Anti-Fouling and Anti-Biofilm Performance of Self-Polishing Waterborne Polyurethane with Gemini Quaternary Ammonium Salts
Abstract
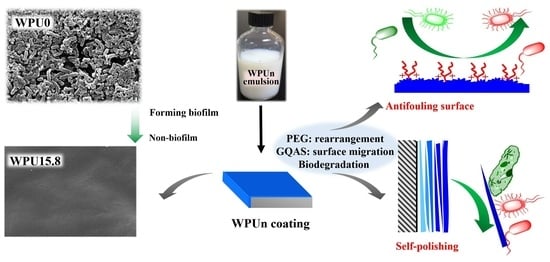
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation WPUn Emulsions and Coatings
2.3. Degradation Assay
2.4. Anti-Fouling Behavior
2.5. Cytotoxicity Assay
2.6. Statistical Analysis
2.7. Characterization
3. Results and Discussion
3.1. Preparation and Characterization of WPUn Emulsions
3.2. Degradation Properties of WPUn
3.3. Anti-Fouling Efficacy
3.3.1. MIC Studies of WPUn Emulsions
3.3.2. Hydrophilic Analysis of WPUn Films
3.3.3. Protein Adsorption Assay
3.3.4. Contact-Active Antibacterial Activity of WPUn Films
3.3.5. Anti-Biofilm Assay
3.4. Cytotoxicity of WPUn Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rzhepishevska, O.; Hakobyan, S.; Ruhal, R.; Gautrot, J.; Barbero, D.; Ramstedt, M. The surface charge of anti-bacterial coatings alters motility and biofilm architecture. Biomater. Sci. 2013, 1, 589–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Liu, T.; Cheng, Y.F.; Guo, N.; Yin, Y. Adhesion of Bacillus subtilis and Pseudoalteromonas lipolytica to steel in a seawater environment and their effects on corrosion. Colloids Surfaces B Biointerfaces 2017, 157, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Schwindt, E.; Gappa, J.L.; Raffo, M.; Tatián, M.; Bortolus, A.; Orensanz, J.; Alonso, G.; Diez, M.; Doti, B.; Genzano, G.; et al. Marine fouling invasions in ports of Patagonia (Argentina) with implications for legislation and monitoring programs. Mar. Environ. Res. 2014, 99, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Fu, X.; Zhang, Y.; Liu, Z.; Jiang, L.; Lei, J. Preparation of waterborne polyurethanes based on the organic solvent-free process. Green Chem. 2016, 18, 412–416. [Google Scholar] [CrossRef]
- Ma, L.; Song, L.; Wang, H.; Fan, L.; Liu, B. Synthesis and characterization of poly(propylene carbonate) glycol-based waterborne polyurethane with a high solid content. Prog. Org. Coat. 2018, 122, 38–44. [Google Scholar] [CrossRef]
- Yang, R.; Zheng, Y.; Shuai, X.; Fan, F.; He, X.; Ding, M.; Li, J.; Tan, H.; Fu, Q. Crosslinking Induced Reassembly of Multiblock Polymers: Addressing the Dilemma of Stability and Responsivity. Adv. Sci. 2020, 7, 1902701. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Shuai, X.; Wang, R.; He, X.; Li, Y.; Ding, M.; Li, J.; Tan, H.; Fu, Q. Clickable and imageable multiblock polymer micelles with magnetically guided and PEG-switched targeting and release property for precise tumor theranosis. Biomaterials 2017, 145, 138–153. [Google Scholar] [CrossRef]
- Xie, Q.; Ma, C.; Liu, C.; Ma, J.; Zhang, G. Poly(dimethylsiloxane)-Based Polyurethane with Chemically Attached Antifoulants for Durable Marine Antibiofouling. ACS Appl. Mater. Interfaces 2015, 7, 21030–21037. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, W.; Zhang, G.; Qian, P.-Y. Environmentally Friendly Antifouling Coatings Based on Biodegradable Polymer and Natural Antifoulant. ACS Sustain. Chem. Eng. 2017, 5, 6304–6309. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Q.; Zhao, J.; Gong, X.; Schlaad, H.; Zhang, G. Betulin-Constituted Multiblock Amphiphiles for Broad-Spectrum Protein Resistance. ACS Appl. Mater. Interfaces 2018, 10, 6593–6600. [Google Scholar] [CrossRef]
