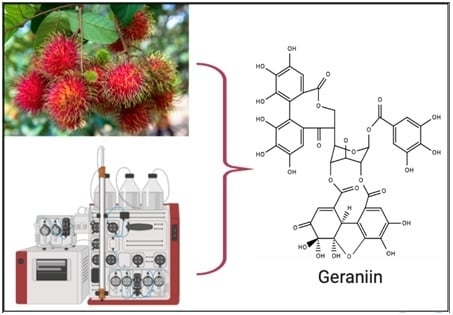
Preliminary Testing of Ultrasound/Microwave-Assisted Extraction (U/M-AE) for the Isolation of Geraniin from Nephelium lappaceum L. (Mexican Variety) Peel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Experimental Design
2.3. Ultrasound/Microwave-Assisted Extraction (U/M-AE) of Soluble Polyphenols
2.4. Separation of Bound Polyphenol Fractions
2.5. Determination of Total Polyphenol Content
2.6. Separation and Partial Purification of Soluble Polyphenol Fractions Using Amberlite XAD-16
2.7. Separation and Isolation of Ellagitannins by Preparative HPLC
2.8. Identification of Polyphenolic Compounds by HPLC/ESI/MS Analysis
3. Results and Discussion
3.1. Soluble Polyphenol Content in RP Extract
3.2. Bound Polyphenol Content in RP Extract
3.3. Isolation of Ellagitannin
3.4. Identification of Bioactive Compounds Present in RP Extract
Author Contributions
Funding
Conflicts of Interest
References
- Castillo-Vera, A.; López-Guillén, G.; Sandoval-Esquivez, A. La historia del cultivo de rambutan (Nepheliumlapacceum L.) en México. Agroproductividad 2017, 10, 53–57. [Google Scholar]
- Hernández-Hernández, C.; Aguilar, C.; Rodríguez-Herrera, R.; Flores-Gallegos, A.; Morlett-Chávez, J.; Govea-Salas, M.; Ascacio-Valdés, J. Rambutan (Nepheliumlappaceum L.): Nutritional and functionalproperties. Trends Food Sci. Technol. 2019, 85, 201–210. [Google Scholar] [CrossRef]
- Sagar, N.; Pareek, S.; Sharma, S.; Yahia, E.; Lobo, M. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [Green Version]
- De Monte, C.; Carradori, S.; Granese, A.; Di Pierro, G.; Leonardo, C.; De Nunzio, C. Modern extraction techniques and their impact on the pharmacological profile of Serenoarepens extracts for the treatment of lower urinary tract symptoms. BMC Urol. 2014, 14, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carniel, N.; Filippi, D.; DellossGullich, L.; Bilibio, D.; Bender, J.; Priamo, W. Recovery of Total Polyphenols from Pomegranate and Butia: A Study of Ultrasound-assisted Extraction and Antioxidant Activity. Indian J. Adv. Chem. Sci. 2017, 5, 112–117. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, R.; Zhang, F.; Liu, R. Phenolic Profiles and Antioxidant Activity of Black Rice Bran of Different Commercially Available Varieties. J. Agric. Food Chem. 2010, 58, 7580–7587. [Google Scholar] [CrossRef] [PubMed]
- Sinlgeton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Nitao, J.; Birr, B.; Nair, M.; Herms, D.; Mattson, W. Rapid Quantification of Proanthocyanidins (Condensed Tannins) with a Continuous Flow Analyzer. J. Agric. Food Chem. 2001, 49, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Ascacio-Valdés, J.; Aguilera-Carbó, A.; Buenrostro, J.; Prado-Barragán, A.; Rodríguez-Herrera, R.; Aguilar, C. The complete biodegradation pathway of ellagitanninsby Aspergillusniger in solid-state fermentation. J. Basic Microbiol. 2016, 56, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Zárate, P.; Wong-Paz, J.; Michel, M.; Buenrostro-Figueroa, J.; Díaz, H.; Ascacio, J.; Contreras-Esquivel, J.; Gutiérrez-Sánchez, G.; Aguilar, C. Characterization of Pomegranate-Husk Polyphenols and Semi-Preparative Fractionation of Punicalagin. Phytochem. Anal. 2017, 28, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, L.; Wong-Paz, J.; Buenrostro-Figueroa, J.; Ascacio-Valdés, J.; Aguilera-Carbó, A.; Aguilar, C. Solid-state fermentation of pomegranate husk: Recovery of ellagic acid by SEC and identification of ellagitannins by HPLC/ESI/MS. Food Biosci. 