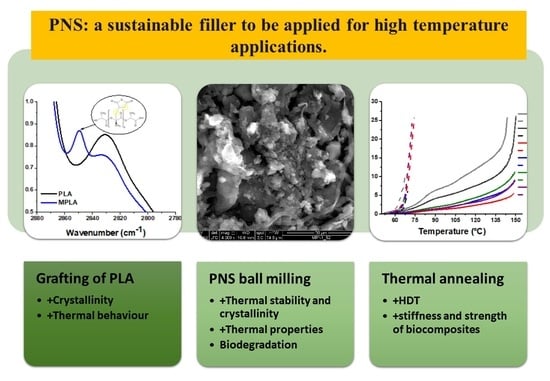
Thermomechanical Properties and Biodegradation Behavior of Itaconic Anhydride-Grafted PLA/Pecan Nutshell Biocomposites †
Abstract
:1. Introduction
2. Experimental Section
2.1. Raw Materials
2.2. Ball Milling of the Biomass
2.3. Modification of PLA via Radical Grafting with Itaconic Anhydride
2.3.1. Determination of Grafting Degree
2.3.2. Determination of Molecular Weight
2.4. Biocomposite Preparation
2.5. Characterization of the Biocomposites
2.5.1. Thermogravimetric Analysis (TGA)
2.5.2. Differential Scanning Calorimetry (DSC)
2.5.3. Heat Deflection Temperature (HDT)
2.5.4. Scanning Electron Microscopy (SEM)
2.5.5. Mechanical Tests
2.5.6. Soil Burial of Biocomposites
3. Results and Discussion
3.1. Characterization of IA-Grafted PLA
3.2. Characterization of MPLA Biocomposites
3.2.1. Thermal Characterization
3.2.2. Thermomechanical Properties
3.2.3. SEM Analysis of Cryofractured Surfaces
3.2.4. Mechanical Properties
3.3. Soil Burial Biodegradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Yu, J.; Chen, L.X.L. The Greenhouse Gas Emissions and Fossil Energy Requirement of Bioplastics from Cradle to Gate of a Biomass Refinery. Environ. Sci. Technol. 2008, 42, 6961–6966. [Google Scholar] [CrossRef] [PubMed]
- Sanusi, O.M.; Benelfellah, A.; Bikiaris, D.N.; Aït Hocine, N. Effect of Rigid Nanoparticles and Preparation Techniques on the Performances of Poly(Lactic Acid) Nanocomposites: A Review. Polym. Adv. Technol. 2021, 32, 444–460. [Google Scholar] [CrossRef]
- Pilz, H.; Brandt, B.; Fehringer, R. The Impact of Plastics on Life Cycle Energy Consumption and Greenhouse Gas Emissions in Europe. Available online: https://plasticseurope.org/wp-content/uploads/2021/10/201009-Denkstatt-Report.pdf. (accessed on 1 January 2020).
- Pimentel, J.; Rodríguez, G.; Gil, I. Synthesis of Alternative Cost-Effective Process Flowsheets for Lactic Acid Valorization by Means of the P-Graph Methodology. Ind. Eng. Chem. Res. 2020, 59, 5921–5930. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Taher, H. A Review on the Lactic Acid Fermentation from Low-Cost Renewable Materials: Recent Developments and Challenges. Environ. Technol. Innov. 2020, 20, 101138. [Google Scholar] [CrossRef]
- Mukherjee, T.; Kao, N. PLA Based Biopolymer Reinforced with Natural Fibre: A Review. J. Polym. Environ. 2011, 19, 714–725. [Google Scholar] [CrossRef]
- Mahmoud Zaghloul, M.Y.; Yousry Zaghloul, M.M.; Yousry Zaghloul, M.M. Developments in Polyester Composite Materials–An in-Depth Review on Natural Fibres and Nano Fillers. Compos. Struct. 2021, 278, 114698. [Google Scholar] [CrossRef]
- Mahmoud Zaghloul, M.Y.; Yousry Zaghloul, M.M.; Yousry Zaghloul, M.M. Physical Analysis and Statistical Investigation of Tensile and Fatigue Behaviors of Glass Fiber-Reinforced Polyester via Novel Fibers Arrangement. J. Compos. Mater. 2022, 002199832211411. [Google Scholar] [CrossRef]
