Facile Synthesis of Self-Adhesion and Ion-Conducting 2-Acrylamido-2-Methylpropane Sulfonic Acid/Tannic Acid Hydrogels Using Electron Beam Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AMPS/TA Hydrogel by E-Beam Irradiation
2.3. Measurements
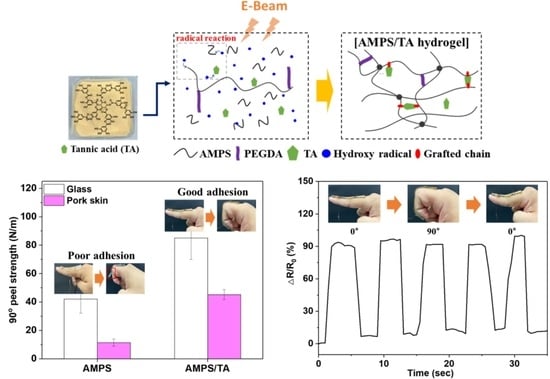
3. Results and Discussion
3.1. Characterization of AMPS/TA Hydrogels Prepared Using E-Beam Irradiation
3.2. Adhesion and Tensile Properties of AMPS/TA Hydrogels
3.3. Mechanical Properties of AMPS/TA Hydrogels
3.4. Ionic Conductivity and Swelling Properties of AMPS/TA Hydrogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Auriemma, M.; Piscitelli, A.; Pasquino, R.; Cerruti, P.; Malinconico, M.; Grizzuti, N. Blending poly(3-hydroxybutyrate) with tannic acid: Influence of a polyphenolic natural additive on the rheological and thermal behavior. Eur. Polym. J. 2015, 63, 123–131. [Google Scholar] [CrossRef]
- Jafari, H.; Ghaffari-Bohlouli, P.; Niknezhad, S.V.; Abedi, A.; Izadifar, Z.; Mohammadinejad, R.; Varma, R.S.; Shavandi, A. Tannic acid: A versatile polyphenol for design of biomedical hydrogels. J. Mater. Chem. B 2022, 10, 5873–5912. [Google Scholar] [CrossRef] [PubMed]
- Saveleva, M.S.; Ivanov, A.N.; Chibrikova, J.A.; Abalymov, A.A.; Surmeneva, M.A.; Surmenev, R.A.; Parakhonskiy, B.V.; Lomova, M.V.; Skirtach, A.G.; Norkin, I.A. Osteogenic Capability of Vaterite-Coated Nonwoven Polycaprolactone Scaffolds for In Vivo Bone Tissue Regeneration. Macromol. Biosci. 2021, 21, 2100266. [Google Scholar] [CrossRef] [PubMed]
- Shutava, T.; Prouty, M.; Kommireddy, D.; Lvov, Y. pH responsive decomposable layer-by-layer nanofilms and capsules on the basis of tannic acid. Macromolecules 2005, 38, 2850–2858. [Google Scholar] [CrossRef]
- Erel-Unal, I.; Sukhishvili, S.A. Hydrogen-bonded multilayers of a neutral polymer and a polyphenol. Macromolecules 2008, 41, 3962–3970. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Zhang, Q.; Jin, Z. Tannic acid-based multifunctional hydrogels with facile adjustable adhesion and cohesion contributed by polyphenol supramolecular chemistry. ACS Omega 2017, 2, 6668–6676. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Jin, Z. Tough, swelling-resistant, self-healing, and adhesive dual-cross-linked hydrogels based on polymer-tannic acid multiple hydrogen bonds. Macromolecules 2018, 51, 1696–1705. [Google Scholar] [CrossRef]
- Fan, H.; Wang, L.; Feng, X.; Bu, Y.; Wu, D.; Jin, Z. Supramolecular Hydrogel Formation Based on Tannic Acid. Macromolecules 2017, 50, 666–676. [Google Scholar] [CrossRef]