- Yuan, P.; Qiu, X.; Wang, X.; Tian, R.; Wang, L.; Bai, Y.; Liu, S.; Chen, X. Substrate-Independent Coating with Persistent and Stable Antifouling and Antibacterial Activities to Reduce Bacterial Infection for Various Implants. Adv. Healthc. Mater. 2019, 8, e1801423. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Xue, H.; Zhang, Z.; Chen, S.; Jiang, S. A Switchable Biocompatible Polymer Surface with Self-Sterilizing and Nonfouling Capabilities. Angew. Chem. Int. Ed. 2008, 47, 8831–8834. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wang, Y.; Gnutt, P.; Wanka, R.; Krause, L.M.K.; Finlay, J.A.; Clare, A.S.; Rosenhahn, A. Layer-by-Layer Deposited Hybrid Polymer Coatings Based on Polysaccharides and Zwitterionic Silanes with Marine Antifouling Properties. ACS Appl. Bio Mater. 2021, 4, 2385–2397. [Google Scholar] [CrossRef]
- Wanka, R.; Koschitzki, F.; Puzovic, V.; Pahl, T.; Manderfeld, E.; Hunsucker, K.Z.; Swain, G.W.; Rosenhahn, A. Synthesis and Characterization of Dendritic and Linear Glycol Methacrylates and Their Performance as Marine Antifouling Coatings. ACS Appl. Mater. Interfaces 2021, 13, 6659–6669. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Liang, X.; Yang, J.; Zhou, S. Sol–gel-derived hard coatings from tetraethoxysilane and organoalkoxysilanes bearing zwitterionic and isothiazolinone groups and their antifouling behaviors. J. Mater. Chem. B 2022, 10, 406–417. [Google Scholar] [CrossRef]
- He, B.; Du, Y.; Wang, B.; Zhao, X.; Liu, S.; Ye, Q.; Zhou, F. Self-healing polydimethylsiloxane antifouling coatings based on zwitterionic polyethylenimine-functionalized gallium nanodroplets. Chem. Eng. J. 2022, 427, 131019. [Google Scholar] [CrossRef]
- Buskens, P.; Wouters, M.; Rentrop, C.; Vroon, Z. A brief review of environmentally benign antifouling and foul-release coatings for marine applications. J. Coat. Technol. Res. 2013, 10, 29–36. [Google Scholar] [CrossRef]
- An, Y.; Friedman, R. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 1998, 43, 338–348. [Google Scholar] [CrossRef]
- Genzer, J.; Efimenko, K. Recent developments in superhydrophobic surfaces and their relevance to marine fouling: A review. Biofouling 2006, 22, 339–360. [Google Scholar] [CrossRef]
- Hao, X.; Wang, W.; Yang, Z.; Yue, L.; Sun, H.; Wang, H.; Guo, Z.; Cheng, F.; Chen, S. pH responsive antifouling and antibacterial multilayer films with Self-healing performance. Chem. Eng. J. 2019, 356, 130–141. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, J.; Kim, J.; Jang, Y.; Shin, K.; Ha, E.; Ryu, S.; Kim, B.-G.; Wooh, S.; Char, K. Development of Multimodal Antibacterial Surfaces Using Porous Amine-Reactive Films Incorporating Lubricant and Silver Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 6550–6560. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Yan, W.; Yang, J.; Bai, Y.; Qian, H.; Lou, Y.; Ju, P.; Zhang, D. Matrine@chitosan-D-proline nanocapsules as antifouling agents with antibacterial properties and biofilm dispersibility in the marine environment. Front. Microbiol. 2022, 13, 950039. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jian, R.; Yang, K.; Bai, W.; Huang, C.; Lin, Y.; Zheng, B.; Wei, F.; Lin, Q.; Xu, Y. Urushiol-based benzoxazine copper polymer with low surface energy, strong substrate adhesion and antibacterial for marine antifouling application. J. Clean. Prod. 2021, 318, 128527. [Google Scholar] [CrossRef]
