Highly Active and Isospecific Styrene Polymerization Catalyzed by Zirconium Complexes Bearing Aryl-substituted [OSSO]-Type Bis(phenolate) Ligands
Abstract
:1. Introduction

2. Experimental Section
2.1. General
2.2. Preparation of Dibenzyl Zirconium(IV) Complex 9
2.3. Preparation of Dibenzyl Zirconium(IV) Complex 10
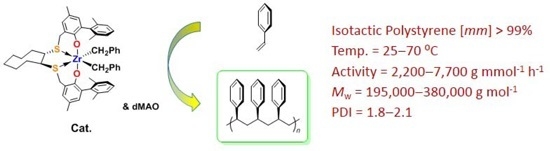
2.4. General Procedure for Styrene Polymerization
2.5. X-ray Crystallographic Analysis
3. Results and Discussion
3.1. Synthesis of Dibenzyl Zirconium(IV) Complexes 9 and 10


3.2. Styrene Polymerization
| Run | Cat. | Temp.(°C) | Time (min) | Activity (g·mmol−1·h−1) | Mw (g·mol−1) | PDI b | (mm) c (%) | Tm d (°C) |
|---|---|---|---|---|---|---|---|---|
| 1 | 9 | 0 | 60 | 50 | 137,000 | 8.6 | >99 | 218.6 |
| 2 | 9 | 25 | 60 | 139 | 156,000 | 3.8 | 96.8 | 209.8 |
| 3 | 9 | 40 | 60 | 182 | 181,000 | 2.9 | 90.7 | - |
| 4 | 9 | 70 | 60 | 618 | 9500 | 2.2 | 87.5 | - |
| 5 | 10 | 0 | 10 | 777 | 257,000 | 3.1 | >99 | 225.3 |
| 6 | 10 | 25 | 10 | 2200 | 380,000 | 2.1 | >99 | 225.8 |
| 7 | 10 | 40 | 5 | 4100 | 338,000 | 1.8 | >99 | 222.6 |
| 8 | 10 | 70 | 5 | 7700 | 195,000 | 1.8 | >99 | 221.4 |

4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tomotsu, N.; Ishihara, N.; Newman, T.H.; Malanga, M.T. Syndiospecific polymerization of styrene. J. Mol. Catal. A 1998, 128, 167–190. [Google Scholar] [CrossRef]
- Malanga, M. Syndiotactic polystyrene materials. Adv. Mater. 2000, 12, 1869–1872. [Google Scholar] [CrossRef]
- Ishihara, N.; Kuramoto, M.; Uoi, M.; (to Idemitsu Kosan Co. Ltd.). Japanese Patent 62187708, 1985.
- Ishihara, N.; Seimiya, T.; Kuramoto, M.; Uoi, M. Crystalline syndiotactic polystyrene. Macromolecules 1986, 19, 2464–2465. [Google Scholar] [CrossRef]
- Luo, Y.; Baldamus, J.; Hou, Z. Scandium half-metallocene-catalyzed syndiospecific styrene polymerization and styrene-ethylene copolymerization: Unprecedented incorporation of syndiotactic styrene-styrene sequences in styrene–ethylene copolymers. J. Am. Chem. Soc. 2004, 126, 13910–13911. [Google Scholar] [CrossRef] [PubMed]
- Kirillov, E.; Lehmann, C.W.; Razavi, A.; Carpentier, J.-F. Highly syndiospecific polymerization of styrene catalyzed by allyl lanthanide complexes. J. Am. Chem. Soc. 2004, 126, 12240–12241. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.-S.; Kirillov, E.; Lehmann, C.W.; Roisnel, T.; Vuillemin, B.; Razavi, A.; Carpentier, J.-F. Allyl ansa-lanthanidocenes: Single-component, single-site catalysts for controlled syndiospecific styrene and styrene–ethylene (co)polymerization. Chem. Eur. J. 2007, 13, 5548–5565. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.-S.; Kirillov, E.; Vuillemin, B.; Razavi, A.; Carpentier, J.-F. Binary ansa-lanthanidocenes/dialkylmagnesium systems versus single-component catalyst: Controlled synthesis of end-capped syndiotactic oligostyrenes. J. Mol. Catal. A 2007, 273, 87–91. [Google Scholar] [CrossRef]
- Jaroschik, F.; Shima, T.; Li, X.; Mori, K.; Ricard, L.; Le Goff, X.-F.; Nief, F.; Hou, Z. Synthesis, characterization, and reactivity of mono(phospholyl) lanthanoid(III) bis(dimethylaminobenzyl) complexes. Organometallics 2007, 26, 5654–5660. [Google Scholar] [CrossRef]