- Agustin-Salazar, S.; Cerruti, P.; Scarinzi, G. 9 Biobased Structural Additives for Polymers. In Sustainability of Polymeric Materials; De Gruyter: Berlin, Germany, 2020; pp. 193–234. ISBN 9783110590586. [Google Scholar]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A Review of Recent Developments in Natural Fibre Composites and Their Mechanical Performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef] [Green Version]
- Agustin-Salazar, S.; Cerruti, P.; Medina-Juárez, L.Á.; Scarinzi, G.; Malinconico, M.; Soto-Valdez, H.; Gamez-Meza, N. Lignin and Holocellulose from Pecan Nutshell as Reinforcing Fillers in Poly (Lactic Acid) Biocomposites. Int. J. Biol. Macromol. 2018, 115, 727–736. [Google Scholar] [CrossRef]
- Berglund, L.; Noël, M.; Aitomäki, Y.; Öman, T.; Oksman, K. Production Potential of Cellulose Nanofibers from Industrial Residues: Efficiency and Nanofiber Characteristics. Ind. Crops Prod. 2016, 92, 84–92. [Google Scholar] [CrossRef]
- Bartos, A.; Nagy, K.; Anggono, J.; Antoni; Purwaningsih, H.; Móczó, J.; Pukánszky, B. Biobased PLA/Sugarcane Bagasse Fiber Composites: Effect of Fiber Characteristics and Interfacial Adhesion on Properties. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106273. [Google Scholar] [CrossRef]
- Agustin-Salazar, S.; Ricciulli, M.; Ambrogi, V.; Cerruti, P.; Scarinzi, G. Effect of Thermal Annealing and Filler Ball-Milling on the Properties of Highly Filled Polylactic Acid/Pecan Nutshell Biocomposites. Int. J. Biol. Macromol. 2022, 200, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Acosta, D.; Rodriguez-Uribe, A.; Álvarez-Chávez, C.R.; Mohanty, A.K.; Misra, M.; López-Cervantes, J.; Madera-Santana, T.J. Physicochemical Characterization and Evaluation of Pecan Nutshell as Biofiller in a Matrix of Poly(Lactic Acid). J. Polym. Environ. 2019, 27, 521–532. [Google Scholar] [CrossRef]
- Álvarez-Chávez, C.R.; Sánchez-Acosta, D.L.; Encinas-Encinas, J.C.; Esquer, J.; Quintana-Owen, P.; Madera-Santana, T.J. Characterization of Extruded Poly(Lactic Acid)/Pecan Nutshell Biocomposites. Int. J. Polym. Sci. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, L.W.; McDonald, A.G. The Effect of Micron Sized Wood Fibers in Wood Plastic Composites. Maderas. Cienc. Tecnol. 2013, 15, 357–374. [Google Scholar] [CrossRef] [Green Version]
- Suaduang, N.; Ross, S.; Ross, G.M.; Pratumshat, S.; Mahasaranon, S. Effect of Spent Coffee Grounds Filler on the Physical and Mechanical Properties of Poly(Lactic Acid) Bio-Composite Films. Mater. Today Proc. 2019, 17, 2104–2110. [Google Scholar] [CrossRef]
- Huber, T.; Misra, M.; Mohanty, A.K. The Effect of Particle Size on the Rheological Properties of Polyamide 6/Biochar Composites. AIP Conf. Proc. 2015, 1664, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Fu, Z.; Li, D.; Wu, M. Effects of Ball Milling Processes on the Microstructure and Rheological Properties of Microcrystalline Cellulose as a Sustainable Polymer Additive. Materials 2018, 11, 1057. [Google Scholar] [CrossRef] [Green Version]