- Yang, W.J.; Neoh, K.-G.; Kang, E.-T.; Teo, S.L.-M.; Rittschof, D. Polymer brush coatings for combating marine biofouling. Prog. Polym. Sci. 2014, 39, 1017–1042. [Google Scholar] [CrossRef]
- Ding, X.; Yang, C.; Lim, T.P.; Hsu, L.Y.; Engler, A.C.; Hedrick, J.L.; Yang, Y.-Y. Antibacterial and antifouling catheter coatings using surface grafted PEG-b-cationic polycarbonate diblock copolymers. Biomaterials 2012, 33, 6593–6603. [Google Scholar] [CrossRef]
- Rauner, N.; Mueller, C.; Ring, S.; Boehle, S.; Strassburg, A.; Schoeneweiss, C.; Wasner, M.; Tiller, J.C. A Coating that Combines Lotus-Effect and Contact-Active Antimicrobial Properties on Silicone. Adv. Funct. Mater. 2018, 28, 1801248. [Google Scholar] [CrossRef]
- Manouras, T.; Koufakis, E.; Vasilaki, E.; Peraki, I.; Vamvakaki, M. Antimicrobial Hybrid Coatings Combining Enhanced Biocidal Activity under Visible-Light Irradiation with Stimuli-Renewable Properties. ACS Appl. Mater. Interfaces 2021, 13, 17183–17195. [Google Scholar] [CrossRef] [PubMed]
- Hympanova, M.; Terlep, S.; Markova, A.; Prchal, L.; Dogsa, I.; Pulkrabkova, L.; Benkova, M.; Marek, J.; Stopar, D. The Antibacterial Effects of New N-Alkylpyridinium Salts on Planktonic and Biofilm Bacteria. Front. Microbiol. 2020, 11, 573951. [Google Scholar] [CrossRef]
- Li, P.; Poon, Y.F.; Li, W.; Zhu, H.-Y.; Yeap, S.H.; Cao, Y.; Qi, X.; Zhou, C.; Lamrani, M.; Beuerman, R.W.; et al. A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat. Mater. 2010, 10, 149–156. [Google Scholar] [CrossRef]
- Ding, M.; He, X.; Wang, Z.; Li, J.; Tan, H.; Deng, H.; Fu, Q.; Gu, Q. Cellular uptake of polyurethane nanocarriers mediated by gemini quaternary ammonium. Biomaterials 2011, 32, 9515–9524. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Li, J.; Gao, Y.; Tan, H.; Wang, K.; Li, J.; Fu, Q. Synthesis and antibacterial characterization of waterborne polyurethanes with gemini quaternary ammonium salt. Sci. Bull. 2015, 60, 1114–1121. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; He, W.; Li, J.; Wang, K.; Li, J.; Tan, H.; Fu, Q. Gemini quaternary ammonium salt waterborne biodegradable polyurethanes with antibacterial and biocompatible properties. Mater. Chem. Front. 2017, 1, 361–368. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Ding, M.; He, W.; Li, J.; Li, J.; Tan, H. Antibacterial and Biocompatible Cross-Linked Waterborne Polyurethanes Containing Gemini Quaternary Ammonium Salts. Biomacromolecules 2018, 19, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, J.; Sun, L.; Kong, F.; Tang, J.; Zhao, X.; Tang, Y.; Zuo, Y. Comparative Study on the Degradation of Two Self-Polishing Antifouling Coating Systems with Copper-Based Antifouling Agents. Coatings 2022, 12, 1156. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Zhang, C.; Feng, H.Y.; Yu, B.; Yang, W.; Pei, X.; Zhou, F. Self-lubricating interpenetrating polymer networks with functionalized nanoparticles enhancement for quasi-static and dynamic antifouling. Chem. Eng. J. 2022, 429, 132300. [Google Scholar] [CrossRef]
