Hydrophobic–Hydrophilic Properties and Characterization of PIM-1 Films Treated by Elemental Fluorine in Liquid Perfluorodecalin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of PIM-1
2.2. Preparation of PIM-1 Samples for Fluorination
2.3. Direct Liquid-Phase Fluorination of PIM-1 Samples
2.4. X-ray Photoelectron Spectroscopy (XPS)
2.5. Scanning and Energy-Dispersive X-ray Spectroscopy (SEM and XEDS)
2.6. Attenuated Total Reflectance Infra-Red Spectroscopy (ATR-IR)
2.7. Contact Angle Measurements and Surface Energy Calculation
3. Results and Discussion
3.1. ATR-IR of the Fluorinated PIM-1 Samples
3.2. XPS Analysis of the Fluorinated PIM-1 Samples
3.3. SEM and XEDS for the Fluorinated PIM-1 Samples
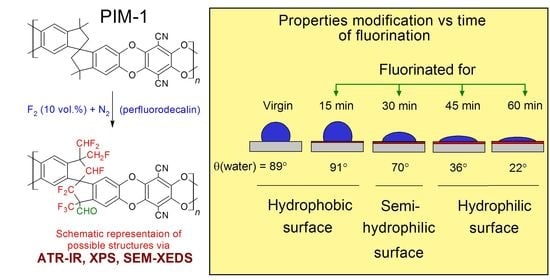
3.4. Surface Properties of Fluorinated PIM-1 Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kharitonov, A.P. Direct fluorination of polymers—From fundamental research to industrial applications. Prog. Org. Coat. 2008, 61, 192–204. [Google Scholar] [CrossRef]
- Nazarov, V.G. Surface Modification of Polymers; Ministry of Education and Science of Russian Federation, Moscow State University of Printing Arts: Moscow, Russia, 2008. [Google Scholar]
- Tressaud, A.; Durand, E.; Labrugčre, C.; Kharitonov, A.P.; Simbirtseva, G.V.; Kharitonova, L.N. Dubois M Surface Modification of Polymers Treated by Various Fluorinating Media. Acta Chim. Slov. 2013, 60, 495–504. [Google Scholar] [PubMed]
- Belov, N.A.; Alentiev, A.Y.; Bogdanova, Y.G.; Vdovichenko, A.Y.; Pashkevich, D.S. Direct Fluorination as Method of Improvement of Operational Properties of Polymeric Materials. Polymers 2020, 12, 2836. [Google Scholar] [CrossRef]
- Blinov, I.A.; Mukhortov, D.A.; Yampolskii, Y.P.; Belov, N.A.; Alentiev, A.Y.; Chirkov, S.V.; Bondarenko, G.N.; Kostina, Y.V.; Legkov, S.A.; Perepuchov, A.M.; et al. Direct fluorination of poly-2,6-dimethyl-1,4-phenylene oxide in perfluorinated liquid medium. J. Fluor. Chem. 2020, 234, 109526. [Google Scholar] [CrossRef]
- Belov, N.A.; Blinov, I.A.; Alentiev, A.Y.; Belokhvostov, V.M.; Mukhortov, D.A.; Chirkov, S.V.; Mazur, A.S.; Kostina, Y.V.; Vozniuk, O.N.; Kurapova, E.S.; et al. Direct fluorination of acetyl and ethyl celluloses in perfluorinated liquid medium. J. Polym. Res. 2020, 27, 290. [Google Scholar] [CrossRef]
- Blinov, I.A.; Belov, N.A.; Suvorov, A.V.; Chirkov, S.V.; Kostina, Y.V.; Mazur, A.S.; Kambur, M.P.; Belochvostov, V.M.; Alentiev, A.Y.; Vozniuk, O.N.; et al. Fluorination of polystyrene by elemental fluorine in liquid media. J. Fluor. Chem. 2021, 246, 109777. [Google Scholar] [CrossRef]
- Belov, N.A.; Nikiforov, R.Y.; Alentiev, A.Y.; Bezgin, D.A.; Blinov, I.A.; Suvorov, A.V.; Kostina, J.V.; Legkov, S.A.; Levin, I.S.; Gringolts, M.L.; et al. Gas Transport and Separation Properties of Polynorbornene Treated with Elemental Fluorine in a Perfluorodecalin Liquid. Membr. Membr. Technol. 2021, 3, 351–364. [Google Scholar] [CrossRef]