- Isa, A.; Minamino, J.; Kojima, Y.; Suzuki, S.; Ito, H.; Makise, R.; Okamoto, M.; Endo, T. The Influence of Dry-Milled Wood Flour on the Physical Properties of Wood Flour/Polypropylene Composites. J. Wood Chem. Technol. 2016, 36, 105–113. [Google Scholar] [CrossRef]
- Ku Marsilla, K.I.; Verbeek, C.J.R. Modification of Poly(Lactic Acid) Using Itaconic Anhydride by Reactive Extrusion. Eur. Polym. J. 2015, 67, 213–223. [Google Scholar] [CrossRef]
- Ku Marsilla, K.I.; Verbeek, C.J.R. Crystallization of Itaconic Anhydride Grafted Poly(Lactic Acid) during Annealing. J. Appl. Polym. Sci. 2017, 134, 1–11. [Google Scholar] [CrossRef]
- Petruš, J.; Kučera, F.; Petrůj, J. Post-Polymerization Modification of Poly(Lactic Acid) via Radical Grafting with Itaconic Anhydride. Eur. Polym. J. 2016, 77, 16–30. [Google Scholar] [CrossRef]
- Petruš, J.; Kučera, F.; Chamradová, I.; Jančář, J. Real-Time Monitoring of Radical Grafting of Poly(Lactic Acid) with Itaconic Anhydride in Melt. Eur. Polym. J. 2018, 103, 378–389. [Google Scholar] [CrossRef]
- Quiroz-Castillo, J.M.; Rodríguez-Félix, D.E.; Grijalva-Monteverde, H.; Del Castillo-Castro, T.; Plascencia-Jatomea, M.; Rodríguez-Félix, F.; Herrera-Franco, P.J. Preparation of Extruded Polyethylene/Chitosan Blends Compatibilized with Polyethylene-Graft-Maleic Anhydride. Carbohydr. Polym. 2014, 101, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, T.; Liu, T.; Liu, T.; Xin, J.; Hiscox, W.C.; Liu, H.; Liu, L.; Zhang, J. Improving Grafting Efficiency of Dicarboxylic Anhydride Monomer on Polylactic Acid by Manipulating Monomer Structure and Using Comonomer and Reducing Agent. Ind. Eng. Chem. Res. 2017, 56, 3920–3927. [Google Scholar] [CrossRef]
- Detyothin, S.; Selke, S.E.M.; Narayan, R.; Rubino, M.; Auras, R. Reactive Functionalization of Poly(Lactic Acid), PLA: Effects of the Reactive Modifier, Initiator and Processing Conditions on the Final Grafted Maleic Anhydride Content and Molecular Weight of PLA. Polym. Degrad. Stab. 2013, 98, 2697–2708. [Google Scholar] [CrossRef]
- le Duigou, A.; Merotte, J.; Bourmaud, A.; Davies, P.; Belhouli, K.; Baley, C. Hygroscopic Expansion: A Key Point to Describe Natural Fibre/Polymer Matrix Interface Bond Strength. Compos. Sci. Technol. 2017, 151, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Maharana, T.; Pattanaik, S.; Routaray, A.; Nath, N.; Sutar, A.K. Synthesis and Characterization of Poly(Lactic Acid) Based Graft Copolymers. React. Funct. Polym. 2015, 93, 47–67. [Google Scholar] [CrossRef]
- Sullins, T.; Pillay, S.; Komus, A.; Ning, H. Hemp Fiber Reinforced Polypropylene Composites: The Effects of Material Treatments. Compos. Part B Eng. 2017, 114, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Kučera, F.; Petruš, J.; Matláková, J.; Jančář, J. Itaconic Anhydride Homopolymerization during Radical Grafting of Poly(Lactic Acid) in Melt. React. Funct. Polym. 2017, 116, 49–56. [Google Scholar] [CrossRef]
