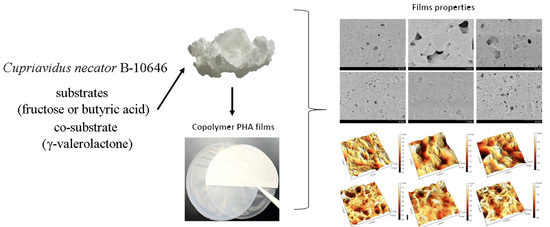
Biosynthesis and Properties of a P(3HB-co-3HV-co-4HV) Produced by Cupriavidus necator B-10646
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Culture Medium and Cultivation Conditions
2.3. PHA Recovery from Cell Biomass
2.4. PHA Chemical Composition
2.5. Physicochemical Properties of PHAs
2.6. Production and Investigation of Polymer Films
2.7. Statistics
3. Results and Discussion
3.1. Synthesis of P(3HB-co-3HV-co-4HV) Copolymers by Bacteria Cupriavidus Necator B-10646
3.2. Physicochemical Properties of P(3HB-co-3HV-co-4HV) Copolymers Depending on the Ratio of Monomers
3.3. Characteristics of Solvent Cast Films Produced from P(3HB-co-3HV-co-4HV) Depending on Monomers Ratio
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 1207–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laversa, J.L.; Bond, A.L. Exceptional and rapid accumulation of anthropogenic debris on one of the world’s most remote and pristine islands. Proc. Natl. Acad. Sci. USA 2017, 114, 6052–6055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojha, N.; Das, N. Microbial production of bioplastics: Current trends and future perspectives. In Bioplastics for Sustainable Development; Springer Verlag: Singapore, 2021; pp. 1–60. [Google Scholar]
- Awasthi, S.K.; Kumar, M.; Kumar, V.; Sarsaiya, S.; Anerao, P.; Ghosh, P.; Singh, L.; Liu, H.; Zhang, Z.; Awasthi, M.K. A comprehensive review on recent advancements in biodegradation and sustainable management of biopolymers. Environ. Pollut. 2022, 307, 119600. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q. Plastics completely synthesized by bacteria: Polyhydroxyalkanoates. In Plastics from Bacteria; Springer: Berlin, Germany, 2010; pp. 17–37. [Google Scholar]
- Sudesh, K. Practical Guide to Microbial Polyhydroxyalkanoates; Smitthes: London, UK, 2010; 158p. [Google Scholar]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoate. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Volova, T.G.; Shishatskaya, E.I.; Sinskey, A.J. Degradable Polymers: Production, Properties, Applications; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2013; 380p. [Google Scholar]
- Chen, G.-Q.; Chen, X.-Y.; Wu, F.-Q.; Chen, J.-C. Polyhydroxyalkanoates (PHA) toward cost competitiveness and functionality. Adv. Ind. Eng. Polym. Res. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Mitra, R.; Xu, T.; Chen, G.-Q.; Xiang, H.; Han, J. An updated overview on the regulatory circuits of polyhydroxyalkanoates synthesis. Microb. Biotechnol. 2021, 15, 1446–1470. [Google Scholar] [CrossRef]
- Tan, D.; Wang, Y.; Tong, Y.; Chen, G.-Q. Grand Challenges for industrializing polyhydroxyalkanoates (PHAs). Trends Biotechnol. 2021, 39, 953–963. [Google Scholar] [CrossRef]
- Koller, M.; Mukherjee, A. A new wave of industrialization of PHA biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Steinbüchel, A.; Valentin, H.E. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 1995, 128, 219–228. [Google Scholar] [CrossRef]
- Koller, M. Chemical and biochemical engineering approaches in manufacturing polyhydroxyalkanoate (PHA) biopolyesters of tailored structure with focus on the diversity of building blocks. Chem. Biochem. Eng. Q. 2018, 32, 413–438. [Google Scholar] [CrossRef]