- Nishiura, M.; Mashiko, T.; Hou, Z. Synthesis and styrene polymerisation catalysis of η5- and η1-pyrrolyl-ligated cationic rare earth metal aminobenzyl complexes. Chem. Commun. 2008, 2019–2021. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Li, X.; Hou, Z.; Assoud, J.; Zhao, R. 1,2-Azaborolyl-ligated half-sandwich complexes of scandium(III) and lutetium(III): Synthesis, structures, and syndiotactic polymerization of styrene. Organometallics 2009, 28, 517–522. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Sun, J. Indenyl abstraction versus alkyl abstraction of [(Indenyl)ScR2(thf)] by [Ph3C] [B(C6F5)4]: Aspecific and syndiospecific styrene polymerization. Chem. Eur. J. 2009, 15, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, F.; Violante, C.D.C.; Roussel, P.; Mortreux, A.; Visseaux, M. Unprecedented dual behaviour of a half-sandwich scandium-based initiator for both highly selective isoprene and styrene polymerisation. Chem. Commun. 2009, 3380–3382. [Google Scholar] [CrossRef] [PubMed]
- Perrin, L.; Sarazin, Y.; Kirillov, E.; Carpentier, J.-F.; Maron, L. On the initiation mechanism of syndiospecific styrene polymerization catalyzed by single-component ansa-lanthanidocenes. Chem. Eur. J. 2009, 15, 3773–3783. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Luo, Y.; Gao, W.; Cui, D. Stereoselective polymerization of styrene with cationic scandium precursors bearing quinolyl aniline ligands. Organometallics 2010, 29, 1916–1923. [Google Scholar] [CrossRef]
- Li, X.; Nishiura, M.; Hu, L.; Mori, K.; Hou, Z. Alternating and random copolymerization of isoprene and ethylene catalyzed by cationic half-sandwich scandium alkyls. J. Am. Chem. Soc. 2009, 131, 13870–13882. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Nishiura, M.; Koshino, H.; Hou, Z. Scandium-catalyzed cyclocopolymerization of 1,5-hexadiene with styrene and ethylene: Efficient synthesis of cyclopolyolefins containing syndiotactic styrene–styrene sequences and methylene-1,3-cyclopentane units. Macromolecules 2011, 44, 6335–6344. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, K.; Nishiura, M.; Hou, Z. Syndiospecific living copolymerization of styrene with ε-caprolactone by scandium catalysts. Macromolecules 2010, 43, 9591–9593. [Google Scholar] [CrossRef]
- Pan, Y.; Rong, W.; Jian, Z.; Cui, D. Ligands dominate highly syndioselective polymerization of styrene by using constrained-geometry-configuration rare-earth metal precursors. Macromolecules 2012, 45, 1248–1253. [Google Scholar] [CrossRef]
- Natta, G.; Pino, P.; Corradini, P.; Danusso, F.; Mantica, E.; Mazzanti, G.; Moraglio, G. Crystalline high polymers of α-olefins. J. Am. Chem. Soc. 1955, 77, 1708–1710. [Google Scholar] [CrossRef]
- Natta, G.; Corradini, P. Kristallstruktur des isotaktischen polystyrols. Makromol. Chem. 1955, 16, 77–80. [Google Scholar] [CrossRef]
- Overberger, C.; Mark, H. A convenient laboratory preparation of isotactic polystyrene. J. Polym. Sci. 1959, 35, 381–389. [Google Scholar] [CrossRef]
- Kern, R.J.; Hurst, H.G.; Richard, W.J. Triethylaluminum–titanium tetrachloride catalysts for preparation of crystalline polystyrene. J. Polym. Sci. 1960, 45, 195–204. [Google Scholar] [CrossRef]
- Xu, G.; Lin, S. A novel NdCl3-modified Ziegler-Natta catalyst for the isotactic-specific polymerization of styrene. Macromol. Rapid Commun. 1994, 15, 873–877. [Google Scholar] [CrossRef]
- Rosário Riberio, M.; Portela, M.F.; Deffieux, A.; Cramail, H.; Rocha, M.F. Isospecific homo- and copolymerization of styrene with ethylene in the presence of VCl3, AlCl3 as catalyst. Macromol. Rapid Commun. 1996, 17, 461–469. [Google Scholar] [CrossRef]