- Avolio, R.; Graziano, V.; Pereira, Y.D.F.; Cocca, M.; Gentile, G.; Errico, M.E.; Ambrogi, V.; Avella, M. Effect of Cellulose Structure and Morphology on the Properties of Poly(Butylene Succinate-Co-Butylene Adipate) Biocomposites. Carbohydr. Polym. 2015, 133, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Longo, A.; Dal Poggetto, G.; Malinconico, M.; Laurienzo, P.; Di Maio, E.; Di Lorenzo, M.L. Enhancement of Crystallization Kinetics of Poly(L-Lactic Acid) by Grafting with Optically Pure Branches. Polymer 2021, 227, 123852. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, Y.; Yi, H.; Haque, F.; Xu, C.; Wang, S.; Uddin, A. Thermal Annealing Dependent Dielectric Properties and Energetic Disorder in PffBT4T-2OD Based Organic Solar Cells. Mater. Sci. Semicond. Process. 2020, 105, 104750. [Google Scholar] [CrossRef]
- Cai, H.; Dave, V.; Gross, R.A.; McCarthy, S.P. Effects of Physical Aging, Crystallinity, and Orientation on the Enzymatic Degradation of Poly(Lactic Acid). J. Polym. Sci. Part B Polym. Phys. 1996, 34, 2701–2708. [Google Scholar] [CrossRef]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of Recent Advances in the Biodegradability of Polyhydroxyalkanoate (PHA) Bioplastics and Their Composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Lim, L.-T.; Auras, R.; Rubino, M. Processing Technologies for Poly(Lactic Acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Ruseckaite, R.A.; Vázquez, A. Degradation of Sisal Fibre/Mater Bi-Y Biocomposites Buried in Soil. Polym. Degrad. Stab. 2006, 91, 3156–3162. [Google Scholar] [CrossRef]
- Siakeng, R.; Jawaid, M.; Asim, M.; Siengchin, S. Accelerated Weathering and Soil Burial Effect on Biodegradability, Colour and Textureof Coir/Pineapple Leaf Fibres/PLA Biocomposites. Polymers 2020, 12, 458. [Google Scholar] [CrossRef] [Green Version]
- Rolere, S.; Monge, S.; Rakotonirina, M.D.; Guillaneuf, Y.; Gigmes, D.; Robin, J.J. Chemical Modification of Poly(Lactic Acid) Induced by Thermal Decomposition of N-Acetoxy-Phthalimide during Extrusion. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 120–129. [Google Scholar] [CrossRef]
- Cocca, M.; Di Lorenzo, M.L.; Malinconico, M.; Frezza, V. Influence of Crystal Polymorphism on Mechanical and Barrier Properties of Poly(l-Lactic Acid). Eur. Polym. J. 2011, 47, 1073–1080. [Google Scholar] [CrossRef]
- Vasanthan, N.; Ly, O. Effect of Microstructure on Hydrolytic Degradation Studies of Poly (l-Lactic Acid) by FTIR Spectroscopy and Differential Scanning Calorimetry. Polym. Degrad. Stab. 2009, 94, 1364–1372. [Google Scholar] [CrossRef]
- Hrabalova, M.; Gregorova, A.; Wimmer, R.; Sedlarik, V.; Machovsky, M.; Mundigler, N. Effect of Wood Flour Loading and Thermal Annealing on Viscoelastic Properties of Poly(Lactic Acid) Composite Films. J. Appl. Polym. Sci. 2010, 118, 1534–1540. [Google Scholar] [CrossRef]
