Multi-Armed Star-Shaped Block Copolymers of Poly(ethylene glycol)-Poly(furfuryl glycidol) as Long Circulating Nanocarriers
Abstract
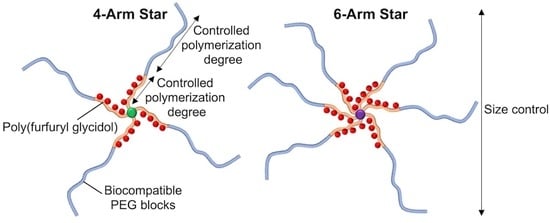
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Polymerization of the 4-Arm PFG-PEG
2.4. Polymerization of the 6-Arm PFG-PEG
2.5. Introduction of a Model Drug via a Diels–Alder Reaction
2.6. Release of Model Drugs by a Retro Diels–Alder Reaction
2.7. Preparation of Fluorescence-Labeled Polymers
2.8. In Vitro Cellular Uptake
2.9. Blood Circulation
3. Results and Discussion
3.1. Synthesis and Characterization of the 4/6-Arm PFG-PEG
3.2. Hydrodynamic Radius Measurement of the 4/6-Arm PFG-PEG
3.3. Time-Dependent Loading and the Release of a Maleimide-Bearing Model Drug
3.4. Loading of Maleimide-Bearing Model Drugs
3.5. Cellular Uptake Evaluation
3.6. Blood Circulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Askarizadeh, A.; Butler, A.E.; Badiee ASahebkar, A. Liposomal nanocarriers for statins: A pharmacokinetic and pharmacodynamics appraisal. J. Cell. Physiol. 2019, 234, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Takenaka, T.; Toh, K.; Wu, S.; Nishihara, H.; Kano, M.R.; Ino, Y.; Nomoto, T.; Matsumoto, Y.; Koyama, H.; et al. Cyclic RGD-Linked Polymeric Micelles for Targeted Delivery of Platinum Anticancer Drugs to Glioblastoma through the Blood–Brain Tumor Barrier. ACS Nano 2013, 7, 8583–8592. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Trzcinska, R.; Trzebicka, B.; Müller, A.H.; Dworak, A.; Tsvetanov, C.B. Loading of polymer nanocarriers: Factors, mechanisms and applications. Prog. Polym. Sci. 2014, 39, 43–86. [Google Scholar] [CrossRef]
- Yoneoka, S.; Park, K.C.; Nakagawa, Y.; Ebara, M.; Tsukahara, T. Synthesis and Evaluation of Thermoresponsive Boron-Containing Poly(N-isopropylacrylamide) Diblock Copolymers for Self-Assembling Nanomicellar Boron Carriers. Polymers 2019, 11, 42. [Google Scholar] [CrossRef] [Green Version]
- Mai, W.X.; Meng, H. Mesoporous silica nanoparticles: A multifunctional nano therapeutic system. Integr. Biol. 2013, 5, 19–28. [Google Scholar] [CrossRef]
- Rappaport, J.; Hanss, B.; Kopp, J.B.; Copeland, T.D.; Bruggeman, L.A.; Coffman, T.M.; Klotman, P.E. Transport of phosphorothioate oligonucleotides in kidney: Implications for molecular therapy. Kidney Int. 1995, 47, 1462–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef]
- Gupta, U.; Agashe, H.B.; Asthana AJain, N.K. Dendrimers: Novel Polymeric Nanoarchitectures for Solubility Enhancement. Biomacromolecules 2006, 7, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Siegwart, D.J.; Lee, H.I.; Sherwood, G.; Peteanu, L.; Hollinger, J.O.; Kataoka, K.; Matyjaszewski, K. Biodegradable Nanogels Prepared by Atom Transfer Radical Polymerization as Potential Drug Delivery Carriers: Synthesis, Biodegradation, in Vitro Release, and Bioconjugation. J. Am. Chem. Soc. 2007, 129, 5939–5945. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.M.; McKenzie, T.G.; Fu, Q.; Wong, E.H.; Xu, J.; An, Z.; Shanmugam, S.; Davis, T.P.; Boyer, C.; Qiao, G.G. Star Polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, E.M.; Tiboni MSoliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada KKataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [Green Version]
- Matyjaszewski, K.; Miller, P.J.; Pyun, J.; Kickelbick, G.; Diamanti, S. Synthesis and Characterization of Star Polymers with Varying Arm Number, Length, and Composition from Organic and Hybrid Inorganic/Organic Multifunctional Initiators. Macromolecules 1999, 32, 6526–6535. [Google Scholar] [CrossRef]
