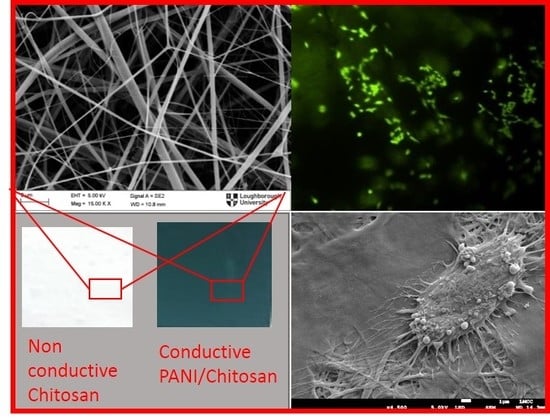
Biocompatibility Assessment of Conducting PANI/Chitosan Nanofibers for Wound Healing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrospinning of Chitosan-Polyaniline Blends
2.2. Water-Proofing of Electrospun Membranes
2.3. Contact Angle Measurement
2.4. Electrical Resistivity
2.5. Cell Culture
2.6. Cell Attachment and Viability Assay
2.7. Cell Proliferation Assay
2.8. Morphological Assessment
3. Results and Discussion
3.1. Fabrication and Neutralization of Chitosan-Polyaniline Electrospun Membranes
3.2. Characterization of Neutralized Electrospun Membranes
3.2.1. Contact Angle
3.2.2. Electrical Conductivity
3.3. Evaluation of Cell Attachment and Viability
3.4. Cell Proliferation
3.5. Cell Morphology Assessment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A




Appendix B

References
- Guimard, N.K.; Gomez, N.; Schmidt, C.E. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 2007, 32, 876–921. [Google Scholar] [CrossRef]
- Qazi, T.H.; Rai, R.; Boccaccini, A.R. Tissue engineering of electrically responsive tissues using polyaniline based polymers: A review. Biomaterials 2014, 35, 9068–9086. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Rouabhia, M.; Zhang, Z. Electrical stimulation modulates osteoblast proliferation and bone protein production through heparin-bioactivated conductive scaffolds. Bioelectromagnetics 2013, 34, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Rouabhia, M.; Park, H.; Meng, S.; Derbali, H.; Zhang, Z. Electrical stimulation promotes wound healing by enhancing dermal fibroblast activity and promoting myofibroblast transdifferentiation. PLoS ONE 2013, 8, e71660. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Raina, D.B.; Pelkonen, M.; Lidgren, L.; Tgil, M.; Kumar, A. Study of in vitro and in vivo bone formation in composite cryogels and the influence of electrical stimulation. Int. J. Biol. Sci. 2015, 11, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Rouabhia, M.; Park, H.J.; Zhang, Z. Electrically activated primary human fibroblasts improve in vitro and in vivo skin regeneration. J. Cell. Physiol. 2016, 231, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Franek, A.; Kostur, R.; Taradaj, J.; Blaszczak, E.; Szlachta, Z.; Dolibog, P.; Dolibog, P.; Polak, A. Effect of high voltage monophasic stimulation on pressure ulcer healing: results from a randomized controlled trial. Wounds 2011, 23, 15–23. [Google Scholar]
- Ahmad, E.T. High-voltage pulsed galvanic stimulation: Effect of treatment duration on healing of chronic pressure ulcers. Ann. Burns Fire Disasters 2008, 21, 124–128. [Google Scholar] [PubMed]
- Myers, B. Wound Management: Principles and Practice, 2nd ed.; Pearson: Tulsa, OK, USA, 2008. [Google Scholar]
- Savina, I.; Shevchenko, R.; Allan, I.; Illsley, M.; Sergey, M. Cryogels as a component of topical wound dressings. In Supermacroporous Cryogels: Biomedical and Biotechnological Applications; Kumar, A., Ed.; CRC Press Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 1–20. [Google Scholar]
- Leung, V.; Ko, F. Biomedical applications of nanofibers. Polym. Adv. Technol. 2011, 22, 350–365. [Google Scholar] [CrossRef]