- Orue, A.; Eceiza, A.; Arbelaiz, A. The Effect of Sisal Fiber Surface Treatments, Plasticizer Addition and Annealing Process on the Crystallization and the Thermo-Mechanical Properties of Poly(Lactic Acid) Composites. Ind. Crops Prod. 2018, 118, 321–333. [Google Scholar] [CrossRef]
- Chen, J.; Deng, C.; Hong, R.; Fu, Q.; Zhang, J. Effect of Thermal Annealing on Crystal Structure and Properties of PLLA/PCL Blend. J. Polym. Res. 2020, 27, 221. [Google Scholar] [CrossRef]
- Běhálek, L.; Maršálková, M.; Lenfeld, P.; Habr, J.; Bobek, J.; Seidl, M. Study of Crystallization of Polylactic Acid Composites And Nanocomposites With Natural Fibres By Dsc Method. In Proceedings of the NANOCON 2013. 5th International Conference, Brno, Czech Republic, 16–18 October 2013; Technical University of Liberec: Brno, Czech Republic, 2013; pp. 1–6. [Google Scholar]
- Ferry, L.; Dorez, G.; Taguet, A.; Otazaghine, B.; Lopez-Cuesta, J.M. Chemical Modification of Lignin by Phosphorus Molecules to Improve the Fire Behavior of Polybutylene Succinate. Polym. Degrad. Stab. 2015, 113, 135–143. [Google Scholar] [CrossRef]
- Tábi, T.; Hajba, S.; Kovács, J.G. Effect of Crystalline Forms (A′ and α) of Poly(Lactic Acid) on Its Mechanical, Thermo-Mechanical, Heat Deflection Temperature and Creep Properties. Eur. Polym. J. 2016, 82, 232–243. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.W.; Li, P.; Ren, X.; Jiang, T.; Yeh, J.-T. Isothermal Crystallization Kinetics and Crystal Structure of Poly(Lactic Acid): Effect of Triphenyl Phosphate and Talc. J. Appl. Polym. Sci. 2010, 118, 3558–3569. [Google Scholar] [CrossRef]
- Gracia-Fernández, C.A.; Gómez-Barreiro, S.; López-Beceiro, J.; Naya, S.; Artiaga, R. New Approach to the Double Melting Peak of Poly(l-Lactic Acid) Observed by DSC. J. Mater. Res. 2012, 27, 1379–1382. [Google Scholar] [CrossRef]
- Solikhin, A.; Hadi, Y.S.; Massijaya, M.Y.; Nikmatin, S. Novel Isolation of Empty Fruit Bunch Lignocellulose Nanofibers Using Different Vibration Milling Times-Assisted Multimechanical Stages. Waste Biomass Valorization 2017, 8, 2451–2462. [Google Scholar] [CrossRef]
- Li, G.; Yang, B.; Han, W.; Li, H.; Kang, Z.; Lin, J. Tailoring the Thermal and Mechanical Properties of Injection-molded Poly (Lactic Acid) Parts through Annealing. J. Appl. Polym. Sci. 2021, 138, 49648. [Google Scholar] [CrossRef]
- Shi, Q.F.; Mou, H.Y.; Li, Q.Y.; Wang, J.K.; Guo, W.H. Influence of Heat Treatment on the Heat Distortion Temperature of Poly(Lactic Acid)/Bamboo Fiber/Talc Hybrid Biocomposites. J. Appl. Polym. Sci. 2012, 123, 2828–2836. [Google Scholar] [CrossRef]
- Bubeck, R.A.; Merrington, A.; Dumitrascu, A.; Smith, P.B. Thermal Analyses of Poly(Lactic Acid) PLA and Micro-Ground Paper Blends. J. Therm. Anal. Calorim. 2018, 131, 309–316. [Google Scholar] [CrossRef]
- Battegazzore, D.; Bocchini, S.; Alongi, J.; Frache, A. Plasticizers, Antioxidants and Reinforcement Fillers from Hazelnut Skin and Cocoa by-Products: Extraction and Use in PLA and PP. Polym. Degrad. Stab. 2014, 108, 297–306. [Google Scholar] [CrossRef]
