Fluorescent Carbon Dioxide-Based Polycarbonates Probe for Rapid Detection of Aniline in the Environment and Its Biomarkers in Urine
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of Monomer M
2.3. Terpolymerization of CO2, PO, and M
3. Results and Discussion
3.1. Monomer Structure Characterization
3.2. CO2 Terpolymerization Reaction
3.3. Confirmation of the Copolymer Structure
3.4. Copolymer Properties
3.4.1. Thermal Properties
3.4.2. Mechanical Properties of the Polymer
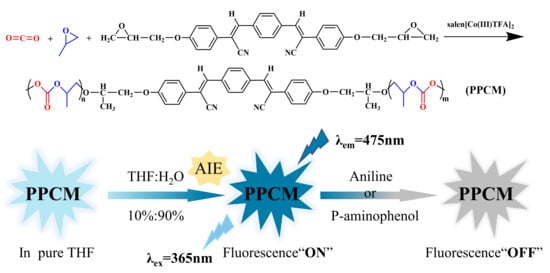
3.4.3. Aggregation-Induced Luminescence Properties of the Polymer
3.5. Applications of Copolymer Probes in the Environment
3.5.1. Recognition of Aniline Compounds via Copolymer PPCM
3.5.2. Anti-Interference Experiments on Aniline Using the Copolymer PPCM
3.5.3. Sensitivity Testing of the Copolymer PPCM to Aniline
3.5.4. Application of Copolymer PPCM in Different Water Samples
3.5.5. Recognition of Aniline Compounds via Polymer PPCM
3.6. Recognition of Aniline Biomarkers in Urine by Polymer PPCM
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aresta, M.; Dibenedetto, A. Editorial: Beyond Current Research Trends in CO2 Utilization. Front. Energy Res. 2022, 10, 814311. [Google Scholar] [CrossRef]
- Batarshin, V.; Gulevatenko, A.; Semiokhin, A. The Use and Utilization of CO2, as Part of the Fight with Greenhouse Effect. IOP Conf. Ser. Earth Environ. Sci. 2021, 666, 032018. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, X.; Chen, X. Advanced Materials and Technologies toward Carbon Neutrality. Acc. Mater. Res. 2022, 3, 913–921. [Google Scholar] [CrossRef]
- Yang, Z.; JingChun, W.; Elmasry, Y.; Alanazi, A.; Armghan, A.; Alanazi, M.; Algelany, A.M.; Wae-hayee, M. Techno-Economic and Multi Objective Optimization of Zero Carbon Emission Biomass Based Supercritical Carbon Dioxide Oxy Combustion System Integrated with Carbon Dioxide Liquefaction System and Solid Oxide Electrolyzer. J. CO2 Util. 2022, 64, 102169. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Nocito, F. The Future of Carbon Dioxide Chemistry. ChemSusChem 2020, 13, 6219–6228. [Google Scholar] [CrossRef]
- Ross, M.B. Carbon Dioxide Recycling Makes Waves. Joule 2019, 3, 1814–1816. [Google Scholar] [CrossRef]
- Lin, H.; Biddinger, E.J. Challenges and Opportunities for Carbon Dioxide Utilization. Energy Technol. 2017, 5, 771–772. [Google Scholar] [CrossRef]
- Overa, S.; Ko, B.H.; Zhao, Y.; Jiao, F. Electrochemical Approaches for CO2 Conversion to Chemicals: A Journey toward Practical Applications. Acc. Chem. Res. 2022, 55, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Shiong, S.C.S.; Liu, Y. Reduction of CO2 to Chemicals and Fuels: Thermocatalysis versus Electrocatalysis. Chem. Eng. J. 2023, 472, 145033. [Google Scholar] [CrossRef]