- Rodrigues PRVieira, R.P. Advances in atom-transfer radical polymerization for drug delivery applications. Eur. Polym. J. 2019, 115, 45–58. [Google Scholar] [CrossRef]
- Inoue, K. Functional dendrimers, hyperbranched and star polymers. Prog. Polym. Sci. 2000, 25, 453–571. [Google Scholar] [CrossRef]
- Vlassopoulos, D.; Fytas, G.; Pakula TRoovers, J. Multiarm star polymers dynamics. J. Phys. Condens. Matter 2001, 13, R855. [Google Scholar] [CrossRef]
- Gevrek, T.N.; Sanyal, A. Furan-containing polymeric Materials: Harnessing the Diels-Alder chemistry for biomedical applications. Eur. Polym. J. 2021, 153, 110514. [Google Scholar] [CrossRef]
- Fujisawa, N.; Takanohashi, M.; Chen, L.; Uto, K.; Matsumoto, Y.; Takeuchi, M.; Ebara, M. A Diels-Alder polymer platform for thermally enhanced drug release toward efficient local cancer chemotherapy. Sci. Technol. Adv. Mater. 2021, 22, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Bi, B.; Ma, M.; Lv, S.; Zhuo, R.; Jiang, X. In-situ forming thermosensitive hydroxypropyl chitin-based hydrogel crosslinked by Diels-Alder reaction for three dimensional cell culture. Carbohydr. Polym. 2019, 212, 368–377. [Google Scholar] [CrossRef] [PubMed]
- St Amant, A.H.; Lemen, D.; Florinas, S.; Mao, S.; Fazenbaker, C.; Zhong, H.; Wu, H.; Gao, C.; Christie, R.J.; Read de Alaniz, J. Tuning the Diels–Alder Reaction for Bioconjugation to Maleimide Drug-Linkers. Bioconjug. Chem. 2018, 29, 2406–2414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimm, B.; HStockmayer, W.H. The Dimensions of Chain Molecules Containing Branches and Rings. J. Chem. Phys. 1949, 17, 1301–1314. [Google Scholar] [CrossRef]
- Morton, M.; Helminiak, T.E.; Gadkary, S.D.; Bueche, F. Preparation and properties of monodisperse branched polystyrene. J. Polym. Sci. 1962, 57, 471–482. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Roovers, J.E.L. Synthesis and solution properties of linear, four-branched, and six-branched star polyisoprenes. J. Polym. Sci. Polym. Phys. Ed. 1974, 12, 2521–2533. [Google Scholar] [CrossRef]
- Zhou, L.L.; Hadjichristidis, N.; Toporowski, P.M.; Roovers, J. Synthesis and Properties of Regular Star Polybutadienes with 32 Arms. Rubber Chem. Technol. 1992, 65, 303–314. [Google Scholar] [CrossRef]
- Burchard, W. Static and Dynamic Light Scattering from Branched Polymers and Biopolymers. In Light Scattering from Polymers; Springer: Berlin/Heidelberg, Germany, 1983; pp. 1–124. [Google Scholar]
- Schmidt, M.; Burchard, W. Translational diffusion and hydrodynamic radius of unperturbed flexible chains. Macromolecules 1981, 14, 210–211. [Google Scholar] [CrossRef]
- Roovers, J.; Zhou, L.L.; Toporowski, P.M.; van der Zwan, M.; Iatrou, H.; Hadjichristidis, N. Regular star polymers with 64 and 128 arms. Models for polymeric micelles. Macromolecules 1993, 26, 4324–4331. [Google Scholar] [CrossRef]
- Brace, C.L. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: What are the differences? Curr. Probl. Diagn. Radiol. 2009, 38, 135–143. [Google Scholar] [CrossRef] [Green Version]






| Sample | Initiator (μmol) | Solvent (mL) | P4-t-Bu (μmol) | FuGE (mmol) | |
|---|---|---|---|---|---|
| TTP-PFG10 | 40 | DMF | (2.5) | 160 | 1.6 |
| TTP-PFG20 | 40 | DMF | (5) | 160 | 3.2 |
| TTP-PFG50 | 40 | DMF | (10) | 160 | 6.4 |
| INO-PFG10 | 33 | DMSO | (3) | 200 | 2.0 |
| Sample | Macro Initiator (μmol) | Solvent (mL) | P4-t-Bu (μmol) | EO (mmol) | ||