- Móczó, J.; Pukánszky, B. Polymer Micro and Nanocomposites: Structure, Interactions, Properties. J. Ind. Eng. Chem. 2008, 14, 535–563. [Google Scholar] [CrossRef]
- Pukánszky, B. Influence of Interface Interaction on the Ultimate Tensile Properties of Polymer Composites. Composites 1990, 21, 255–262. [Google Scholar] [CrossRef]
- Li, M.X.; Kim, S.H.; Choi, S.W.; Goda, K.; Lee, W. Il Effect of Reinforcing Particles on Hydrolytic Degradation Behavior of Poly (Lactic Acid) Composites. Compos. Part B Eng. 2016, 96, 248–254. [Google Scholar] [CrossRef]
- Eskander, S.; Saleh, H.E.M. Biodegradation: Process Mechanism. Biodegrad. Bioremediat. 2017, 8, 1–31. [Google Scholar]
- Serrano-Ruiz, H.; Martin-Closas, L.; Pelacho, A.M. Biodegradable Plastic Mulches: Impact on the Agricultural Biotic Environment. Sci. Total Environ. 2021, 750, 141228. [Google Scholar] [CrossRef]
- Janczak, K.; Hrynkiewicz, K.; Znajewska, Z.; Dąbrowska, G. Use of Rhizosphere Microorganisms in the Biodegradation of PLA and PET Polymers in Compost Soil. Int. Biodeterior. Biodegradation 2018, 130, 65–75. [Google Scholar] [CrossRef]
- Alshehrei, F. Biodegradation of Synthetic and Natural Plastic by Microorganisms. J. Appl. Environ. Microbiol. 2017, 5, 8–19. [Google Scholar] [CrossRef]
- Diez, M.C. BIOLOGICAL ASPECTS INVOLVED IN THE DEGRADATION OF ORGANIC POLLUTANTS. J. Soil Sci. Plant Nutr. 2010, 10, 244–267. [Google Scholar] [CrossRef]
- Chia, W.Y.; Ying Tang, D.Y.; Khoo, K.S.; Kay Lup, A.N.; Chew, K.W. Nature’s Fight against Plastic Pollution: Algae for Plastic Biodegradation and Bioplastics Production. Environ. Sci. Ecotechnology 2020, 4, 100065. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Li, Y.; Fan, R.; Chen, Z.; Chen, J.; Brandon, A.M.; Criddle, C.S.; Zhang, Y.; Wu, W. Biodegradation of Low-Density Polyethylene and Polystyrene in Superworms, Larvae of Zophobas Atratus ( Coleoptera: Tenebrionidae ): Broad and Limited Extent Depolymerization *. Environ. Pollut. 2020, 266, 115206. [Google Scholar] [CrossRef]
- Song, Y.; Qiu, R.; Hu, J.; Li, X.; Zhang, X.; Chen, Y. Science of the Total Environment Biodegradation and Disintegration of Expanded Polystyrene by Land Snails Achatina Fulica. Sci. Total Environ. 2020, 746, 141289. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Sánchez, M.D.R.; Campo-Erazo, S.D.; Villada-Castillo, H.S.; Solanilla-Duque, J.F. Structural Changes of Cassava Starch and Polylactic Acid Films Submitted to Biodegradation Process. Int. J. Biol. Macromol. 2019, 129, 442–447. [Google Scholar] [CrossRef]
- Janczak, K.; Dąbrowska, G.B.; Raszkowska-Kaczor, A.; Kaczor, D.; Hrynkiewicz, K.; Richert, A. Biodegradation of the Plastics PLA and PET in Cultivated Soil with the Participation of Microorganisms and Plants. Int. Biodeterior. Biodegrad. 2020, 155, 105087. [Google Scholar] [CrossRef]








| Mn kDa | Mw kDa | Mw Mn−1 | |