- Ramsay, B.A.; Saracovan, I.; Ramsay, J.A.; Marchessault, R.H. Continuous production of long-side-chain poly-β-hydroxyalkanoates by Pseudomonas oleovorans. Appl. Environ. Microbiol. 1991, 57, 625–629. [Google Scholar] [CrossRef] [Green Version]
- Barbuzzi, T.; Giuffrida, M.; Impallomeni, G.; Carnazza, S.; Ferreri, A.; Guglielmino, S.P.; Ballistreri, A. Microbial synthesis of poly(3-hydroxyalkanoates) by Pseudomonas aeruginosa from fatty acids: Identification of higher monomer units and structural characterization. Biomacromolecules 2004, 5, 2469–2478. [Google Scholar] [CrossRef]
- Impallomeni, G.; Ballistreri, A.; Carnemolla, G.M.; Rizzo, M.G.; Nicolò, M.S.; Guglielmino, S.P. Biosynthesis and structural characterization of polyhydroxyalkanoates produced by Pseudomonas aeruginosa ATCC 27853 from long odd-chain fatty acids. Int. J. Biol. Macromol. 2018, 108, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.L.L.; da Silva, A.C.M.S.; Menezes Filho, J.A.; Druzian, J.I. Impact of different by-products from the biodiesel industry and bacterial strains on the production, composition, and properties of novel polyhydroxyalkanoates containing achiral building blocks. Ind. Crop. Prod. 2015, 69, 212–223. [Google Scholar] [CrossRef]
- Ray, S.; Kalia, V.C. Co-metabolism of substrates by Bacillus thuringiensis regulates polyhydroxyalkanoate co-polymer composition. Bioresour. Technol. 2017, 224, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Olivera, E.R.; Arcos, M.; Naharro, G.; Luengo, J.M. Unusual PHA biosynthesis. In Plastics from Bacteria; Chen, G.G.Q., Ed.; Springer: Heidelberg/Berlin, Germany, 2010; pp. 133–186. [Google Scholar]
- Vandamme, P.; Coenye, T. Taxonomy of the genus Cupriavidus: A tale of lost and found. Int. Syst. Evol. Microbiol. 2004, 54, 2285–2289. [Google Scholar] [CrossRef] [Green Version]
- Tindall, B.J. Rule 15 of the international code of nomenclature of bacteria: A current source of confusion. Int. Syst. Evol. Microbiol. 2008, 58, 1775–1778. [Google Scholar] [CrossRef] [Green Version]
- Senior, P.J.; Dawes, E.A. The regulation of poly-β-hydroxybutyrate metabolism in Azotobacter beijerinckii. Biochem. J. 1973, 134, 225–238. [Google Scholar] [CrossRef]
- Tarrahi, R.; Fathi, Z.; Seydibeyoğlu, M.O.; Doustkhah, E.; Khataee, A. Polyhydroxyalkanoates (PHA): From production to nanoarchitecture. Int. J. Biol. Macromol. 2020, 146, 596–619. [Google Scholar] [CrossRef]
- López, J.C.; Rodríguez, Y.; Pérez, V.; Lebrero, R.; Muñoz, R. CH4-based polyhydroxyalkanoates production: A step further towards a sustainable bioeconomy. In Biotechnological Applications of Polyhydroxyalkanoates; Springer Nature: Singapore, 2019; pp. 283–321. [Google Scholar] [CrossRef]
- Popa, M.S.; Frone, A.N.; Panaitescu, D.M. Polyhydroxybutyrate blends: A solution for biodegradable packaging. Int. J. Biol. Macromol. 2022, 207, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Mukherjee, A. Polyhydroxyalkanoates—Linking properties, applications, and end-of-life options. Chem. Biochem. Eng. 2020, 34, 115–129. [Google Scholar] [CrossRef]
- Dalton, B.; Bhagabati, P.; De Micco, J.; Padamati, R.B.; O’Connor, K. A review on biological synthesis of the biodegradable polymers polyhydroxyalkanoates and the development of multiple applications. Catalysts 2022, 12, 319. [Google Scholar] [CrossRef]
