 0.878 (0.05) | 0.18 | 0.35 | 0.86 (0.05) | 0.26 | 0.39 | 0.86 (0.05) | 0.26 | 0.39 | ||
| Baseline Index Combination (6 Bands, 4 Indices) Applied for Crowns in Low and High Forest Stands with Individual Models | ||||||||||||||||
| Low | 6 | 450, 550, 670 | 800 | 1094, 1205 | NDVI-H, LWVI2, BGI, SR(670,800) | 0.933 (0.01) | 0.32 | 0.49 | 0.93 (0.01) | 0.36 | 0.51 | 0.90 (0.01) | 0.45 | 0.58 | ||
| High | 6 | 450, 550, 670 | 800 | 1094, 1205 | NDVI-H, LWVI2, BGI, SR(670,800) | 0.905 (0.03) | 0.2 | 0.36 | 0.91 (0.03) | 0.23 | 0.36 | 0.88 (0.03) | 0.29 | 0.4 | ||
| Best Index Combinations for Individual Crown Sizes | ||||||||||||||||
| Small | 6 | 550, 685 | 1094, 1205 | 1510, 1680 | LWVI2, mRGI, NDNI | 0.930 (0.01) | 0.36 | 0.51 | 0.92 (0.02) | 0.4 | 0.55 | 0.90 (0.01) | 0.46 | 0.6 | ||
| Medium | 6 | 670 | 800 | 1510, 1680 | 2090, 2210 | NDVI-H, mSWIRVI, NDNI | 0.947 (0.01) | 0.24 | 0.37 | 0.93 (0.01) | 0.29 | 0.42 | 0.90 (0.01) | 0.35 | 0.5 | |
| Large | 6 | 675, 700 | 819 | 1600 | 2090, 2210 | MSI, mSRa, mSWIRVI | 0.897 (0.05) | 0.17 | 0.32 | 0.87 (0.04) | 0.24 | 0.37 | 0.87 (0.04) | 0.24 | 0.37 | |
| Crown | # of | Centre Wavelengths in nm (10 nm bandwidths) | Indices 1 | RF | M5P | LR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size | Bands | VIS | NIR1 | Correlation 4 (Stdev) | MAE 4 | RMSE | Correlation 4 (Stdev) | MAE 4 | RMSE | Correlation 4 (Stdev) | MAE 4 | RMSE | |
| VNIR Index Combinations on All Crown Sizes | |||||||||||||
| All | 6 2 | 550, 685 3, 700, 750 | 900, 970 | mNDVI-A, mRVI, mRGI, GM2, WBI | 0.93 (0.01) | 0.28 | 0.43 | 0.925 (0.01) | 0.29 | 0.44 | 0.89 (0.01) | 0.4 | 0.53 |
| 5 | 550, 685 3, 700, 750 | 900 | mNDVI-A, mRGI, mRVI, GM2 | 0.92 (0.01) | 0.29 | 0.46 | 0.92 (0.01) | 0.31 | 0.45 | 0.88 (0.01) | 0.4 | 0.56 | |
| VNIR Baseline Index Combination Applied on Individual Crown Sizes with Individual Models | |||||||||||||
| Small | 6 2 | 550, 685 3, 700, 750 | 900, 970 | mNDVI-A, mRVI, mRGI, GM2, WBI | 0.90 (0.02) | 0.42 | 0.58 | 0.90 (0.02) | 0.44 | 0.6 | 0.89 (0.02) | 0.47 | 0.62 |
| Medium | 6 2 | 550, 685 3, 700, 750 | 900, 970 | mNDVI-A, mRVI, mRGI, GM2, WBI | 0.94 (0.01) | 0.27 | 0.38 | 0.94 (0.01) | 0.29 | 0.4 | 0.92 (0.01) | 0.35 | 0.45 |
| Large | 6 2 | 550, 685 3, 700, 750 | 900, 970 | mNDVI-A, mRVI, mRGI, GM2, WBI | 0.88 (0.04) | 0.24 | 0.34 | 0.86 (0.04) | 0.27 | 0.38 | 0.87 (0.04) | 0.27 | 0.37 |
| VNIR Index Combinations in a RF Regression Selected for Different Crown Sizes Separately | |||||||||||||
| Small | 5 | 531, 570, 685 | 900, 970 | mNDVI-A, PRI, WBI | 0.90(0.02) | 0.43 | 0.61 | 0.89(0.02) | 0.45 | 0.63 | 0.84(0.02) | 0.59 | 0.77 |
| Medium | 6 | 440, 670, 690 | 800, 900, 970 | NDVI-H, WBI, LIC2 | 0.94(0.01) | 0.26 | 0.39 | 0.93(0.01) | 0.43 | 0.45 | 0.89(0.01) | 0.38 | 0.54 |