- He, J.; Janáky, C. Recent Advances in Solar-Driven Carbon Dioxide Conversion: Expectations versus Reality. ACS Energy Lett. 2020, 5, 1996–2014. [Google Scholar] [CrossRef] [PubMed]
- Biswal, T.; Shadangi, K.P.; Sarangi, P.K.; Srivastava, R.K. Conversion of Carbon Dioxide to Methanol: A Comprehensive Review. Chemosphere 2022, 298, 134299. [Google Scholar] [CrossRef]
- Ding, P.; Zhao, H.; Li, T.; Luo, Y.; Fan, G.; Chen, G.; Gao, S.; Shi, X.; Lu, S.; Sun, X. Metal-Based Electrocatalytic Conversion of CO2 to Formic Acid/Formate. J. Mater. Chem. A 2020, 8, 21947–21960. [Google Scholar] [CrossRef]
- Raza, A.; Ikram, M.; Guo, S.; Baiker, A.; Li, G. Green Synthesis of Dimethyl Carbonate from CO2 and Methanol: New Strategies and Industrial Perspective. Adv. Sustain. Syst. 2022, 6, 2200087. [Google Scholar] [CrossRef]
- Gürtler, C. Sustainable Carbon Sources for the Chemical Industry—New Products Based on CO2 as a Building Block for Polyurethane Plastics. Chem. Ing. Tech. 2018, 90, 1141. [Google Scholar] [CrossRef]
- Scharfenberg, M.; Seiwert, J.; Scherger, M.; Preis, J.; Susewind, M.; Frey, H. Multiarm Polycarbonate Star Polymers with a Hyperbranched Polyether Core from CO2 and Common Epoxides. Macromolecules 2017, 50, 6577–6585. [Google Scholar] [CrossRef]
- Qin, Y.; Sheng, X.; Liu, S.; Ren, G.; Wang, X.; Wang, F. Recent Advances in Carbon Dioxide Based Copolymers. J. CO2 Util. 2015, 11, 3–9. [Google Scholar] [CrossRef]
- Kozak, C.M.; Ambrose, K.; Anderson, T.S. Copolymerization of Carbon Dioxide and Epoxides by Metal Coordination Complexes. Coord. Chem. Rev. 2018, 376, 565–587. [Google Scholar] [CrossRef]
- Dhapte, V.; Gaikwad, N.; More, P.V.; Banerjee, S.; Dhapte, V.V.; Kadam, S.; Khanna, P.K. Transparent ZnO/Polycarbonate Nanocomposite for Food Packaging Application. Nanocomposites 2015, 1, 106–112. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, X.; Chen, Z.; Jiang, H. Palmitoylated Cellulose Nanocrystal/Polycarbonate Composite with High Mechanical Performance and Good Transparency. J. Appl. Polym. Sci. 2023, 140, e53298. [Google Scholar] [CrossRef]
- Pang, X.; Chen, M.; Fu, J.; Lin, Z.; Li, Y.; Wu, J.; Yan, J.; Chen, X.; Ge, J. Eugenol Polysiloxane-Polycarbonate/Graphene Nanocomposite: Enhanced in Thermostability and Barrier Property. Nanomaterials 2019, 9, 1747. [Google Scholar] [CrossRef]
- Zong, Q.; Zhou, S.; Ye, J.; Peng, X.; Wu, H.; Li, M.; Ye, X.; Tian, N.; Sun, W.; Zhai, Y. Aliphatic Polycarbonate-Based Hydrogel Dressing for Wound Healing. J. Drug Deliv. Sci. Technol. 2023, 79, 104083. [Google Scholar] [CrossRef]
- Cui, S.; Li, L.; Wang, Q. Fabrication of (PPC/NCC)/PVA Composites with Inner-Outer Double Constrained Structure and Improved Glass Transition Temperature. Carbohydr. Polym. 2018, 191, 35–43. [Google Scholar] [CrossRef]
