Potent Application of Scrap from the Modified Natural Rubber Production as Oil Absorbent
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Preparation of the Rubber Foams
2.3. Measurement of Curing Characteristics
2.4. Measurement of Relative Foam Density and Expansion Ration
2.5. Measurement of Hardness
2.6. Oil Absorbency
2.7. Diffusion Studies
2.8. Optical Image and Scanning Electron Microscopy
2.9. Thermogravimetric Analysis (TGA)
2.10. Fourier Transform Infrared-Spectroscopic Analysis (FT-IR)
3. Results and Discussion
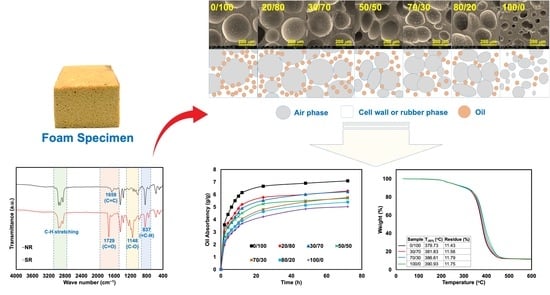
3.1. Characterization of NR and SR
3.2. Cure Characteristics
3.3. Physical Properties, Appearance, and Morphologies
3.4. Oil Absorbency
3.5. Diffusion Study
3.6. Transport Mechanism
3.7. Thermal Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mardiyati, Y.; Fauza, A.N.; Rachman, O.A.; Steven, S.; Santosa, S.P. A Silica–Lignin Hybrid Filler in a Natural Rubber Foam Composite as a Green Oil Spill Absorbent. Polymers 2022, 14, 2930. [Google Scholar] [CrossRef]
- Passow, U.; Lee, K. Future oil spill response plans require integrated analysis of factors that influence the fate of oil in the ocean. Curr. Opin. Chem. Eng. 2022, 36, 100769. [Google Scholar] [CrossRef]
- Dong, J.; Asif, Z.; Shi, Y.; Zhu, Y.; Chen, Z. Climate Change Impacts on Coastal and Offshore Petroleum Infrastructure and the Associated Oil Spill Risk: A Review. J. Mar. Sci. Eng. 2022, 10, 849. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.; Lü, Y.; Liu, Y.; Yang, D.; Li, Q.; Qiu, F. Recent progress and future prospects of oil-absorbing materials. Chin. J. Chem. Eng. 2019, 27, 1282–1295. [Google Scholar] [CrossRef]
- Chin, C.C.; Musbah, N.D.L.; Abdullah, I.; Lazim, A.M. Characterization and Evaluation of Prudent Liquid Natural Rubber-Based Foam for Oil Spill Control Application. Arab. J. Sci. Eng. 2018, 43, 6097–6108. [Google Scholar] [CrossRef]
- Jadhav, A.C.; Jadhav, N.C. Graft copolymerization of methyl methacrylate on Meizotropis Pellita fibres and their applications in oil absorbency. Iran. Polym. J. 2021, 30, 9–24. [Google Scholar] [CrossRef]
- Ratcha, A.; Yoosuk, B.; Kongparakul, S. Grafted Methyl Methacrylate and Butyl Methacrylate onto Natural Rubber Foam for Oil Sorbent. Adv. Mater. Res. 2013, 844, 385–390. [Google Scholar] [CrossRef]
- Kanagaraj, L.; Bao, C.A.; Ying, C.S.; Ing, K. Mechanical Properties and Thermal Stability of methyl methacrylate grafted latex and natural rubber latex foam blends. J. Eng. Sci. Technol. 2019, 14, 3616–3627. [Google Scholar]
- Charoeythornkhajhornchai, P.; Samthong, C.; Boonkerd, K.; Somwangthanaroj, A. Effect of azodicarbonamide on microstructure, cure kinetics and physical properties of natural rubber foam. J. Cell. Plast. 2016, 53, 287–303. [Google Scholar] [CrossRef]
- Lee, E.-K.; Choi, S.-Y. Preparation and characterization of natural rubber foams: Effects of foaming temperature and carbon black content. Korean J. Chem. Eng. 2007, 24, 1070–1075. [Google Scholar] [CrossRef]