- Belov, N.A.; Blinov, I.A.; Suvorov, A.V.; Nikiforov, R.Y.; Chirkov, S.V.; Alentiev, A.Y.; Kambur, M.P.; Kostina, Y.V.; Levin, I.S.; Shapagin, A.V.; et al. Gas Permeability of Cellulose Acetate Films Treated with Fluorine in Perfluorodecalin. Membr. Membr. Technol. 2021, 3, 114–123. [Google Scholar] [CrossRef]
- Alentiev, A.Y.; Levin, I.S.; Buzin, M.I.; Belov, N.A.; Nikiforov, R.Y.; Chirkov, S.V.; Blagodatskikh, I.V.; Kechekyan, A.S.; Kechekyan, P.A.; Bekeshev, V.G.; et al. Gas transport parameters, density and free volume of nanocrystalline poly-2,6-dimethylphenylene oxide. Polymer 2021, 226, 123804. [Google Scholar] [CrossRef]
- Puleo, A.C.; Muruganandam, N.; Paul, D.R. Gas sorption and transport in substituted polystyrenes. J. Polym. Sci. Part B Polym. Phys. 1989, 27, 2385–2406. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Loginov, B.A. Direct fluorination of polymer final products: From fundamental study to practical application. Russ. J. Gen. Chem. 2009, 79, 635–641. [Google Scholar] [CrossRef]
- Ma, X.; Li, K.; Zhu, Z.; Dong, H.; Lv, J.; Wang, Y.; Pinnau, I.; Li, J.; Chen, B.; Han, Y. High-performance polymer molecular sieve membranes prepared by direct fluorination for efficient helium enrichment. J. Mater. Chem. A 2021, 9, 18313–18322. [Google Scholar] [CrossRef]
- Anokhina, T.S.; Yushkin, A.A.; Budd, P.M.; Volkov, A.V. Application of PIM-1 for solvent swing adsorption and solvent recovery by nanofiltration. Sep. Purif. Technol. 2015, 156, 683–690. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Feng, W.; Zhu, L.; Zhang, L. PIM-1 pore-filled thin film composite membranes for tunable organic solvent nanofiltration. J. Membr. Sci. 2020, 601, 117951. [Google Scholar] [CrossRef]
- Wu, X.M.; Guo, H.; Soyekwo, F.; Zhang, Q.G.; Lin, C.X.; Liu, Q.L.; Zhu, A.M. Pervaporation Purification of Ethylene Glycol Using the Highly Permeable PIM-1 Membrane. J. Chem. Eng. Data 2016, 61, 579–586. [Google Scholar] [CrossRef]
- Yong, W.F.; Salehian, P.; Zhang, L.; Chung, T.-S. Effects of hydrolyzed PIM-1 in polyimide-based membranes on C2–C4 alcohols dehydration via pervaporation. J. Membr. Sci. 2017, 523, 430–438. [Google Scholar] [CrossRef]
- Wu, X.M.; Zhang, Q.G.; Soyekwo, F.; Liu, Q.L.; Zhu, A.M. Pervaporation removal of volatile organic compounds from aqueous solutions using the highly permeable PIM-1 membrane. AIChE J. 2016, 62, 842–851. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Bogdanova, Y.G.; Dolzhikov, V.D. Relationship between Energy Characteristics of Surface of Polymeric Membranes and Their Transport Properties. Russ. J. Appl. Chem. 2018, 91, 1311–1321. [Google Scholar] [CrossRef]