- Jankowski, P.; Ogonczyk, D.; Kosinski, A.; Lisowski, W.; Garstecki, P. Hydrophobic Modification of Polycarbonate for Reproducible and Stable Formation of Biocompatible Microparticles. Lab Chip 2011, 11, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Li, Q.; Xu, C.; Li, R.; Wang, H.; Bu, Z.; Lin, T. Wool Powder: An Efficient Additive to Improve Mechanical and Thermal Properties of Poly(Propylene Carbonate). Compos. Sci. Technol. 2017, 153, 119–127. [Google Scholar] [CrossRef]
- Li, X.; Meng, L.; Zhang, Y.; Qin, Z.; Meng, L.; Li, C.; Liu, M. Research and Application of Polypropylene Carbonate Composite Materials: A Review. Polymers 2022, 14, 2159. [Google Scholar] [CrossRef]
- Liang, Y.F.; Xia, Y.; Zhang, S.Z.; Wang, X.L.; Xia, X.H.; Gu, C.D.; Wu, J.B.; Tu, J.P. A Preeminent Gel Blending Polymer Electrolyte of Poly(Vinylidene Fluoride-Hexafluoropropylene)-Poly(Propylene Carbonate) for Solid-State Lithium Ion Batteries. Electrochim. Acta 2019, 296, 1064–1069. [Google Scholar] [CrossRef]
- Han, D.; Guo, Z.; Chen, S.; Xiao, M.; Peng, X.; Wang, S.; Meng, Y. Enhanced Properties of Biodegradable Poly(Propylene Carbonate)/Polyvinyl Formal Blends by Melting Compounding. Polymers 2018, 10, 771. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Wang, W.-Z.; Wang, L.; Li, L.-L.; Zhang, K.-Y.; Zhao, S.-D. Poly(Propylene Carbonate) Networks with Excellent Properties: Terpolymerization of Carbon Dioxide, Propylene Oxide, and 4,4′-(Hexafluoroisopropylidene) Diphthalic Anhydride. e-Polymers 2021, 21, 511–519. [Google Scholar] [CrossRef]
- Lee, J.; Pan, J.; Chun, J.; Won, Y.-Y. Unexpected Conformational Behavior of Poly(Poly(Ethylene Glycol) Methacrylate)-Poly(Propylene Carbonate)-Poly(Poly(Ethylene Glycol) Methacrylate) (PPEGMA-PPC-PPEGMA) Amphiphilic Block Copolymers in Micellar Solution and at the Air-Water Interface. J. Colloid Interface Sci. 2020, 566, 304–315. [Google Scholar] [CrossRef]
- Chesterman, J.P.; Hughes, T.C.; Amsden, B.G. Reversibly Photo-Crosslinkable Aliphatic Polycarbonates Functionalized with Coumarin. Eur. Polym. J. 2018, 105, 186–193. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Y.; Zhang, N.; Zhang, L.; Darensbourg, D.J. One-Pot Synthesis of Ion-Containing CO2-Based Polycarbonates Using Protic Ionic Liquids as Chain Transfer Agents. Macromolecules 2018, 51, 9122–9130. [Google Scholar] [CrossRef]
- Alagi, P.; Zapsas, G.; Hadjichristidis, N.; Hong, S.C.; Gnanou, Y.; Feng, X. All-Polycarbonate Graft Copolymers with Tunable Morphologies by Metal-Free Copolymerization of CO2 with Epoxides. Macromolecules 2021, 54, 6144–6152. [Google Scholar] [CrossRef]
- Beharaj, A.; Ekladious, I.; Grinstaff, M.W. Poly(Alkyl Glycidate Carbonate)s as Degradable Pressure-Sensitive Adhesives. Angew. Chem. Int. Ed. 2019, 58, 1407–1411. [Google Scholar] [CrossRef]