- Li, W.; Shanti, R.M.; Tuan, R.S. Electrospinning technology for nanofibrous scaffolds in tissue engineering. In Nanotechnologies for the Life Sciences; Kumar, C.S.S.R., Ed.; Wiley-VCH: Hoboken, NJ, USA, 2006; Volume 9, pp. 135–187. [Google Scholar]
- Xia, Y.; Lu, X.; Zhu, H. Natural silk fibroin/polyaniline (core/shell) coaxial fiber: Fabrication and application for cell proliferation. Compos. Sci. Technol. 2013, 77, 37–41. [Google Scholar] [CrossRef]
- Yarin, A.L.; Zussman, E. Upward needleless electrospinning of multiple nanofibers. Polymer 2004, 45, 2977–2980. [Google Scholar] [CrossRef]
- Balogh, A.; Cselkó, R.; Démuth, B.; Verreck, G.; Mensch, J.; Marosi, G.; Nagy, Z.K. Alternating current electrospinning for preparation of fibrous drug delivery systems. Int. J. Pharm. 2015, 495, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Balogh, A.; Farkas, B.; Domokos, A.; Farkas, A.; Démuth, B.; Borbás, E.; Nagy, B.; Marosi, G.; Nagy, Z.K. Controlled-release solid dispersions of Eudragit® FS 100 and poorly soluble spironolactone prepared by electrospinning and melt extrusion. Eur. Polym. J. 2017, 95, 406–417. [Google Scholar] [CrossRef]
- Kostakova, E.; Seps, M.; Pokorny, P.; Lukas, D. Study of polycaprolactone wet electrospinning process. Express Polym. Lett. 2014, 8, 554–564. [Google Scholar] [CrossRef] [Green Version]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Electrical stimulation: A novel tool for tissue engineering. Tissue Eng. Part B Rev. 2013, 19, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.K.; Park, S.J.; Kim, M.S.; Kang, C.M.; Kim, J.I.; Kim, C.H. Fabrication of sonicated chitosan nanofiber mat with enlarged porosity for use as hemostatic materials. Carbohydr. Polym. 2013, 97, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Marcasuzaa, P.; Reynaud, S.; Ehrenfeld, F.; Khoukh, A.; Desbrieres, J. Chitosan-graft-polyaniline-based hydrogels: Elaboration and properties. Biomacromolecules 2010, 11, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Reis, R.L.; Mano, J.F. Graft copolymerized chitosan—Present status and applications. Carbohydr. Polym. 2005, 62, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Li, P.; Guo, B.; Ma, P.X. Antibacterial and conductive injectable hydrogels based on quaternized chitosan-graft-polyaniline/oxidized dextran for tissue engineering. Acta Biomater. 2015, 26, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Alvarez-perez, M.A.; Borriello, A.; Napolitano, T.; Ambrosio, L. Conductive PANi/PEGDA macroporous hydrogels for nerve regeneration. Adv. Healthc. Mater. 2013, 2, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Suhaimi, H.; Wang, S.; Thornton, T.; Das, D.B. On glucose diffusivity of tissue engineering membranes and scaffolds. Chem. Eng. Sci. 2015, 126, 244–256. [Google Scholar] [CrossRef] [Green Version]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: Advances, challenges, and future prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.C.; Ray, S.; Gizdavic-Nikolaidis, M.; Uy, B.; Swift, S.; Jin, J.; Cooney, R.P. Nanostructured bioactive material based on polycaprolactone and polyaniline fiber-scaffolds. Synth. Met. 2014, 198, 41–50. [Google Scholar] [CrossRef]
- Ma, X.; Ge, J.; Li, Y.; Guo, B.; Ma, P.X. Nanofibrous electroactive scaffolds from a chitosan-grafted-aniline tetramer by electrospinning for tissue engineering. RSC Adv. 2014, 4, 13652–13661. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, H.; Wang, S.; Feng, L.; Ji, Y.; Tao, L.; Li, S.; Wei, Y. Cellular responses of aniline oligomers: A preliminary study. Toxicol. Res. 2012, 1, 201–205. [Google Scholar] [CrossRef]