|---|---|---|---|---|---|---|
| TTP-PFG10-PEG150 | TTP10 | 40 | DMF | (5) | 160 | 9.60 |
| TTP-PFG10-PEG500 | DMF | (10) | 160 | 19.2 | ||
| TTP-PFG10-PEG1000 | DMF | (20) | 160 | 38.4 | ||
| TTP-PFG20-PEG70 | TTP20 | 40 | DMF | (3.5) | 160 | 6.4 |
| TTP-PFG20-PEG90 | DMF | (7) | 160 | 12.8 | ||
| TTP-PFG20-PEG430 | DMF | (10) | 160 | 25.6 | ||
| TTP-PFG50-PEG60 | TTP50 | 40 | DMF | (3.5) | 160 | 6.40 |
| TTP-PFG50-PEG150 | DMF | (7) | 160 | 12.8 | ||
| INO-PFG10-PEG60 | INO10 | 33 | DMSO | (6) | 200 | 12 |
| INO-PFG10-PEG120 | DMSO | (13) | 200 | 24 | ||
| Sample | Aimed DP | Evaluated DP a | Mw b | |||
|---|---|---|---|---|---|---|
| PFG | PEG | PFG | PEG | PFG (kDa) | PFG-PEG (kDa) | |
| TTP-PFG10-PEG150 | 10 | 150 | 10.9 | 154 | 7.11 | 34.3 |
| TTP-PFG10-PEG500 | 500 | 539 | 102 | |||
| TTP-PFG10-PEG1000 | 1000 | 1060 | 194 | |||
| TTP-PFG20-PEG70 | 20 | 70 | 24.2 | 71.2 | 15.3 | 27.9 |
| TTP-PFG20-PEG90 | 90 | 93 | 31.7 | |||
| TTP-PFG20-PEG430 | 430 | 430 | 91.1 | |||
| TTP-PFG50-PEG60 | 40 | 60 | 51.1 | 61.5 | 31.9 | 42.7 |
| TTP-PFG50-PEG150 | 150 | 151 | 58.5 | |||
| INO-PFG10-PEG60 | 10 | 60 | 13.7 | 66 | 12.9 | 30.3 |
| INO-PFG10-PEG120 | 120 | 122 | 45.1 | |||
| Sample | Average Hydrodynamic Radius in Water ± S.D. (nm) a | PDI | Average Hydrodynamic Radius in DMF ± S.D. (nm) b | PDI | Rh (nm) c |
|---|---|---|---|---|---|
| TTP-PFG10-PEG150 | 31 ± 1 | 0.104 | - | 5.33 | |
| TTP-PFG10-PEG500 | 30 ± 1 | 0.251 | - | 9.52 | |
| TTP-PFG10-PEG1000 | 23 ± 3 | 0.288 | - | 12.4 | |
| TTP-PFG20-PEG70 | 76 ± 4 | 0.236 | 5 ± 1 | 0.070 | 4.00 |
| TTP-PFG20-PEG90 | 52 ± 7 | 0.411 | 4 ± 1 | 0.069 | 4.42 |
| TTP-PFG20-PEG430 | 42 ± 1 | 0.213 | 5 ± 1 | 0.226 | 8.94 |
| TTP-PFG50-PEG60 | 85 ± 1 | 0.146 | 5 ± 1 | 0.229 | 4.42 |
| TTP-PFG50-PEG150 | 50 ± 2 | 0.179 | 5 ± 1 | 0.301 | 5.50 |
| INO-PFG10-PEG60 | 23 ± 1 | 0.295 | 4 ± 1 | 0.451 | 3.64 |
| INO-PFG10-PEG120 | 22 ± 1 | 0.371 | 6 ± 1 | 0.856 | 4.96 |
| Sample | No. of Furfuryl Units | Introduction No. a | Introduction Ratio a | ||
|---|---|---|---|---|---|
| pMal | MalC | pMal (%) | MalC (%) | ||
| TTP-PFG50-PEG60 | 204 | 137 | 196 | 67.2 | 96.1 |
| INO-PFG10-PEG120 | 82 | 71 | 71 | 86.6 | 86.6 |
| Sample | Initial Radius in Water (nm) | Radius after pMal in Water (nm) | Radius after MalC in Water (nm) | After MalC in PBS at pH 8 (nm) |
|---|---|---|---|---|
| TTP-PFG50-PEG60 | 85 | 130 | 96 | 2.28 |
| INO-PFG10-PEG120 | 22 | 27 | 25 | 4.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagawa, Y.; Ushidome, K.; Masuda, K.; Igarashi, K.; Matsumoto, Y.; Yamasoba, T.; Anraku, Y.; Takai, M.; Cabral, H. Multi-Armed Star-Shaped Block Copolymers of Poly(ethylene glycol)-Poly(furfuryl glycidol) as Long Circulating Nanocarriers. Polymers 2023, 15, 2626. https://doi.org/10.3390/polym15122626
Nakagawa Y, Ushidome K, Masuda K, Igarashi K, Matsumoto Y, Yamasoba T, Anraku Y, Takai M, Cabral H. Multi-Armed Star-Shaped Block Copolymers of Poly(ethylene glycol)-Poly(furfuryl glycidol) as Long Circulating Nanocarriers. Polymers. 2023; 15(12):2626. https://doi.org/10.3390/polym15122626
Chicago/Turabian StyleNakagawa, Yasuhiro, Kotaro Ushidome, Keita Masuda, Kazunori Igarashi, Yu Matsumoto, Tatsuya Yamasoba, Yasutaka Anraku, Madoka Takai, and Horacio Cabral. 2023. "Multi-Armed Star-Shaped Block Copolymers of Poly(ethylene glycol)-Poly(furfuryl glycidol) as Long Circulating Nanocarriers" Polymers 15, no. 12: 2626. https://doi.org/10.3390/polym15122626