- Cyriac, A.; Lee, S.H.; Varghese, J.K.; Park, J.H.; Jeon, J.Y.; Kim, S.J.; Lee, B.Y. Preparation of Flame-Retarding Poly(Propylene Carbonate). Green Chem. 2011, 13, 3469–3475. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Tsai, F.-T. Postpolymerization Functionalization of Copolymers Produced from Carbon Dioxide and 2-Vinyloxirane: Amphiphilic/Water-Soluble CO2-Based Polycarbonates. Macromolecules 2014, 47, 3806–3813. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Wang, Y. Terpolymerization of Propylene Oxide and Vinyl Oxides with CO2: Copolymer Cross-Linking and Surface Modification via Thiol–Ene Click Chemistry. Polym. Chem. 2015, 6, 1768–1776. [Google Scholar] [CrossRef]
- Ma, H.; Qi, C.; Cheng, C.; Yang, Z.; Cao, H.; Yang, Z.; Tong, J.; Yao, X.; Lei, Z. AIE-Active Tetraphenylethylene Cross-Linked N-Isopropylacrylamide Polymer: A Long-Term Fluorescent Cellular Tracker. ACS Appl. Mater. Interfaces 2016, 8, 8341–8348. [Google Scholar] [CrossRef] [PubMed]
- Lenora, C.U.; Hu, N.; Furgal, J.C. Thermally Stable Fluorogenic Zn(II) Sensor Based on a Bis(Benzimidazole)Pyridine-Linked Phenyl-Silsesquioxane Polymer. ACS Omega 2020, 5, 33017–33027. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.-H.; Yu, K.; Huang, J.; Liu, F.; Zhang, Z.-Y.; Chen, S.-P.; Zhang, F.; Guan, S.-P.; Qiu, L. Ratiometric Fluorescence Detection of Sulfide Ions Based on Lanthanide Coordination Polymer Using Guanosine Diphosphate as Ligand. Colloids Surf. B Biointerfaces 2021, 204, 111796. [Google Scholar] [CrossRef] [PubMed]
- Su, H.-J.; Wu, F.-I.; Tseng, Y.-H.; Shu, C.-F. Color Tuning of a Light-Emitting Polymer: Polyfluorene-Containing Pendant Amino-Substituted Distyrylarylene Units. Adv. Funct. Mater. 2005, 15, 1209–1216. [Google Scholar] [CrossRef]
- Tian, W.; Lin, T.; Chen, H.; Wang, W. Configuration-Controllable E/Z Isomers Based on Tetraphenylethene: Synthesis, Characterization, and Applications. ACS Appl. Mater. Interfaces 2019, 11, 6302–6314. [Google Scholar] [CrossRef]
- Arumugam, R.; Nayak, P.; Dey, B.; Kannan, R.; Venkatasubbaiah, K.; Chandrasekhar, V. 2-Hydroxyphenyl Benzimidazoles and Their Boron Complexes: Synthesis, Structure, Aggregation-Induced Emission and Picric Acid Sensing. Dalton Trans. 2023, 52, 7926–7935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, G.; Chen, J.; Zhang, Y.; Sun, Q.; Xue, S.; Yang, W. Unexpected Emission Behaviors of Rhodamine Derivatives in PVA After UV–Green Light Excitation. Adv. Opt. Mater. 2023, 11, 2301147. [Google Scholar] [CrossRef]
- Xiao, H.; Shi, Q.-X.; Su, M.; Sun, X.-L.; Bao, H.; Wan, W.-M. One-Pot Synthesis of Stimuli-Responsive Fluorescent Polymers through Polymerization-Induced Emission. ACS Macro Lett. 2023, 12, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hande, P.E.; Samui, A.B.; Kulkarni, P.S. Selective Nanomolar Detection of Mercury Using Coumarin Based Fluorescent Hg(II)—Ion Imprinted Polymer. Sens. Actuators B Chem. 2017, 246, 597–605. [Google Scholar] [CrossRef]