2018, 22, 99–104. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, H.; Zhuang, Y. Preparation of Free, Soluble Conjugate, and Insoluble-Bound Phenolic Compounds from Peels of Rambutan (Nepheliumlappaceum) and Evaluation of Antioxidant Activitiesin vitro. J. Food Sci. 2012, 77, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Soquetta, M.; Terra, L.; Bastos, C. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA-J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Liao, X.; Hu, F.; Chen, Z. Identification and Quantitation of the Bioactive Components in Osmanthusfragrans Fruits by HPLC-ESI-MS/MS. J. Agric. Food Chem. 2018, 66, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, U.; Ling, L.; Manaharan, T.; Appleton, D. Rapid isolation of geraniin from Nepheliumlappaceum rind waste and its anti-hyperglycemic activity. Food Chem. 2011, 127, 21–27. [Google Scholar] [CrossRef]
- Mendez-Flores, A.; Hernández-Almanza, A.; Sáenz-Galindo, A.; Morlett-Chávez, J.; Aguilar, C.; Ascacio-Valdés, J. Ultrasound-assisted extraction of antioxidant polyphenolic compounds from Nepheliumlappaceum L. (Mexican variety) husk. Asian Pac. J. Trop. Med. 2018, 11, 676–681. [Google Scholar] [CrossRef]






| ID | Mass/Volume Ratio (g/mL) | Water/Ethanol Ratio (%) |
|---|---|---|
| 1 | 1:16 | 0 |
| 2 | 1:16 | 70 |
| 3 | 1:8 | 0 |
| 4 | 1:8 | 70 |
| 5 | 1:12 | 30 |
| Purification | ID | Retention Time (min) | Compounds | Mass(m/z)[M-H]− | MS2 | Group/Family |
|---|---|---|---|---|---|---|
| Amberlite XAD-16 | 1 | 25.66 | Corilagin | 634 | 481,301,275 | Ellagitannin |
| 2 | 26.55 | Geraniin | 952 | 933,301,169 | Ellagitannin | |
| 3 | 28.5 | Punigluconin | 802 | 649,347,348 | Ellagitannin | |
| 4 | 29.85 | Ellagicacidpentoside | 433 | 299,300,287,125 | Ellagitannin | |
| 5 | 31.65 | Ellagicacid | 302 | 257, 229,185 | Ellagitannin | |
| 6 | 33.07 | Tetragalloyglucose | 789 | 617,465,635 | Hydroxybenzoic acid | |
| 7 | 33.5 | Pedunculagin | 785 | 301,481,765 | Ellagitannin | |
| Preparative HPLC | 1 | 27.45 | Geraniin | 952 | 933,301,169 | Ellagitannin |
| 2 | 30.55 | Ellagicacid | 302 | 257,229,185 | Ellagitannin | |
| 3 | 32.75 | Ellagicacidpentoside | 433 | 299,300,287,125 | Ellagitannin |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Hernández, C.; Aguilar, C.N.; Flores-Gallegos, A.C.; Sepúlveda, L.; Rodríguez-Herrera, R.; Morlett-Chávez, J.; Govea-Salas, M.; Ascacio-Valdés, J. Preliminary Testing of Ultrasound/Microwave-Assisted Extraction (U/M-AE) for the Isolation of Geraniin from Nephelium lappaceum L. (Mexican Variety) Peel. Processes 2020, 8, 572. https://doi.org/10.3390/pr8050572
Hernández-Hernández C, Aguilar CN, Flores-Gallegos AC, Sepúlveda L, Rodríguez-Herrera R, Morlett-Chávez J, Govea-Salas M, Ascacio-Valdés J. Preliminary Testing of Ultrasound/Microwave-Assisted Extraction (U/M-AE) for the Isolation of Geraniin from Nephelium lappaceum L. (Mexican Variety) Peel. Processes. 2020; 8(5):572. https://doi.org/10.3390/pr8050572
Chicago/Turabian StyleHernández-Hernández, Cristian, Cristóbal Noé Aguilar, Adriana Carolina Flores-Gallegos, Leonardo Sepúlveda, Raúl Rodríguez-Herrera, Jesús Morlett-Chávez, Mayela Govea-Salas, and Juan Ascacio-Valdés. 2020. "Preliminary Testing of Ultrasound/Microwave-Assisted Extraction (U/M-AE) for the Isolation of Geraniin from Nephelium lappaceum L. (Mexican Variety) Peel" Processes 8, no. 5: 572. https://doi.org/10.3390/pr8050572