- Song, F.; Wang, J.; Zhang, L.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Liu, P.; Duan, J. Synergistically Improved Antifouling Efficiency of a Bioinspired Self-renewing Interface via a Borneol/Boron Acrylate Polymer. J. Colloid Interface Sci. 2022, 612, 459–466. [Google Scholar] [CrossRef]
- Dai, G.; Xie, Q.; Ma, C.; Zhang, G. Biodegradable Poly(ester-co-acrylate) with Antifoulant Pendant Groups for Marine Anti-Biofouling. ACS Appl. Mater. Interfaces 2019, 11, 11947–11953. [Google Scholar] [CrossRef]
- Xu, W.; Ma, C.; Ma, J.; Gan, T.; Zhang, G. Marine Biofouling Resistance of Polyurethane with Biodegradation and Hydrolyzation. ACS Appl. Mater. Interfaces 2014, 6, 4017–4024. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Han, S.; Han, J.; Jiang, D. Poly(propylene carbonate) polyurethane self-polishing coating for marine antifouling application. J. Appl. Polym. Sci. 2016, 133, 43667. [Google Scholar] [CrossRef]
- Ma, C.; Xu, L.; Xu, W.; Zhang, G. Degradable polyurethane for marine anti-biofouling. J. Mater. Chem. B 2013, 1, 3099–3106. [Google Scholar] [CrossRef]
- Porter, J.R.; Henson, A.; Popat, K.C. Biodegradable poly(ε-caprolactone) nanowires for bone tissue engineering applications. Biomaterials 2009, 30, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wu, J.; Mather, P.T. Polyhedral Oligomeric Silsesquioxane (POSS) Suppresses Enzymatic Degradation of PCL-Based Polyurethanes. Biomacromolecules 2011, 12, 3066–3077. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Ling, Q.; Zhao, W.; Ma, Y.; Bai, P.; Wei, Q.; Li, H.; Zhao, C. Modification of polyethersulfone membrane by grafting bovine serum albumin on the surface of polyethersulfone/poly(acrylonitrile-co-acrylic acid) blended membrane. J. Membr. Sci. 2009, 329, 46–55. [Google Scholar] [CrossRef]
- Bakhshi, H.; Yeganeh, H.; Mehdipour-Ataei, S.; Shokrgozar, M.A.; Yari, A.; Saeedi-Eslami, S.N. Synthesis and characterization of antibacterial polyurethane coatings from quaternary ammonium salts functionalized soybean oil based polyols. Mater. Sci. Eng. C 2013, 33, 153–164. [Google Scholar] [CrossRef]
- Kolter, L.A.P.a.R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 1998, 30, 285–293. [Google Scholar]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Melchior, M.; Fink-Gremmels, J.; Gaastra, W. Comparative assessment of the antimicrobial susceptibility of Staphylococcus aureus isolates from bovine mastitis in biofilm versus planktonic culture. J. Vet. Med. Ser. B 2006, 53, 326–332. [Google Scholar] [CrossRef]
- Smułek, W.; Siejak, P.; Fathordoobady, F.; Masewicz, Ł.; Guo, Y.; Jarzębska, M.; Kitts, D.; Kowalczewski, P.; Baranowska, H.; Stangierski, J.; et al. Whey Proteins as a Potential Co-Surfactant with Aesculus hippocastanum L. as a Stabilizer in Nanoemulsions Derived from Hempseed Oil. Molecules 2021, 26, 5856. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, F.; Zhao, J.; Wang, Z.; Wang, R.; Zheng, Y.; Liu, H.; Peng, C.; Li, J.; Tan, H.; et al. Intrinsically fluorescent polyureas toward conformation-assisted metamorphosis, discoloration and intracellular drug delivery. Nat. Commun. 2022, 13, 4551. [Google Scholar] [CrossRef]