- Najib, N.N.; Ariff, Z.M.; Bakar, A.A.; Sipaut, C.S. Correlation between the acoustic and dynamic mechanical properties of natural rubber foam: Effect of foaming temperature. Mater. Des. 2011, 32, 505–511. [Google Scholar] [CrossRef]
- Ramasamy, S.; Ismail, H.; Munusamy, Y. Effect of rice husk powder on compression behavior and thermal stability of natural rubber latex foam. BioResources 2013, 8, 4258–4269. [Google Scholar] [CrossRef]
- Panploo, K.; Chalermsinsuwan, B.; Poompradub, S. Natural rubber latex foam with particulate fillers for carbon dioxide adsorption and regeneration. RSC Adv. 2019, 9, 28916–28923. [Google Scholar] [CrossRef] [Green Version]
- Ariff, Z.M.; Zakaria, Z.; Tay, L.H.; Lee, S.Y. Effect of foaming temperature and rubber grades on properties of natural rubber foams. J. Appl. Polym. Sci. 2007, 107, 2531–2538. [Google Scholar] [CrossRef]
- Baru, F.; Saiwari, S.; Hayeemasae, N. Classification of natural rubber foam grades by optimising the azodicarbonamide content. Polímeros 2022, 32, e2022014. [Google Scholar] [CrossRef]
- Yao, K.D.; Peng, T.; Feng, H.B.; He, Y.Y. Swelling kinetics and release characteristic of crosslinked chitosan: Polyether polymer network (semi-IPN) hydrogels. J. Polym. Sci. Part A: Polym. Chem. 1994, 32, 1213–1223. [Google Scholar] [CrossRef]
- Kalkornsurapranee, E.; Sahakaro, K.; Kaesaman, A.; Nakason, C. From a laboratory to a pilot scale production of natural rubber grafted with PMMA. J. Appl. Polym. Sci. 2009, 114, 587–597. [Google Scholar] [CrossRef]
- Nakason, C.; Pechurai, W.; Sahakaro, K.; Kaesaman, A. Rheological, thermal, and curing properties of natural rubber-g-poly(methyl methacrylate). J. Appl. Polym. Sci. 2006, 99, 1600–1614. [Google Scholar] [CrossRef]
- Thiraphattaraphun, L.; Kiatkamjornwong, S.; Prasassarakich, P.; Damronglerd, S. Natural rubber-g-methyl methacrylate/poly(methyl methacrylate) blends. J. Appl. Polym. Sci. 2001, 81, 428–439. [Google Scholar] [CrossRef]
- Jin, J.; Noordermeer, J.W.M.; Dierkes, W.K.; Blume, A. The origin of marching modulus of silica-filled tire tread compounds. Rubber Chem. Technol. 2020, 93, 378–394. [Google Scholar] [CrossRef] [Green Version]
- Harpell, G.A.; Gallagher, R.B.; Novits, M.F. Use of azo foaming agents to produce reinforced elastomeric foams. Rubber Chem. Technol. 1977, 50, 678–687. [Google Scholar] [CrossRef]
- Bhatti, A.S.; Dollimore, D.; Goddard, R.J.; O’Donnell, G. The thermal decomposition of azodicarbonamide. Thermochim. Acta 1984, 76, 63–77. [Google Scholar] [CrossRef]
- Guan, L.T.; Du, F.G.; Wang, G.Z.; Chen, Y.K.; Xiao, M.; Wang, S.J.; Meng, Y.Z. Foaming and chain extension of completely biodegradable poly(propylene carbonate) using DPT as blowing agent. J. Polym. Res. 2007, 14, 245–251. [Google Scholar] [CrossRef]
- Phomrak, S.; Nimpaiboon, A.; Newby, B.-M.Z.; Phisalaphong, M. Natural Rubber Latex Foam Reinforced with Micro- and Nanofibrillated Cellulose via Dunlop Method. Polymers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Lee, H.-K.; Chung, T.-K.; Kim, S.-C.; Kim, H.-G.; Choi, K.-M.; Kim, Y.-M.; Han, D.-H. Influence of the type of curing agent on swelling behaviour of natural rubber foam. J. Korea Acad.-Ind. Coop. Soc. 2008, 9, 1775–1781. [Google Scholar]