- Belov, N.A.; Pashkevich, D.S.; Alentiev, A.Y.; Tressaud, A. Effect of Direct Fluorination on the Transport Properties and Swelling of Polymeric Materials: A Review. Membranes 2021, 11, 713. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Razorenov, D.Y.; Blagodatskikh, I.V.; Muranov, A.V.; Starannikova, L.E.; Alent’ev, A.Y.; Nikiforov, R.Y.; Yampol’skii, Y.P. Polymer with Intrinsic Microporosity PIM-1: New Methods of Synthesis and Gas Transport Properties. Polym. Sci. Ser. B 2019, 61, 605–612. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Blagodatskikh, I.V.; Muranov, A.V.; Volkova, Y.A.; Razorenov, D.Y.; Ponomarev, I.I.; Skupov, K.M. Dimethyl sulfoxide as a green solvent for successful precipitative polyheterocyclization based on nucleophilic aromatic substitution, resulting in high molecular weight PIM-1. Mendeleev Commun. 2016, 26, 362–364. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Blagodatskikh, I.V.; Muranov, A.V.; Volkova, Y.A.; Razorenov, D.Y.; Ponomarev, I.I.; Skupov, K.M. Ultrasonic Activation of PIM-1 Synthesis and Properties of Polymers Obtained by Precipitation Polyheterocyclization in Dimethyl Sulfoxide. Polym. Sci. Ser. C 2020, 62, 259–265. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, Y.; Wu, H.; Ren, Y.; Guo, Z.; Liang, X.; Wu, Y.; Liu, Y.; Jiang, Z. Surface functionalization of Polymers of Intrinsic Microporosity (PIMs) membrane by polyphenol for efficient CO2 separation. Green. Chem. Eng. 2021, 2, 70–76. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; Wiley: Chichester, UK; New York, NY, USA, 1992. [Google Scholar]
- Kloubek, J. Development of methods for surface free energy determination using contact angles of liquids on solids. Adv. Colloid Interface Sci. 1992, 38, 99–142. [Google Scholar] [CrossRef]
- Lee, L.-H. Correlation between Lewis Acid-Base Surface Interaction Components and Linear Solvation Energy Relationship Solvatochromic α and β Parameters. Langmuir 1996, 12, 1681–1687. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Poteryaeva, Z.A.; Belyaev, S.S.; Nifant’ev, I.E.; Shlyakhtin, A.V.; Kostina, Y.V.; Cherevan’, A.S.; Efimov, M.N.; Bondarenko, G.N.; Sivtsov, E.V. Controlled synthesis of polyacrylonitrile via reversible addition-fragmentation chain-transfer pseudoliving radical polymerization and its thermal behavior. Polym. Sci. Ser. B 2011, 53, 391–403. [Google Scholar] [CrossRef]
- Cherevan’, A.S.; Kostina, Y.V.; Bondarenko, G.N. Solvent effect in the formation of polyconjugated system during pyrolysis of polyacrylonitrile. Russ. Chem. Bull. 2012, 61, 259–263. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Moskvin, Y.L. Direct fluorination of polystyrene films. J. Fluor. Chem. 1998, 91, 87–93. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Moskvin, Y.L.; Kolpakov, G.A. Application of interference spectroscopy for studying kinetics of chemical reactions in optically transparent films. Polym. Sci. USSR 1985, 27, 739–743. [Google Scholar] [CrossRef]
- Harnett, E.M.; Alderman, J.; Wood, T. The surface energy of various biomaterials coated with adhesion molecules used in cell culture. Colloids Surf. B Biointerfaces 2007, 55, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; Wiley: New York, NY, USA, 1997. [Google Scholar]