- Jeong, S.I.; Jun, I.D.; Choi, M.J.; Nho, Y.C.; Lee, Y.M.; Shin, H. Development of electroactive and elastic nanofibers that contain polyaniline and poly(l-lactide-co-ε-caprolactone) for the control of cell adhesion. Macromol. Biosci. 2008, 8, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Dong, Y.Q.; Sengothi, K.; Tan, K.L.; Kang, E.T. In-vivo tissue response to polyaniline. Synth. Met. 1999, 102, 1313–1314. [Google Scholar] [CrossRef]
- Humpolicek, P.; Kasparkova, V.; Saha, P.; Stejskal, J. Biocompatibility of polyaniline. Synth. Met. 2012, 162, 722–727. [Google Scholar] [CrossRef]
- Abidian, M.R.; Kim, D.H.; Martin, D.C. Conducting-polymer nanotubes for controlled drug release. Adv. Mater. 2006, 18, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Sangsanoh, P.; Supaphol, P. Stability improvement of electrospun chitosan nanofibrous membranes in neutral or weak basic aqueous solutions. Biomacromolecules 2006, 7, 2710–2714. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Isogai, A.; Onabe, F.; Usuda, M. Dissolving states of cellulose and chitosan in trifluoroacetic acid. J. Appl. Polym. Sci. 1992, 45, 1857–1863. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Ocio, M.J.; Lagaron, J.M. Development of active antimicrobial fiber based chitosan polysaccharide nanostructures using electrospinning. Eng. Life Sci. 2008, 8, 303–314. [Google Scholar] [CrossRef]
- Nizioł, J.; Gondek, E.; Plucinski, K.J. Characterization of solution and solid state properties of polyaniline processed from trifluoroacetic acid. J. Mater. Sci. Mater. Electron. 2012, 23, 2194–2201. [Google Scholar] [CrossRef]
- Angammana, C.J.; Jayaram, S.H. Analysis of the effects of solution conductivity on electrospinning process and fiber morphology. IEEE Trans. Ind. Appl. 2011, 47, 1109–1117. [Google Scholar] [CrossRef]
- Moutsatsou, P.; Coopman, K.; Smith, M.B.; Georgiadou, S. Conductive PANI fibers and determining factors for the electrospinning window. Polymer 2015, 77, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Terada, D.; Kobayashi, H.; Zhang, K.; Tiwari, A.; Yoshikawa, C.; Hanagata, N. Transient charge-masking effect of applied voltage on electrospinning of pure chitosan nanofibers from aqueous solutions. Sci. Technol. Adv. Mater. 2012, 13, 015003. [Google Scholar] [CrossRef] [PubMed]
- Ninan, N.; Muthiah, M.; Park, I.K.; Kalarikkal, N.; Elain, A.; Wong, T.W.; Thomas, S.; Grohens, Y. Wound healing analysis of pectin/carboxymethyl cellulose/microfibrillated cellulose based composite scaffolds. Mater. Lett. 2014, 132, 34–37. [Google Scholar] [CrossRef]
- Bober, P.; Humpolíček, P.; Pacherník, J.; Stejskal, J.; Lindfors, T. Conducting polyaniline based cell culture substrate for embryonic stem cells and embryoid bodies. RSC Adv. 2015, 5, 50328–50335. [Google Scholar] [CrossRef]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Rouabhia, M.; Zhang, Z.; De, D.; De, F.; Laval, U. Electrical stimulation in tissue regeneration. In Applied Biomedical Engineering; InTech: Rijeka, Croatia, 2011; pp. 37–62. [Google Scholar]