- Palmeiro-Sanchez, T.; O’Flaherty, V.; Lens, P.N.L. Polyhydroxyalkanoate bio-production and its rise as biomaterial of the future. J. Biotechnol. 2022, 348, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Marova, I.; Snajdar, O.; Mravcova, L.; Svoboda, Z. Production of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) by Cupriavidus necator from waste rapeseed oil using propanol as a precursor of 3-hydroxyvalerate. Biotechnol. Lett. 2010, 32, 1925–1932. [Google Scholar] [CrossRef] [Green Version]
- Ng, L.M.; Sudesh, K. Identification of a new polyhydroxyalkanoate (PHA) producer Aquitalea sp. USM4 (JCM 19919) and characterization of its PHA synthase. J. Biosci. Bioeng. 2016, 122, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Koller, M. Polyhydroxyalkanoate biosynthesis at the edge of water activity-haloarchaea as biopolyester factories. Bioengineering 2019, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.H.; Loo, C.Y.; Nomura, C.T.; Sudesh, K. Biosynthesis of polyhydroxyalkanoate copolymers from mixtures of plant oils and 3-hydroxyvalerate precursors. Bioresour. Technol. 2008, 99, 6844–6851. [Google Scholar] [CrossRef]
- Koller, M.; Salerno, A.; Strohmeier, K.; Schober, S.; Mittelbach, M.; Illieva, V.; Chiellini, E.; Braunegg, G. Novel precursors for production of 3-hydroxyvalerate-containing poly [(R)-hydroxyalkanoate]s. Biocatal. Biotransform. 2014, 32, 161–167. [Google Scholar] [CrossRef]
- Obruca, S.; Benesova, P.; Petrik, S.; Oborna, J.; Prikryl, R.; Marova, I. Production of polyhydroxyalkanoates using hydrolysate of spent coffee grounds. Process Biochem. 2014, 49, 1409–1414. [Google Scholar] [CrossRef]
- Koller, M.; Hesse, P.; Fasl, H.; Stelzer, F.; Braunegg, G. Study on the effect of levulinic acid on whey-based biosynthesis of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) by Hydrogenophaga pseudoflava. Appl. Food Biotechnol. 2017, 4, 65–78. [Google Scholar] [CrossRef]
- Gomez, J.G.C.; Rodrigues, M.F.A.; Alli, R.C.P.; Torres, B.B.; Netto, C.L.; Oliveira, M.S.; Da Silva, L.F. Evaluation of soil gram-negative bacteria yielding polyhydroxyalkanoic acids from carbohydrates and propionic acid. Appl. Microbiol. Biotechnol. 1996, 45, 785–791. [Google Scholar] [CrossRef]
- Oliveira-Filho, E.R.; Gomez, J.G.C.; Taciro, M.K.; Silva, L.F. Burkholderia sacchari (synonym Paraburkholderia sacchari): An industrial and versatile bacterial chassis for sustainable biosynthesis of polyhydroxyalkanoates and other bioproducts. Bioresour. Technol. 2021, 337, 125472. [Google Scholar] [CrossRef]
- Martínez-Abad, A.; Cabedo, L.; Oliveira, C.S.; Hilliou, L.; Reis, M.; Lagarón, J.M. Characterization of polyhydroxyalkanoate blends incorporating unpurified biosustainably produced poly (3-hydroxybutyrate-co-3-hydroxyvalerate). J. Appl. Polym. Sci. 2016, 133, 42633. [Google Scholar] [CrossRef]
- Meléndez-Rodríguez, B.; Torres-Giner, S.; Reis, M.A.; Silva, F.; Matos, M.; Cabedo, L.; Lagarón, J.M. Blends of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) with fruit pulp biowaste derived poly (3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) for organic recycling food packaging. Polymers 2021, 13, 1155. [Google Scholar] [CrossRef] [PubMed]
- Valentin, H.E.; Schönebaum, A.; Steinbüchel, A. Identification of 4-hydroxyvaleric acid as a constituent of biosynthetic polyhydroxyalkanoic acids from bacteria. Appl. Microbiol. Biotechnol. 1992, 36, 507–514. [Google Scholar] [CrossRef]