- Sun, Q.; Fan, Y.; Liao, S.; Liu, J.; Wan, F.; Xu, J. Isospecific polymerization of styrene with modified Ziegler-type catalysts. Polymer 2001, 42, 4087–4090. [Google Scholar] [CrossRef]
- Kern, R.J. Homogeneous synthesis of isotactic polystyrene using n-butyllithium initiator. Nature 1960, 187, 410. [Google Scholar] [CrossRef]
- Cazzaniga, L.; Cohen, R.E. Anionic synthesis of isotactic polystyrene. Macromolecules 1989, 22, 4125–4128. [Google Scholar] [CrossRef]
- Makino, T.; Hogen-Esch, T.E. Anionic synthesis of highly isotactic polystyrene in hexane in the presence of lithium hydroxides. Macromolecules 1999, 32, 5712–5714. [Google Scholar] [CrossRef]
- Maréchal, J.-M.; Carlotti, S.; Shcheglova, L.; Deffieux, A. Stereospecific anionic polymerization of styrene initiated by R2Mg/ROMt ‘ate’ complexes. Polymer 2004, 45, 4641–4646. [Google Scholar] [CrossRef]
- Ascenso, J.R.; Dias, A.R.; Gomes, P.T.; Romao, C.C.; Tkatchenko, I.; Revillon, A.; Pham, Q.T. Isospecific oligo-/polymerization of styrene with soluble cationic nickel complexes. The influence of phosphorus(III) ligands. Macromolecules 1996, 29, 4172–4179. [Google Scholar] [CrossRef]
- Crossetti, G.L.; Bormioli, C.; Ripa, A.; Giarrusso, A.; Porri, L. Polymerization of styrene to isotactic polymer with MAO-Ni(acac)2. Examination of the factors that influence activity and stereospecificity. Macromol. Rapid Commun. 1997, 18, 801–808. [Google Scholar] [CrossRef]
- Po, R.; Cardi, N.; Santi, R.; Romano, A.M.; Zannoni, C.; Spera, S. Polymerization of styrene with nickel complex/methylaluminoxane catalytic systems. J. Polym. Sci., Part A Polym. Chem. 1998, 36, 2119–2126. [Google Scholar] [CrossRef]
- Arai, T.; Ohtsu, T.; Suzuki, S. Homo- and copolymerization of styrene by bridged zirconocene complexes with benzindenyl ligands. Polym. Prepr. 1998, 39, 220–221. [Google Scholar]
- Arai, T.; Suzuki, S.; Ohtsu, T. Homo- and copolymerization of styrene by bridged zirconocene complex with benz indenyl ligand. ACS Symp. Ser. 2009, 749, 66–80. [Google Scholar]
- Capacchione, C.; Proto, A.; Ebeling, H.; Mülhaupt, R.; Möller, K.; Spaniol, T.P.; Okuda, J. Ancillary ligand effect on single-site styrene polymerization: Isospecificity of Group 4 metal bis(phenolate) catalysts. J. Am. Chem. Soc. 2003, 125, 4964–4965. [Google Scholar] [CrossRef] [PubMed]
- Capacchione, C.; Manivannan, R.; Barone, M.; Beckerle, K.; Centore, R.; Oliva, L.; Proto, A.; Tuzi, A.; Spaniol, T.P.; Okuda, J. Isospecific styrene polymerization by chiral titanium complexes that contain a tetradentate [OSSO]-Type bis(phenolato) ligand. Organometallics 2005, 24, 2971–2982. [Google Scholar] [CrossRef]
- Hohberger, C.; Spaniol, T.P.; Okuda, J. Living polymerization by bis(phenolate) zirconium catalysts: Synthesis of isotactic polystyrene-block-polybutadiene copolymers. Macromol. Chem. Phys. 2014, 215, 2001–2006. [Google Scholar] [CrossRef]
- Proto, A.; Avagliano, A.; Saviello, D.; Ricciardi, R.; Capacchione, C. Living, isoselective polymerization of styrene and formation of stereoregular block copolymers via sequential monomer addition. Macromolecules 2010, 43, 5919–5921. [Google Scholar] [CrossRef]
- Capacchione, C.; Saviello, D.; Ricciardi, R.; Proto, A. Living, isoselective polymerization of 4-methyl-1,3-pentadiene and styrenic monomers and synthesis of highly stereoregular block copolymers via sequential monomer addition. Macromolecules 2011, 44, 7940–7947. [Google Scholar] [CrossRef]