|---|---|---|---|
| PLA | 48.1 | 70.0 | 1.4 |
| MPLA | 42.2 | 68.9 | 1.6 |
| Before Annealing | After Annealing | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tg | Tc | ΔHc | Tm | ΔHm | ΔHm * | Tg | Tc | ΔHc | Tm | ΔHm | ΔHm * | |
| °C | °C | J g−1 | °C | J g−1 | J g−1 | °C | °C | J g−1 | °C | J g−1 | J g−1 | |
| PLA | 58.8 | 110.2 | 23.6 | 147.0 | 21.5 | 24.9 | 62.8 | - | - | - | 150.5 | 28.8 |
| 153.0 | 3.4 | |||||||||||
| MPLA | 64.1 | 104.3 | 28.3 | 140.9 | 6.9 | 28.4 | - | - | - | - | 150.9 | 32.0 |
| 153.8 | 21.7 | |||||||||||
| MPN1 | 60.0 | 104.1 | 32.2 | 140.5 | 14.3 | 34.6 | - | - | - | - | 150.3 | 37.7 |
| 154.3 | 20.1 | |||||||||||
| MPN2 | 62.4 | 103.3 | 32.4 | 145.9 | 14.8 | 34.3 | - | - | - | - | 151.7 | 41.8 |
| 154.1 | 19.6 | |||||||||||
| MPN3 | 61.0 | 105.7 | 37.9 | 146.1 | 13.0 | 36.2 | - | - | - | - | 152.1 | 37.8 |
| 154.3 | 22.0 | |||||||||||
| MPN4 | 63.6 | 103.8 | 39.4 | 146.1 | 12.2 | 32.4 | - | - | - | - | 150.9 | 43.3 |
| 154.5 | 23.4 | |||||||||||
| Nitrogen | Air | |||||
|---|---|---|---|---|---|---|
| Tonset °C | Tmax °C | Char. Yield * wt.% | Tonset °C | Tmax °C | Char. Yield * wt.% | |
| PLA | 268.1 | 304.6 | 2.7 | 297.6 | 338.6 | 0.0 |
| MPLA | 268.5 | 292.5 | 3.9 | 316.0 | 356.8 | 0.0 |
| MPN1 | 272.9 | 306.1 | 20.6 | 281.0 | 357.0 | 3.4 |
| MPN2 | 277.2 | 319.9 | 24.8 | 280.0 | 360.0 | 0.0 |
| MPN3 | 275.3 | 327.7 | 24.1 | 280.4 | 362.0 | 2.8 |
| MPN4 | 273.6 | 323.5 | 24.0 | 272.3 | 360.0 | 1.3 |
| Before | After Annealing | ||
|---|---|---|---|
| HDT °C | HDT °C | Strain * % | |
| PLA | 63.1 ± 1.8 | 77.1 ± 1.5 | 18.5 ± 1.6 |
| MPLA | 67.9 ± 2.1 | 82.9 ± 1.4 | 11.7 ± 1.2 |
| MPN1 | 66.3 ± 1.6 | 130.7 ± 0.9 | 3.4 ± 1.1 |
| MPN2 | 66.8 ± 2.2 | 115.2 ± 1.6 | 5.9 ± 0.7 |
| MPN3 | 66.1 ± 1.2 | 106.7 ± 1.3 | 7.6 ± 1.3 |
| MPN4 | 66.1 ± 1.2 | 117.6 ± 2.0 | 5.7 ± 1.4 |
| FLEXURAL | IMPACT | ||||
|---|---|---|---|---|---|
| Stress at Break MPa | Modulus MPa | Strain at Break % | Strength N | Resilience KJ m−2 | |
| Before annealing | |||||
| MPLA | 78.2 ± 3.8 | 3646 ± 235 | 2.6 ± 0.5 | 103.8 ± 6.7 | 2.1 ± 0.3 |
| MPN1 | 61.5 ± 3.3 | 4824 ± 232 | 1.2 ± 0.2 | 98.7 ± 12.7 | 0.8 ± 0.1 |
| MPN2 | 52.6 ± 5.8 | 4562 ± 415 | 1.1 ± 0.1 | 95.9 ± 10.4 | 0.7 ± 0.1 |
| MPN3 | 48.5 ± 6.4 | 4053 ± 199 | 1.2 ± 0.1 | 82.1 ± 8.1 | 0.5 ± 0.1 |
| MPN4 | 48.9 ±6.7 | 4082 ± 196 | 1.3 ± 0.1 | 72.7 ± 13.1 | 0.6 ± 0.1 |
| After annealing | |||||
| MPLA | 86.5 ± 3.8 | 3696 ± 26 | 1.8 ± 0.2 | 109.2 ± 24.2 | 2.1 ± 0.3 |
| MPN1 | 68.4 ± 3.2 | 4851 ± 76 | 1.4 ± 0.1 | 103.1 ± 19.6 | 0.9 ± 0.1 |
| Time Weeks | Tonset °C | Tmax °C | Char. Yield * wt.% | Tonset °C | Tmax °C | Char. Yield * wt.% | |
|---|---|---|---|---|---|---|---|
| Nitrogen | Air | ||||||
| PLA | 0 | 268.1 | 304.6 | 2.7 | 297.6 | 338.6 | - |
| 27 | 269.2 | 305.6 | 5.5 | 261.3 | 294.4 | 1.1 | |
| 52 | 272.2 | 299.4 | 2.4 | 265.3 | 301.0 | 0.2 | |
| MPLA | 0 | 268.5 | 292.5 | 3.9 | 316.0 | 357.8 | 0.3 |