- Valentin, H.E.; Steinbüchel, A. Accumulation of poly (3-hydroxybutyric acid-co-3-hydroxyvaleric acid-co-hydroxyvaleric acid) by mutants and recombinant strains of Alcaligenes eutrophus. J. Environ. Polym. Degrad. 1995, 3, 169–175. [Google Scholar] [CrossRef]
- Muzaiyanah, A.R.; Amirul, A.A. Studies on the microbial synthesis and characterization of polyhydroxyalkanoates containing 4-hydroxyvalerate using γ-valerolactone. Appl. Biochem. Biotechnol. 2013, 170, 1194–1215. [Google Scholar] [CrossRef]
- Tanadchangsaeng, N.; Yu, J. Miscibility of natural polyhydroxyalkanoate blend with controllable material properties. J. Appl. Polym. Sci. 2013, 129, 2004–2016. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Cai, J.Y.; Liu, Z.; Zheng, Y.; Wang, H.; Li, Q.; He, N. Biosynthesis and thermal properties of PHBV produced from levulinic acid by Ralstonia eutropha. PLoS ONE 2013, 8, e60318. [Google Scholar] [CrossRef]
- Sheu, D.S.; Chen, Y.L.L.; Jhuang, W.J.; Chen, H.Y.; Jane, W.N. Cultivation temperature modulated the monomer composition and polymer properties of polyhydroxyalkanoate synthesized by Cupriavidus sp. L7L from levulinate as sole carbon source. Int. J. Biol. Macromol. 2018, 118, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Novackova, I.; Kucera, D.; Porizka, J.; Pernicova, I.; Sedlacek, P.; Koller, M.; Kovalcik, A.; Obruca, S. Adaptation of Cupriavidus necator to levulinic acid for enhanced production of P(3HB-co-3HV) copolyesters. Biochem. Eng. J. 2019, 151, 107350. [Google Scholar] [CrossRef]
- Ashby, R.D.; Ashby, R.D.; Solaiman, D.K.Y.; Strahan, G.D.; Zhu, C.; Tappel, R.C.; Nomura, C.T. Glycerine and levulinic acid: Renewable co-substrates for the fermentative synthesis of short-chain poly (hydroxyalkanoate) biopolymers. Biores. Technol. 2012, 118, 272–280. [Google Scholar] [CrossRef]
- Irorere, V.U.; Bagheriasl, S.; Blevins, M.; Kwiecien, I.; Stamboulis, A.; Radecka, I. Electrospun Fibres of polyhydroxybutyrate synthesized by Ralstonia eutropha from different carbon sources. Int. J. Polym. Sci. 2014, 2014, 705359. [Google Scholar] [CrossRef] [Green Version]
- Sanhueza, C.; Diaz-Rodriguez, P.; Villegas, P.; González, A.; Seeger, M.; Suárez-González, J.; Concheiro, A.; Alvarez-Lorenzo, C.; Acevedo, F. Influence of the carbon source on the properties of poly-(3)-hydroxybutyrate produced by Paraburkholderia xenovorans LB400 and its electrospun fibers. Int. Biol. Macromol. 2020, 152, 11–20. [Google Scholar] [CrossRef]
- Shishatskaya, E.; Nemtsev, I.; Lukyanenko, A.; Vasiliev, A.; Kiselev, E.; Sukovatyi, A.; Volova, T. Polymer films of poly-3-hydroxybutyrate synthesized by Cupriavidus necator from different carbon sources. J. Polym. Environ. 2021, 29, 837–850. [Google Scholar] [CrossRef]
- Volova, T.; Kiselev, E.; Nemtsev, I.; Lukyanenko, A.; Sukovatyi, A.; Kuzmin, A.; Ryltseva, G.; Shishatskaya, E. Properties of degradable PHAs with different monomer compositions. Int. Biol. Macromol. 2021, 182, 98–114. [Google Scholar] [CrossRef]
- Zhila, N.O.; Sapozhnikova, K.Y.; Kiselev, E.G.; Vasiliev, A.D.; Nemtsev, I.V.; Shishatskaya, E.I.; Volova, T.G. Properties of Degradable Polyhydroxyalkanoates (PHAs) Synthesized by a New Strain, Cupriavidus necator IBP/SFU-1, from Various Carbon Sources. Polymers 2021, 13, 3142. [Google Scholar] [CrossRef]