- Sae-oui, P.; Sirisinha, C.; Thepsuwan, U.; Thapthong, P. Influence of accelerator type on properties of NR/EPDM blends. Polym. Test. 2007, 26, 1062–1067. [Google Scholar] [CrossRef]
- Kee, D.D.; Liu, Q.; Hinestroza, J. Viscoelastic (Non-Fickian) Diffusion. Can. J. Chem. Eng. 2005, 83, 913–929. [Google Scholar] [CrossRef]
- Bengfort, M.; Malchow, H.; Hilker, F.M. The Fokker–Planck law of diffusion and pattern formation in heterogeneous environments. J. Math. Biol. 2016, 73, 683–704. [Google Scholar] [CrossRef]
- Samarasinghe, I.H.K.; Walpalage, S.; Edirisinghe, D.G.; Egodage, S.M. The use of diisopropyl xanthogen polysulfide as a potential accelerator in efficient sulfur vulcanization of natural rubber compounds. J. Appl. Polym. Sci. 2022, 139, e52063. [Google Scholar] [CrossRef]












| Characteristics | Values | |
|---|---|---|
| NR | SR | |
| Ash content | 0.02% | 0.04% |
| Volatile matter | 0.52% | 0.67% |
| Acetone extract | 2.67% | 14.99% |
| Soluble fraction | 98.7% | 96.5% |
| Nitrogen content | 0.26% | Not detected |
| Original plasticity (Po) | 40.3 | 72.1 |
| Moony viscosity (ML 1 + 4 at 100 °C) | 77.4 | 113.1 |
| Mixing Sequence | Ingredients | Amount (phr) | Mixing Time (min) |
|---|---|---|---|
| 1 | SR/NR * | 100 | 5 |
| 2 | Stearic acid | 1 | 1 |
| 3 | TMQ | 1 | 1 |
| 4 | ZnO | 5 | 1 |
| 5 | CaCO3 | 10 | 3 |
| 6 | TDAE oil | 10 | 2 |
| 7 | CBS | 2.5 | 1 |
| 8 | ADC | 5 | 1.5 |
| 9 | Sulfur | 0.5 | 1 |
| SR/NR (phr/phr) | Relative Foam Density (RFD) | Expansion Ratio | Porosity (1 − RFD) | Hardness (Shore OO) |
|---|---|---|---|---|
| 0/100 | 0.57 ± 0.01 | 1.75 | 0.43 | 58 ± 0.71 |
| 20/80 | 0.59 ± 0.02 | 1.70 | 0.41 | 61 ± 0.44 |
| 30/70 | 0.66 ± 0.01 | 1.52 | 0.34 | 64 ± 0.50 |
| 50/50 | 0.70 ± 0.01 | 1.44 | 0.30 | 69 ± 0.26 |
| 70/30 | 0.72 ± 0.02 | 1.38 | 0.28 | 81 ±0.56 |
| 80/20 | 0.75 ± 0.01 | 1.34 | 0.25 | 86 ± 0.52 |
| 100/0 | 0.84 ± 0.01 | 1.19 | 0.16 | 91 ± 0.59 |
| SR/NR (phr/phr) | n | k |
|---|---|---|
| 0/100 | 0.7348 | 0.0575 |
| 20/80 | 0.7566 | 0.0517 |
| 30/70 | 0.7587 | 0.0512 |
| 50/50 | 0.8074 | 0.0420 |
| 70/30 | 0.8178 | 0.0394 |
| 80/20 | 0.8524 | 0.0344 |
| 100/0 | 0.8668 | 0.0297 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thitithammawong, A.; Saiwari, S.; Salaeh, S.; Hayeemasae, N. Potent Application of Scrap from the Modified Natural Rubber Production as Oil Absorbent. Polymers 2022, 14, 5066. https://doi.org/10.3390/polym14235066
Thitithammawong A, Saiwari S, Salaeh S, Hayeemasae N. Potent Application of Scrap from the Modified Natural Rubber Production as Oil Absorbent. Polymers. 2022; 14(23):5066. https://doi.org/10.3390/polym14235066
Chicago/Turabian StyleThitithammawong, Anoma, Sitisaiyidah Saiwari, Subhan Salaeh, and Nabil Hayeemasae. 2022. "Potent Application of Scrap from the Modified Natural Rubber Production as Oil Absorbent" Polymers 14, no. 23: 5066. https://doi.org/10.3390/polym14235066