| Large | 5 | 531, 645 | 860, 900, 970 | mNDVI-A, WBI, MLO | 0.89(0.05) | 0.18 | 0.34 | 0.86(0.05) | 0.39 | 0.39 | 0.85(0.05) | 0.26 | 0.39 |
| VNIR Baseline Index Combination Applied in Individual Models for Different Stand Situations | |||||||||||||
| Low | 6 2 | 550, 685 3, 700, 750 | 900, 970 | mNDVI-A, mRVI, mRGI, GM2, WBI | 0.93 (0.01) | 0.34 | 0.51 | 0.92 (0.01) | 0.37 | 0.53 | 0.89 (0.01) | 0.47 | 0.61 |
| High | 6 2 | 550, 685 3, 700, 750 | 900, 970 | mNDVI-A, mRVI, mRGI, GM2, WBI | 0.90 (0.03) | 0.21 | 0.37 | 0.89 (0.03) | 0.24 | 0.38 | 0.87 (0.02) | 0.29 | 0.42 |
| Name | Equation | Name, Description | Literature |
|---|---|---|---|
| SR800 | Simple Ratio 800/670 chlorophyll concentration and LAI | [108] | |
| SR708 | Simple Ratio 670/708 chlorophyll concentration and LAI | [112] | |
| RDVI | Renormalized Difference VI chlorophyll concentration and LAI | [113] | |
| NDVI | Normalized Difference VI chlorophyll concentration and LAI | [114] | |
| mNDWI-Hyp | Modified Normalized Difference Water Index–Hyperion vegetation canopy water content and canopy structure | [115] |
| Crown/Stand Situation | Random Forest Regression | M5P Regression | Linear Regression | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Corre-Lation 1 | MAE | RMSE | Corre-Lation 1 | MAE | RMSE | Corre-Lation 1 | MAE | RMSE | |
| All Crowns | 0.93 (0.01) | 0.27 | 0.43 | 0.93 (0.01) | 0.29 | 0.44 | 0.91 (0.01) | 0.36 | 0.49 |
| Small Crowns | 0.92 (0.02) | 0.39 | 0.56 | 0.91 (0.02) | 0.42 | 0.58 | 0.91 (0.02) | 0.46 | 0.59 |
| Medium Crowns | 0.94 (0.01) | 0.25 | 0.39 | 0.94 (0.01) | 0.29 | 0.42 | 0.92 (0.01) | 0.35 | 0.46 |
| Large Crowns | 0.89 (0.05) | 0.19 | 0.34 | 0.84 (0.05) | 0.27 | 0.41 | 0.87 (0.05) | 0.25 | 0.37 |
| Low Stands | 0.93 (0.01) | 0.33 | 0.49 | 0.93 (0.01) | 0.35 | 0.5 | 0.91 (0.01) | 0.43 | 0.57 |
| High Stands | 0.90 (0.03) | 0.21 | 0.36 | 0.92 (0.01) | 0.23 | 0.36 | 0.89 (0.01) | 0.28 | 0.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meiforth, J.J.; Buddenbaum, H.; Hill, J.; Shepherd, J. Monitoring of Canopy Stress Symptoms in New Zealand Kauri Trees Analysed with AISA Hyperspectral Data. Remote Sens. 2020, 12, 926. https://doi.org/10.3390/rs12060926
Meiforth JJ, Buddenbaum H, Hill J, Shepherd J. Monitoring of Canopy Stress Symptoms in New Zealand Kauri Trees Analysed with AISA Hyperspectral Data. Remote Sensing. 2020; 12(6):926. https://doi.org/10.3390/rs12060926
Chicago/Turabian StyleMeiforth, Jane J., Henning Buddenbaum, Joachim Hill, and James Shepherd. 2020. "Monitoring of Canopy Stress Symptoms in New Zealand Kauri Trees Analysed with AISA Hyperspectral Data" Remote Sensing 12, no. 6: 926. https://doi.org/10.3390/rs12060926