- Volova, T.G.; Shishatskaya, E.I. Cupriavidus eutrophus Bacterial Strain VKPM B-10646-A Producer of Polyhydroxyalkanoates and a Method of Their Production (Cupriavidus eutrophus Shtamm Bakterii VKPM B-10646-Produtsent Poligidroksialkanoatov i Sposob Ikh Polucheniya). RU2439143C1, 10 January 2012. (In Russian). [Google Scholar]
- Schlegel, H.G.; Kaltwasser, H.; Gottschalk, G. A submersion method for culture of hydrogen-oxidizing bacteria: Growth physiological studies. Arch. Microbiol. 1961, 38, 209–222. [Google Scholar]
- Volova, T.; Kiselev, E.; Shishatskaya, E.; Zhila, N.; Boyandin, A.; Syrvacheva, D.; Vinogradova, O.; Kalacheva, G.; Vasiliev, A.; Peterson, I. Cell growth and PHA accumulation from CO2 and H2 of a hydrogen-oxidizing bacterium, Cupriavidus eutrophus B-10646. Bioresour. Technol. 2013, 146, 215–222. [Google Scholar] [CrossRef]
- Ermakov, A.I.; Arasimovich, V.V.; Smirnova-Ikonnikova, M.I.; Yarosh, N.P.; Lukovnikova, G.A. Metody Biokhimicheskogo Issledovaniya Rastenii (Methods of Biochemical of Biochemical Plant Research); Kolos Publishers: Sankt Petersburg, Russia, 1972; 456p. (in Russian) [Google Scholar]
- Kiselev, E.G. Technical and Technological Bases of Biosynthesis of Reserve Polyhydroxyalkanoates by Hydrogen Bacteria. Ph.D. Thesis, Siberian Federal University, Krasnoyarsk, Russia, 2012. [Google Scholar]
- Sharma, V.; Sehgal, R.; Gupta, R. Polyhydroxyalkanoate (PHA): Properties and modifications. Polym. J. 2021, 212, 123–161. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khalil, S.; Abid, S. Recent progress in development and chemical modification of poly (hydroxybutyrate)-based blends for potential medical applications. Int. J. Biol. Macromol. 2020, 160, 77–100. [Google Scholar] [CrossRef]
- ISO 468:1982; Surface Roughness. Parameters, Their Values, and General Rules for Specifying Requirements. ISO: Geneva, Switzerland, 1982.
- Volova, T.G.; Zhila, N.O.; Shishatskaya, E.I.; Mironov, P.V.; Vasil’ev, A.D.; Sukovatyi, A.G.; Sinskey, A.J. The physicochemical properties of polyhydroxyalkanoates with different chemical structures. Polym. Sci. Ser. A 2013, 55, 427–437. [Google Scholar] [CrossRef]
- Kiselev, E.G.; Vasiliev, A.D.; Volova, T.G. Synthesis and characterization of multicomponent PHAs. J. Sib. Fed. Univ. Biol. 2021, 14, 97–113. [Google Scholar] [CrossRef]
- Volova, T.; Peterson, I.; Kiselev, E.; Shishatskaya, E.; Menshikova, O.; Vasiliev, A.; Zhila, N.; Thomas, S. Biosynthesis and properties of P(3HB/3HV/3H4MV) produced by using Cupriavidus eutrophus B-10646. J. Chem. Technol. Biotechnol. 2019, 94, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Green, P.R.; Kemper, J.; Schechtman, L.; Guo, L.; Satkowski, M.; Fiedler, S.; Steinbüchel, A.; Rehm, B.H.A. Formation of short chain length/medium chain length polyhydroxyalkanoate copolymers by fatty acid β-oxidation inhibited Ralstonia eutropha. Biomacromolecules 2002, 3, 208–213. [Google Scholar] [CrossRef]
- Volova, T.G.; Syrvacheva, D.A.; Zhila, N.O.; Sukovatiy, A.G. Synthesis of P(3HB-co-3HHx) copolymers containing high molar fraction of 3-hydroxyhexanoate monomer by Cupriavidus eutrophus B10646. J. Chem. Technol. Biotechnol. 2016, 91, 416–425. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, Z.; Marchessault, R.H.; Ramsay, J.A.; Ramsay, B.A. Biosynthesis and properties of medium-chain-length polyhydroxyalkanoates with enriched content of the dominant monomer. Biomacromolecules 2012, 13, 2926–2932. [Google Scholar] [CrossRef]