| 27 | 288.4 | 309.3 | 0.3 | 276.0 | 309.5 | 9.4 | |
| 52 | 305.2 | 331.8 | 0.6 | 276.9 | 331.8 | 6.7 | |
| MPN1 | 0 | 272.9 | 306.1 | 20.6 | 281.0 | 356.0 | 3.4 |
| 27 | 276.8 | 319.7 | 22.5 | 287.7 | 311.7 | 6.6 | |
| 52 | 266.6 | 301.5 | 25.9 | 265.3 | 296.6 | 8.2 | |
| MPN2 | 0 | 277.2 | 319.9 | 24.8 | 280.0 | 391.3 | - |
| 27 | 270.8 | 320.9 | 20.4 | 259.2 | 353.1 | 3.8 | |
| 52 | 276.8 | 320.3 | 19.3 | 269.9 | 343.3 | - | |
| MPN3 | 0 | 275.3 | 327.7 | 24.1 | 280.4 | 360.0 | 2.8 |
| 27 | 253.9 | 331.3 | 23.3 | 254.2 | 348.6 | 3.0 | |
| 52 | 258.7 | 311.9 | 22.5 | 254.8 | 355.3 | 0.5 | |
| MPN4 | 0 | 273.6 | 323.5 | 24.0 | 272.3 | 358.4 | 1.3 |
| 27 | 271.7 | 324 | 23.9 | 250.1 | 305.3 | 4.7 | |
| 52 | 248.4 | 303.3 | 23.7 | 247.4 | 333.2 | 3.2 | |
| Time | Tg | Tc | ΔHc * | Tm | ΔHm * | |
|---|---|---|---|---|---|---|
| Weeks | °C | °C | J g−1 | °C | J g−1 | |
| PLA | 0 | 58.8 | 110.2 | 23.6 | 147.0 | 24.9 |
| 153.0 | ||||||
| 27 | 63.0 | 109.7 | 15.4 | 152.5 | 31.6 | |
| 52 | 61.3 | 107.7 | 28.1 | 153.6 | 35.9 | |
| MPLA | 0 | 64.1 | 104.3 | 28.3 | 140.9 | 28.4 |
| 153.8 | ||||||
| 27 | 64.7 | 105.86 | 24.3 | 146.5 | 34.0 | |
| 151.67 | ||||||
| 52 | 63.85 | 90.75 | 23.45 | 144.65 | 36.92 | |
| 152.14 | ||||||
| MPN1 | 0 | 60.00 | 104.14 | 32.22 | 140.47 | 34.64 |
| 154.32 | ||||||
| 27 | 63.37 | 104.01 | 32.20 | 145.70 | 39.38 | |
| 153.44 | ||||||
| 52 | 60.28 | 97.71 | 8.45 | 144.20 | 31.96 | |
| 152.44 | ||||||
| MPN2 | 0 | 62.43 | 103.31 | 32.44 | 145.86 | 34.30 |
| 154.08 | ||||||
| 27 | 62.79 | 101.42 | 25.70 | 145.31 | 37.94 | |
| 152.67 | ||||||
| 52 | 63.84 | 113.39 | 20.10 | 140.84 | 65.10 | |
| 150.06 | ||||||
| MPN3 | 0 | 61.05 | 105.72 | 37.9 | 146.10 | 36.20 |
| 154.31 | ||||||
| 27 | 62.38 | 98.59 | 20.90 | 143.12 | 40.68 | |
| 151.85 | ||||||
| 52 | 62.11 | 99.15 | 2.94 | 142.43 | 30.24 | |
| 151.58 | ||||||
| MPN4 | 0 | 63.56 | 103.81 | 39.36 | 146.14 | 32.40 |
| 154.54 | ||||||
| 27 | 61.78 | 99.88 | 28.58 | 144.57 | 45.64 | |
| 152.47 | ||||||
| 52 | 60.37 | 88.78 | 1.22 | 141.55 | 40.60 | |
| 150.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agustin-Salazar, S.; Ricciulli, M.; Ambrogi, V.; Cerruti, P.; Scarinzi, G. Thermomechanical Properties and Biodegradation Behavior of Itaconic Anhydride-Grafted PLA/Pecan Nutshell Biocomposites. Polymers 2022, 14, 5532. https://doi.org/10.3390/polym14245532
Agustin-Salazar S, Ricciulli M, Ambrogi V, Cerruti P, Scarinzi G. Thermomechanical Properties and Biodegradation Behavior of Itaconic Anhydride-Grafted PLA/Pecan Nutshell Biocomposites. Polymers. 2022; 14(24):5532. https://doi.org/10.3390/polym14245532
Chicago/Turabian StyleAgustin-Salazar, Sarai, Marco Ricciulli, Veronica Ambrogi, Pierfrancesco Cerruti, and Gennaro Scarinzi. 2022. "Thermomechanical Properties and Biodegradation Behavior of Itaconic Anhydride-Grafted PLA/Pecan Nutshell Biocomposites" Polymers 14, no. 24: 5532. https://doi.org/10.3390/polym14245532