- du Toit, F.J.; Sanderson, R.D. Surface fluorination of polypropylene. J. Fluor. Chem. 1999, 98, 107–114. [Google Scholar] [CrossRef]
- Bogdanova, Y.G.; Antonova, L.V.; Silnikov, V.N.; Khanova MYu Senokosova, E.A.; Barbarash, L.S. Impact of Modification on the Energy Characteristics of Surfaces and Matrix Properties of the New Effective Polymer Vascular Implants. Key Eng. Mater. 2021, 899, 342–354. [Google Scholar]










| Fluorination Time, min | An | |||||||
|---|---|---|---|---|---|---|---|---|
| А2955 | А2928 | А1445 | А1309 | А1263 | А874 | А752 | А734 | |
| 0 (virgin) | 2.26 | 1.56 | 23.87 | 13.17 | 21.98 | 8.43 | 7.12 | 2.67 |
| 0 (PFD) | 2.99 | 2.27 | 23.15 | 12.83 | 21.53 | 7.68 | 5.29 | 2.08 |
| 15 | 2.74 | 2.67 | 23.32 | 12.68 | 21.35 | 7.60 | 5.47 | 2.22 |
| 30 | 2.51 | 1.94 | 23.06 | 12.36 | 21.23 | 7.93 | 5.61 | 2.24 |
| 45 | 2.30 | 1.44 | 25.90 | 13.48 | 24.04 | 8.76 | 7.18 | 2.73 |
| 60 | 2.14 | 1.17 | 26.10 | 13.69 | 24.14 | 9.39 | 7.23 | 3.03 |
| Fluorination Time, min | C | O | N | F |
|---|---|---|---|---|
| 0 (virgin) | 82.3 | 13.5 | 3.9 | 0.2 |
| 0 (PFD) | 82.8 | 12.8 | 2.9 | 1.5 |
| 15 | 69.9 | 16.8 | 3.0 | 10.4 |
| 30 | 60.9 | 18.9 | 2.0 | 18.2 |
| 45 | 59.3 | 18.9 | 2.6 | 19.2 |
| 60 | 56.3 | 21.3 | 2.4 | 20.0 |
| Sample | θ(H2O), deg | θ(CH2I2), deg | γdSV mJ∙m−2 | γpSV, mJ∙m−2 | γSV, mJ∙m−2 |
|---|---|---|---|---|---|
| PIM-1 | 89 | 25 | 46 | 1 | 47 |
| PIM-F-0-PFD | 93 | 26 | 47 | close to zero | 47 |
| PIM-F-15 | 91 | 67 | 21 | 4 | 25 |
| PIM-F-30 | 70 | 38 | 34 | 9 | 43 |
| PIM-F-45 | 36 | - * | - * | - * | - * |
| PIM-F-60 | 22 | - * | - * | - * | - * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belov, N.A.; Alentiev, A.Y.; Pashkevich, D.S.; Voroshilov, F.A.; Dvilis, E.S.; Nikiforov, R.Y.; Chirkov, S.V.; Syrtsova, D.A.; Kostina, J.V.; Ponomarev, I.I.; et al. Hydrophobic–Hydrophilic Properties and Characterization of PIM-1 Films Treated by Elemental Fluorine in Liquid Perfluorodecalin. Polymers 2022, 14, 5152. https://doi.org/10.3390/polym14235152
Belov NA, Alentiev AY, Pashkevich DS, Voroshilov FA, Dvilis ES, Nikiforov RY, Chirkov SV, Syrtsova DA, Kostina JV, Ponomarev II, et al. Hydrophobic–Hydrophilic Properties and Characterization of PIM-1 Films Treated by Elemental Fluorine in Liquid Perfluorodecalin. Polymers. 2022; 14(23):5152. https://doi.org/10.3390/polym14235152
Chicago/Turabian StyleBelov, Nikolay A., Aleksandr Yu. Alentiev, Dmitrii S. Pashkevich, Fedor A. Voroshilov, Edgar S. Dvilis, Roman Yu. Nikiforov, Sergey V. Chirkov, Daria A. Syrtsova, Julia V. Kostina, Igor I. Ponomarev, and et al. 2022. "Hydrophobic–Hydrophilic Properties and Characterization of PIM-1 Films Treated by Elemental Fluorine in Liquid Perfluorodecalin" Polymers 14, no. 23: 5152. https://doi.org/10.3390/polym14235152