- Rodrigues, A.-S.; Kirillov, E.; Roisnel, T.; Razavi, A.; Vuillemin, B.; Carpentier, J.-F. Highly isospecific styrene polymerization catalyzed by single-component bridged bis(indenyl) allyl yttrium and neodymium complexes. Angew. Chem., Int. Ed. 2007, 46, 7240–7243. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, L.; Rodrigues, A.-S.; Kirillov, E.; Sarazin, Y.; Okuda, J.; Perrin, L.; Maron, L.; Carpentier, J.-F. Isoselective styrene polymerization catalyzed by ansa-bis(indenyl) allyl rare earth complexes. Stereochemical and mechanistic aspects. Macromolecules 2011, 44, 3312–3322. [Google Scholar] [CrossRef]
- Nakata, N.; Toda, T.; Ishii, A. Recent advances in the chemistry of Group 4 metal complexes incorporating [OSSO]-type bis(phenolato) ligands as post-metallocene catalysts. Polym. Chem. 2011, 2, 1597–1610. [Google Scholar] [CrossRef]
- Nakata, N.; Ishii, A. Precise polymerization of α-olefins using a mixed donor-type ligand containing oxygen and sulfur atoms. Koubunshi Ronbunshu 2015, 72, 285–294. [Google Scholar] [CrossRef]
- Toda, T.; Nakata, N.; Matsuo, T.; Ishii, A. Synthesis and structures of dialkyl zirconium complexes with an [OSSO]-type bis(phenolate) ligand bearing a trans-1,2-cyclooctanediylbis(thio) unit. J. Organomet. Chem. 2011, 696, 1258–1261. [Google Scholar] [CrossRef]
- Nakata, N.; Toda, T.; Ishii, A.; Matsuo, T. Titanium complexes supported by an [OSSO]-Type bis(phenolato) ligand based on a trans-cyclooctanediyl platform: Synthesis, structures, and 1-hexene polymerization. Inorg. Chem. 2012, 51, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Nakata, N.; Ishii, A.; Matsuo, T. Synthesis, structure, and 1‑hexene polymerization catalytic ability of Group 5 metal complexes incorporating an [OSSO]-type ligand. ACS Catal. 2013, 3, 1764–1767. [Google Scholar] [CrossRef]
- Nakata, N.; Saito, Y.; Ishii, A. Neutral methyl and cationic aluminum complexes supported by a trans-1,2-cyclooctanediyl-bridged [OSSO]-type bis(phenolato) ligand: Synthesis, structures, and use in catalysis for propylene oxide polymerization. Organometallics 2014, 33, 1840–1844. [Google Scholar] [CrossRef]
- Ishii, A.; Toda, T.; Nakata, N.; Matsuo, T. Zirconium complex of an [OSSO]-type diphenolate ligand bearing trans-1,2-cyclooctanediylbis(thio) core: Synthesis, structure, and isospecific 1-hexene polymerization. J. Am. Chem. Soc. 2009, 131, 13566–13567. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Nakata, N.; Matsuo, T.; Ishii, A. Extremely active α-olefin polymerization and copolymerization with ethylene catalyzed by a dMAO-activated zirconium(IV) dichloro complex having an [OSSO]-type ligand. RSC Adv. 2015, 8, 88826–88831. [Google Scholar] [CrossRef]
- Nakata, N.; Toda, T.; Matsuo, T.; Ishii, A. Controlled isospecific polymerization of α‑olefins by hafnium complex incorporating with a trans-cyclooctanediyl-bridged [OSSO]-type bis(phenolate) ligand. Macromolecules 2013, 46, 6758–6764. [Google Scholar] [CrossRef]
- Nakata, N.; Saito, Y.; Watanabe, T.; Ishii, A. Completely isospecific polymerization of 1-hexene catalyzed by hafnium(IV) dichloro complex incorporating with an [OSSO]-type bis(phenolate) ligand. Top. Catal. 2014, 57, 918–922. [Google Scholar] [CrossRef]
- Takano, M.; Ito, K.; Ishii, A.; Nakata, N.; Kawauchi, F. Jpn. Kokai Tokkyo Koho JP 2013166735 29 August 2013.