- Kong, X.; Li, M.; Zhang, Y.; Yin, Y.; Lin, W. Engineering an AIE N2H4 Fluorescent Probe Based on α-Cyanostilbene Derivative with Large Stokes Shift and Its Versatile Applications in Solution, Solid-State and Biological Systems. Sens. Actuators B Chem. 2021, 329, 129232. [Google Scholar] [CrossRef]
- Lin, S.; Gutierrez-Cuevas, K.G.; Zhang, X.; Guo, J.; Li, Q. Fluorescent Photochromic α-Cyanodiarylethene Molecular Switches: An Emerging and Promising Class of Functional Diarylethene. Adv. Funct. Mater. 2021, 31, 2007957. [Google Scholar] [CrossRef]
- Park, J.-M.; Kim, D.W.; Chung, H.Y.; Kwon, J.E.; Hong, S.H.; Choi, T.-L.; Park, S.Y. A Stereoregular β-Dicyanodistyrylbenzene (β-DCS)-Based Conjugated Polymer for High-Performance Organic Solar Cells with Small Energy Loss and High Quantum Efficiency. J. Mater. Chem. A 2017, 5, 16681–16688. [Google Scholar] [CrossRef]
- Hang, C.; Wu, H.-W.; Zhu, L.-L. π-Conjugated Cyanostilbene-Based Optoelectric Functional Materials. Chin. Chem. Lett. 2016, 27, 1155–1165. [Google Scholar] [CrossRef]
- Bakier, Y.M.; Ghali, M.; Elkun, A.; Beltagi, A.M.; Zahra, W.K. Static Interaction between Colloidal Carbon Nano-Dots and Aniline: A Novel Platform for Ultrasensitive Detection of Aniline in Aqueous Media. Mater. Res. Bull. 2021, 134, 111119. [Google Scholar] [CrossRef]
- Zhang, F.; Hou, W.; Yang, Z.; Wang, Z.; Chen, R.; Drioli, E.; Wang, X.; Cui, Z. Treatment of Aniline Wastewater by Membrane Distillation and Crystallization. Membranes 2023, 13, 561. [Google Scholar] [CrossRef]
- Wang, W.-Z.; Zhang, K.-Y.; Jia, X.-G.; Wang, L.; Li, L.-L.; Fan, W.; Xia, L. A New Dinuclear Cobalt Complex for Copolymerization of CO2 and Propylene Oxide: High Activity and Selectivity. Molecules 2020, 25, 4095. [Google Scholar] [CrossRef]
- Wang, W.-Z.; Zhao, C.; Li, L.-L.; Liu, S.; Zhang, Y.-L.; Luo, L. Preparation of Carbon Dioxide, Propylene Oxide, and Norbornene Dianhydride Terpolymers Catalyzed via Dinuclear Cobalt Complexes: Effective Improvement of Thermal, Mechanical, and Degradation Properties. Polymer 2022, 256, 125188. [Google Scholar] [CrossRef]
- Liang, J.; Ye, S.; Wang, W.; Fan, C.; Wang, S.; Han, D.; Liu, W.; Cui, Y.; Hao, L.; Xiao, M.; et al. Performance Tailorable Terpolymers Synthesized from Carbon Dioxide, Phthalic Anhydride and Propylene Oxide Using Lewis Acid-Base Dual Catalysts. J. CO2 Util. 2021, 49, 101558. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Han, W.; Huang, J.; Zhang, Y.; Zhao, C.; Li, L. A One-Pot Strategy for the Preparation of Fire-Retardant Poly(Propylene Carbonate) by Terpolymerization of CO2, Propylene Oxide and Chlorendic Anhydride. Mater. Today Commun. 2023, 34, 105179. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Feng, Y.; Yu, Z.-Q.; Wang, L.; Ren, X.-K.; Liu, Y. Aggregation-Mediated Photo-Responsive Luminescence of Cyanostilbene Based Cruciform AIEgens. J. Mater. Chem. C 2021, 9, 975–981. [Google Scholar] [CrossRef]