- Ding, M.; Li, J.; Fu, X.; Zhou, J.; Tan, H.; Gu, Q.; Fu, Q. Synthesis, Degradation, and Cytotoxicity of Multiblock Poly(ε-caprolactone urethane)s Containing Gemini Quaternary Ammonium Cationic Groups. Biomacromolecules 2009, 10, 2857–2865. [Google Scholar] [CrossRef]
- Song, N.-J.; Jiang, X.; Li, J.-H.; Pang, Y.; Li, J.; Tan, H.; Fu, Q. The degradation and biocompatibility of waterborne biodegradable polyurethanes for tissue engineering. Chin. J. Polym. Sci. 2013, 31, 1451–1462. [Google Scholar] [CrossRef]
- Xie, Q.; Pan, J.; Ma, C.; Zhang, G. Dynamic surface antifouling: Mechanism and systems. Soft Matter 2019, 15, 1087–1107. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W. Lipoteichoic acid and lipids in the membrane of Staphylococcus aureus. Med. Microbiol. Immunol. 1994, 183, 61–76. [Google Scholar] [CrossRef]
- Beveridge, T.J. Structures of Gram-Negative Cell Walls and Their Derived Membrane Vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, W.; Deng, H.; Zhang, W.; Kang, J.; Zhang, C. Enhanced Mechanical Properties and Functional Performances of Cationic Waterborne Polyurethanes Enabled by Different Natural Phenolic Acids. ACS Sustain. Chem. Eng. 2020, 8, 17447–17457. [Google Scholar] [CrossRef]








| Samples | Molar Ratio of IPDI/PEG/PCL/EG12/Lysine | Dosage of EG12 (wt%) | Mn (g/mol) | Mw/Mn | Zeta Potential (mV) | Tg (°C) |
|---|---|---|---|---|---|---|
| WPU0 | 2.05:0.33:0.67:0:1 | 0 | 62,419 | 1.41 | −4.92 ± 0.38 | −55.27 |
| WPU8.5 | 2.05:0.33:0.67:0.25:0.75 | 8.5 | 93,688 | 2.09 | 1.07 ± 0.49 | −55.52 |
| WPU15.8 | 2.05:0.33:0.67:0.5:0.5 | 15.8 | 55,265 | 1.53 | 2.89 ± 0.79 | −55.34 |
| WPU22.3 | 2.05:0.33:0.67:0.75:0.25 | 22.3 | 39,613 | 1.40 | 3.68 ± 0.36 | −55.39 |
| WPU30 | 2.05:0.33:0.67:1:0 | 30.0 | 39,251 | 1.53 | 5.40 ± 0.88 | −55.12 |
| Samples | E. coli | S. aureus |
|---|---|---|
| WPU0 | >8000 (0 *) | >8000 (0 *) |
| WPU8.5 | 197.65 (16.8 *) | 98.85 (8.4 *) |
| WPU15.8 | 91.40 (14.4 *) | 45.7 (7.2 *) |
| WPU22.3 | 47.65 (10.6 *) | 23.85 (5.3 *) |
| WPU30 | 44.90 (12.6 *) | 11.25 (3.2 *) |
| EG12 | 16.00 | 4.00 |
| DTAB | 64.00 | 16.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ge, T.; Li, Y.; Lu, J.; Du, H.; Yan, L.; Tan, H.; Li, J.; Yin, Y. Anti-Fouling and Anti-Biofilm Performance of Self-Polishing Waterborne Polyurethane with Gemini Quaternary Ammonium Salts. Polymers 2023, 15, 317. https://doi.org/10.3390/polym15020317
Zhang Y, Ge T, Li Y, Lu J, Du H, Yan L, Tan H, Li J, Yin Y. Anti-Fouling and Anti-Biofilm Performance of Self-Polishing Waterborne Polyurethane with Gemini Quaternary Ammonium Salts. Polymers. 2023; 15(2):317. https://doi.org/10.3390/polym15020317
Chicago/Turabian StyleZhang, Yi, Tao Ge, Yifan Li, Jinlin Lu, Hao Du, Ling Yan, Hong Tan, Jiehua Li, and Yansheng Yin. 2023. "Anti-Fouling and Anti-Biofilm Performance of Self-Polishing Waterborne Polyurethane with Gemini Quaternary Ammonium Salts" Polymers 15, no. 2: 317. https://doi.org/10.3390/polym15020317