- Hasan, T.; Ioku, A.; Nishii, K.; Shiono, T.; Ikeda, T. Syndiospecific living polymerization of propene with [t-BuNSiMe2Flu]TiMe2 using MAO as cocatalyst. Macromolecules 2001, 34, 3142–3145. [Google Scholar] [CrossRef]
- Rong, Y.; Al-Harbi, A.; Parkin, G. Highly variable Zr-CH2-Ph bond angles in tetrabenzylzirconium: Analysis of benzyl ligand coordination modes. Organometallics 2012, 31, 8208–8217. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97. In Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Cohen, A.; Yeori, A.; Goldberg, I.; Kol, M. Group 4 complexes of a new [OSSO]-Type dianionic ligand. Coordination chemistry and preliminary polymerization catalysis studies. Inorg. Chem. 2007, 46, 8114–8116. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Goldberg, I.; Venditto, V.; Kol, M. Oscillating non-metallocenes—From stereoblock-isotactic polypropylene to isotactic polypropylene via zirconium and hafnium dithiodiphenolate catalysts. Eur. J. Inorg. Chem. 2011, 5219–5223. [Google Scholar] [CrossRef]
- Buffet, J.-C.; Okuda, J. Group 4 metal initiators for the controlled stereoselective polymerization of lactide monomers. Chem. Commun. 2011, 47, 4796–4798. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Thevenon, A.; Brosmer, J.L.; Yu, I.; Khan, S.I.; Mehrkhodavandi, P.; Diaconescu, P.L. Redox control of Group 4 metal ring-opening polymerization activity toward l‑lactide and ε‑caprolactone. J. Am. Chem. Soc. 2014, 136, 11264–11267. [Google Scholar] [CrossRef] [PubMed]
- Konkol, M.; Nabika, M.; Kohno, T.; Hino, T.; Miyatake, T. Synthesis, structure and α-olefin polymerization activity of Group 4 metal complexes with [OSSO]-type bis(phenolate) ligands. J. Organomet. Chem. 2011, 696, 1792–1802. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakata, N.; Toda, T.; Saito, Y.; Watanabe, T.; Ishii, A. Highly Active and Isospecific Styrene Polymerization Catalyzed by Zirconium Complexes Bearing Aryl-substituted [OSSO]-Type Bis(phenolate) Ligands. Polymers 2016, 8, 31. https://doi.org/10.3390/polym8020031
Nakata N, Toda T, Saito Y, Watanabe T, Ishii A. Highly Active and Isospecific Styrene Polymerization Catalyzed by Zirconium Complexes Bearing Aryl-substituted [OSSO]-Type Bis(phenolate) Ligands. Polymers. 2016; 8(2):31. https://doi.org/10.3390/polym8020031
Chicago/Turabian StyleNakata, Norio, Tomoyuki Toda, Yusuke Saito, Takanori Watanabe, and Akihiko Ishii. 2016. "Highly Active and Isospecific Styrene Polymerization Catalyzed by Zirconium Complexes Bearing Aryl-substituted [OSSO]-Type Bis(phenolate) Ligands" Polymers 8, no. 2: 31. https://doi.org/10.3390/polym8020031