- You, L.; Zha, D.; Anslyn, E.V. Recent Advances in Supramolecular Analytical Chemistry Using Optical Sensing. Chem. Rev. 2015, 115, 7840–7892. [Google Scholar] [CrossRef] [PubMed]
- Sparano, B.A.; Koide, K. Fluorescent Sensors for Specific RNA: A General Paradigm Using Chemistry and Combinatorial Biology. J. Am. Chem. Soc. 2007, 129, 4785–4794. [Google Scholar] [CrossRef] [PubMed]
- Mallick, A.; Garai, B.; Addicoat, M.A.; Petkov, P.S.; Heine, T.; Banerjee, R. Solid State Organic Amine Detection in a Photochromic Porous Metal Organic Framework. Chem. Sci. 2015, 6, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lin, Y.; Liu, J.; Shi, W.; Yang, G.; Cheng, P. Rapid Detection of the Biomarkers for Carcinoid Tumors by a Water Stable Luminescent Lanthanide Metal–Organic Framework Sensor. Adv. Funct. Mater. 2018, 28, 1707169. [Google Scholar] [CrossRef]
- Shihana, F.; Dawson, A.H.; Buckley, N.A. A Bedside Test for Methemoglobinemia, Sri Lanka. Bull. World Health Organ. 2016, 94, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.-J.; Yan, B. The Point-of-Care Colorimetric Detection of the Biomarker of Phenylamine in the Human Urine Based on Tb3+ Functionalized Metal-Organic Framework. Anal. Chim. Acta 2018, 1012, 82–89. [Google Scholar] [CrossRef] [PubMed]
















| Polymer | n[PO]:n[M] | Yield (%) | Mn (g/mol) b | PDI c |
|---|---|---|---|---|
| PPCM1 | 100:1 | 32 | 6352 | 1.43 |
| PPCM2 | 100:2 | 55 | 8570 | 1.14 |
| PPCM3 | 100:3 | 70 | 14577 | 1.27 |
| PPCM4 | 100:4 | 68 | 8530 | 1.64 |
| PPCM5 | 100:5 | 65 | 7335 | 1.86 |
| Sample | Spiked (M) | Found (M) | Recovery (%) |
|---|---|---|---|
| Tap water | 0.10 | 0.109 | 109.0 |
| 0.50 | 0.514 | 102.8 | |
| 1.0 | 0.963 | 96.3 | |
| River water | 0.10 | 0.104 | 104.0 |
| 0.50 | 0.508 | 101.6 | |
| 1.0 | 1.085 | 108.5 | |
| Waste water | 0.10 | 0.097 | 97.0 |
| 0.50 | 0.516 | 103.2 | |
| 1.0 | 0.942 | 94.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, W.-Z.; Zhang, Z.-P.; Du, C.-B.; Li, L.-L.; Zhao, C.; Li, H.-J.; Huang, Q. Fluorescent Carbon Dioxide-Based Polycarbonates Probe for Rapid Detection of Aniline in the Environment and Its Biomarkers in Urine. Polymers 2024, 16, 541. https://doi.org/10.3390/polym16040541
Liu Y, Wang W-Z, Zhang Z-P, Du C-B, Li L-L, Zhao C, Li H-J, Huang Q. Fluorescent Carbon Dioxide-Based Polycarbonates Probe for Rapid Detection of Aniline in the Environment and Its Biomarkers in Urine. Polymers. 2024; 16(4):541. https://doi.org/10.3390/polym16040541
Chicago/Turabian StyleLiu, Yun, Wen-Zhen Wang, Zhi-Ping Zhang, Chun-Bao Du, Lei-Lei Li, Chen Zhao, Hong-Jiu Li, and Qing Huang. 2024. "Fluorescent Carbon Dioxide-Based Polycarbonates Probe for Rapid Detection of Aniline in the Environment and Its Biomarkers in Urine" Polymers 16, no. 4: 541. https://doi.org/10.3390/polym16040541