- Meng, Z.; He, Y.; Wang, F.; Hang, R.; Zhang, X.; Huang, X.; Yao, X. Enhancement of Antibacterial and Mechanical Properties of Photocurable ϵ-Poly-l-lysine Hydrogels by Tannic Acid Treatment. ACS Appl. Bio Mater. 2021, 4, 2713–2722. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, X.; Pan, M.; Yuan, J.; Jia, Z.; Zhu, L. A Robust, Tough and Multifunctional Polyurethane/Tannic Acid Hydrogel Fabricated by Physical-Chemical Dual Crosslinking. Polymers 2020, 12, 239. [Google Scholar] [CrossRef]
- Bakhtawara; Faizan, S.; Shah, L.A. Adhesion tuning of hydrogels via cross-linker for the junction of solid surfaces in dry and wet conditions. Surf. Interfaces 2022, 28, 101659. [Google Scholar] [CrossRef]
- Cui, C.; Zhang, S. Preparation, Characterization and Performance Evaluation of a Novel Scale Inhibiting and Dispersing Copolymer Containing Natural Tannin. J. Polym. Environ. 2020, 28, 1869–1879. [Google Scholar] [CrossRef]
- Seo, H.S.; Bae, J.Y.; Kwon, K.; Shin, S. Synthesis and Assessment of AMPS-Based Copolymers Prepared via Electron-Beam Irradiation for Ionic Conductive Hydrogels. Polymers 2022, 14, 2547. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Demeter, M.; Călina, I.; Scărișoreanu, A.; Micutz, M. E-Beam Cross-Linking of Complex Hydrogels Formulation: The Influence of Poly(Ethylene Oxide) Concentration on the Hydrogel Properties. Gels 2021, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Călina, I.; Demeter, M.; Scărișoreanu, A.; Sătulu, V.; Mitu, B. One Step e-Beam Radiation Cross-Linking of Quaternary Hydrogels Dressings Based on Chitosan-Poly(Vinyl-Pyrrolidone)-Poly(Ethylene Glycol)-Poly(Acrylic Acid). Int. J. Mol. Sci. 2020, 21, 9236. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; Okubayashi, S.; Elsisi, H.; Abouelenin, S. Electron beam irradiation treatment of textiles materials: A review. J. Polym. Res. 2022, 29, 117. [Google Scholar] [CrossRef]
- Chikh, L.; Girard, S.; Teyssie, D.; Fichet, O. Proton conducting PAMPS networks: From flexible to rigid materials. J. Appl. Polym. Sci. 2008, 107, 3672–3680. [Google Scholar] [CrossRef]
- Zhang, C.; Easteal, A.J. Study of poly(acrylamide-co-2-acrylamido-2-methylpropane sulfonic acid) hydrogels made using gamma radiation initiation. J. Appl. Polym. Sci. 2003, 89, 1322–1330. [Google Scholar] [CrossRef]
- Nizam El-Din, H.M. Surface coating on cotton fabrics of new multilayer formulations based on superabsorbent hydrogels synthesized by gamma radiation designed for diapers. J. Appl. Polym. Sci. 2012, 125, 180–186. [Google Scholar] [CrossRef]
- ASTM D638; Standard Test Method for Tensile Properties of Plastics. ASTM: West Conshohocken, PA, USA, 2014.