- Zhang, P.; Liu, Z.T.; He, G.X.; Liu, J.P.; Feng, J. Low-voltage direct-current stimulation is safe and promotes angiogenesis in rabbits with myocardial infarction. Cell Biochem. Biophys. 2011, 59, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Kohl, P.; Gourdie, R.G. Fibroblast-myocyte electrotonic coupling: Does it occur in native cardiac tissue? J. Mol. Cell. Cardiol. 2014, 70, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Edmondson, D.; Veiseh, O.; Matsen, F.A.; Zhang, M. Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials 2005, 26, 6176–6184. [Google Scholar] [CrossRef] [PubMed]
- Khalili, A.A.; Ahmad, M.R. A Review of cell adhesion studies for biomedical and biological applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef] [PubMed]
- Gümüşderelioglu, M.; Dalkiranoǧlu, S.; Aydin, R.S.T.; Çakmak, S. A novel dermal substitute based on biofunctionalized electrospun PCL nanofibrous matrix. J. Biomed. Mater. Res. Part A 2011, 98, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Pelipenko, J.; Kocbek, P.; Govedarica, B.; Rošic, R.; Baumgartner, S.; Kristl, J. The topography of electrospun nanofibers and its impact on the growth and mobility of keratinocytes. Eur. J. Pharm. Biopharm. 2013, 84, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Bidez, P.R.; Li, S.; Macdiarmid, A.G.; Venancio, E.C.; Wei, Y.; Lelkes, P.I. Polyaniline, an electroactive polymer, supports adhesion and proliferation of cardiac myoblasts. J. Biomater. Sci. Polym. Ed. 2006, 17, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Gizdavic-Nikolaidis, M.; Ray, S.; Bennett, J.R.; Easteal, A.J.; Cooney, R.P. Electrospun functionalized polyaniline copolymer-based nanofibers with potential application in tissue engineering. Macromol. Biosci. 2010, 10, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Kamkin, A.; Kiseleva, I.; Lozinsky, I.; Scholz, H. Electrical interaction of mechanosensitive fibroblasts and myocytes in the heart. Basic Res. Cardiol. 2005, 100, 337–345. [Google Scholar] [CrossRef] [PubMed]








| PANI/CS Ratio | Electrospinning Parameters | Nanofibre Diameter | |||||
|---|---|---|---|---|---|---|---|
| Voltage (kV) | Flow Rate (mL/h) | Tip to Collector Distance (cm) | Relative Humidity (RH) (%) | Average Diameter (nm) | SD * | RSD (%) ** | |
| 0 (Pure CS) | 26 | 1 | 13 | 35 | 111 | 64 | 53 |
| 1:3 | 26 | 1 | 16 | 35 | 116 | 44 | 38 |
| 3:5 | 28.5 | 0.3 | 16 | 20 | 130 | 123 | 95 |
| 1:1 | 29.5 | 0.3 | 16 | 20 | 160 | 126 | 78 |
| PANI/CS Ratio | Contact Angle (°) | |
|---|---|---|
| Average | SD | |
| 0—Pure CS | 41.3 | 6.72 |
| 1:3 | 46.67 | 6.47 |
| 3:5 | 53.09 | 8.17 |
| 1:1 | 70.34 | 6.35 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moutsatsou, P.; Coopman, K.; Georgiadou, S. Biocompatibility Assessment of Conducting PANI/Chitosan Nanofibers for Wound Healing Applications. Polymers 2017, 9, 687. https://doi.org/10.3390/polym9120687
Moutsatsou P, Coopman K, Georgiadou S. Biocompatibility Assessment of Conducting PANI/Chitosan Nanofibers for Wound Healing Applications. Polymers. 2017; 9(12):687. https://doi.org/10.3390/polym9120687
Chicago/Turabian StyleMoutsatsou, Panagiota, Karen Coopman, and Stella Georgiadou. 2017. "Biocompatibility Assessment of Conducting PANI/Chitosan Nanofibers for Wound Healing Applications" Polymers 9, no. 12: 687. https://doi.org/10.3390/polym9120687