- David, T.R.; Austin, D.C.C. A technique for measuring the alternating current electrical conductivity of hydrogels. J. Br. Contact Lens 1995, 18, 115–118. [Google Scholar] [CrossRef]
- Wang, J.; Yu, X.; Wang, C.; Xiang, K.; Deng, M.; Yin, H. PAMPS/MMT composite hydrogel electrolyte for solid-state supercapacitors. J. Alloys Compd. 2017, 709, 596–601. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, W.; Fan, Q.; Ma, H.; Yin, Y.; Long, Y.; Guan, J. Polyphenol-Mediated Synthesis of Superparamagnetic Magnetite Nanoclusters for Highly Stable Magnetically Responsive Photonic Crystals. Adv. Funct. Mater. 2023, 33, 2303470. [Google Scholar] [CrossRef]
- Kord Forooshani, P.; Lee, B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 9–33. [Google Scholar] [CrossRef]
- Schmidt, G.; Smith, K.H.; Miles, L.J.; Gettelfinger, C.K.; Hawthorne, J.A.; Fruzyna, E.C.; Wilker, J.J. Tunable Tannic Acid–Zein Adhesives for Bonding Different Substrates. Adv. Sustain. Syst. 2022, 6, 2100392. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Liu, J.; Liu, R.; Luo, J. Synthesis of acrylated tannic acid as bio-based adhesion promoter in UV-curable coating with improved corrosion resistance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 644, 128834. [Google Scholar] [CrossRef]
- Rao, K.M.; Uthappa, U.T.; Kim, H.J.; Han, S.S. Tissue Adhesive, Biocompatible, Antioxidant, and Antibacterial Hydrogels Based on Tannic Acid and Fungal-Derived Carboxymethyl Chitosan for Wound-Dressing Applications. Gels 2023, 9, 354. [Google Scholar] [CrossRef]
- Jin, S.; Kim, Y.; Son, D.; Shin, M. Tissue Adhesive, Conductive, and Injectable Cellulose Hydrogel Ink for On-Skin Direct Writing of Electronics. Gels 2022, 8, 336. [Google Scholar] [CrossRef]
- Cui, C.; Shao, C.; Meng, L.; Yang, J. High-Strength, Self-Adhesive, and Strain-Sensitive Chitosan/Poly(acrylic acid) Double-Network Nanocomposite Hydrogels Fabricated by Salt-Soaking Strategy for Flexible Sensors. ACS Appl. Mater. Interfaces 2019, 11, 39228–39237. [Google Scholar] [CrossRef]
- Shao, C.; Wang, M.; Meng, L.; Chang, H.; Wang, B.; Xu, F.; Yang, J.; Wan, P. Mussel-Inspired Cellulose Nanocomposite Tough Hydrogels with Synergistic Self-Healing, Adhesive, and Strain-Sensitive Properties. Chem. Mater. 2018, 30, 3110–3121. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Wang, Z.; Zhu, X.; Liu, J.; Min, X.; Cao, T.; Fan, X. Smart Composite Hydrogels with pH-Responsiveness and Electrical Conductivity for Flexible Sensors and Logic Gates. Polymers 2019, 11, 1564. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Gao, Z.; Li, X.; Gao, G. An amylopectin-enabled skin-mounted hydrogel wearable sensor. J. Mater. Chem. B 2021, 9, 1082–1088. [Google Scholar] [CrossRef] [PubMed]











| Sample Code | AMPS Salt Solution (g) | H2O (g) | TA | PEGDA | ||
|---|---|---|---|---|---|---|
| (g) | (phm *) | (g) | (phm *) | |||
| TA0 | 80 (40/40) | 20 | - | - | 0.08 | 0.2 |
| TA1 | 20.1 | 0.1 | 0.25 | |||
| TA3 | 20.3 | 0.3 | 0.75 | |||
| TA5 | 20.5 | 0.5 | 1.25 | |||
| TA7 | 20.7 | 0.7 | 1.75 | |||
| TA9 | 20.9 | 0.9 | 2.25 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-W.; Jang, N.-G.; Seo, H.-S.; Kwon, K.; Shin, S. Facile Synthesis of Self-Adhesion and Ion-Conducting 2-Acrylamido-2-Methylpropane Sulfonic Acid/Tannic Acid Hydrogels Using Electron Beam Irradiation. Polymers 2023, 15, 3836. https://doi.org/10.3390/polym15183836
Park H-W, Jang N-G, Seo H-S, Kwon K, Shin S. Facile Synthesis of Self-Adhesion and Ion-Conducting 2-Acrylamido-2-Methylpropane Sulfonic Acid/Tannic Acid Hydrogels Using Electron Beam Irradiation. Polymers. 2023; 15(18):3836. https://doi.org/10.3390/polym15183836
Chicago/Turabian StylePark, Hee-Woong, Nam-Gyu Jang, Hyun-Su Seo, Kiok Kwon, and Seunghan Shin. 2023. "Facile Synthesis of Self-Adhesion and Ion-Conducting 2-Acrylamido-2-Methylpropane Sulfonic Acid/Tannic Acid Hydrogels Using Electron Beam Irradiation" Polymers 15, no. 18: 3836. https://doi.org/10.3390/polym15183836