- Gao, J.; Vo, M.T.; Ramsay, J.A.; Ramsay, B.A. Overproduction of MCL-PHA with high 3-hydroxydecanoate content. Biotechnol. Bioeng. 2018, 115, 390–400. [Google Scholar] [CrossRef]
- Gorenflo, V.; Schmack, G.; Vogel, R.; Steinbüchel, A. High-titer production of monomeric hydroxyvalerates from levulinic acid in Pseudomonas putida. J. Biotechnol. 2009, 139, 61–67. [Google Scholar] [CrossRef]
- Cha, D.; Ha, H.S.; Lee, S.K. Metabolic engineering of Pseudomonas putida for the production of various types of short-chain-length polyhydroxyalkanoates from levulinic acid. Bioresour. Technol. 2020, 309, 123332. [Google Scholar] [CrossRef]
- Scandola, M.; Ceccorulli, G.; Pizzoli, M.; Gazzano, M. Study of the crystal phase and crystallization rate of bacterial poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Macromolecules 1992, 25, 1405–1410. [Google Scholar] [CrossRef]
- Hench, L.L.; Jones, J.R. Biomaterials, Artificial Organs and Tissue Engineering; Technosphera: Moscow, Russia, 2007; 304p. [Google Scholar]
- Zhang, J.; Shishatskaya, E.I.; Volova, T.G.; da Silva, L.F.; Chen, G.Q. Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater. Sci. Eng. C 2018, 86, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Srivastava, J.K.; Chandel, A.K.; Sharma, L.; Mallick, N.; Singh, S.P. Biomedical applications of microbially engineered polyhydroxyalkanoates: An insight into recent advances, bottlenecks, and solutions. Appl. Microbiol. Biotechnol. 2019, 103, 2007–2032. [Google Scholar] [CrossRef]
- Kalia, V.; Gogante, P.; Cinelli, P.; Seggiani, V.A.; Alaverex, A.; Lazzeri, A. Processing and thermomechanical properties of PHA. In The Handbook of Polyhydroxyalkanoates. Postsynthetic Treatment, Processing and Applications, 1st ed.; Koller, M., Ed.; CRS Press: Boca Raton, FL, USA, 2020; pp. 91–118. [Google Scholar]
- Asare, E.; Gregory, D.A.; Frisker, A.; Marcello, E.; Paxinou, A.; Taylor, C.S.; Haucock, J.W.; Roy, I. Polyhydroxyalkanoates, their processing and biomedical applications. In The Handbook of Polyhydroxyalkanoates. Postsynthetic Treatment, Processing and Applications, 1st ed.; Koller, M., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 255–284. [Google Scholar]
- Volova, T.G.; Kiselev, E.G.; Demidenko, A.V.; Zhila, N.O.; Nemtsev, I.V.; Lukyanenko, A.V. Production and properties of microbial polyhydroxyalkanoates synthesized from hydrolysates of Jerusalem artichoke tubers and vegetative biomass. Polymers 2022, 14, 132. [Google Scholar] [CrossRef]








| Strain, Substrate | X, g/L | PHA, % | 3HB | 3HV | 4HV | Mw, kDa | Đ | Tmelt, °C | Tdegr, °C | Tg, °C | Cx, % | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. eutrophus H16, 4-hydroxyvaleric acid | - | 52 | 39.9 | 53.9 | 6.3 | - | - | - | - | - | - | [41] |
| A. eutrophus H16, γ-valerolactone | - | 73 | 41 | 54.1 | 4.9 | - | - | - | - | - | - | [41] |
| A. eutrophus NCIB 11599, 4-hydroxyvaleric acid | - | 45 | 23.9 | 67.3 | 8.8 | - | - | - | - | - | - | [42] |
| C. necator USMAA2-4, oleic acid + γ-valerolactone | - | - | 8 | 91 | 1 | 510 | 2.6 | 95.5 | 281 | −25.1 | 9.7 | [43] |
| C. necator USMAA2-4, oleic acid + γ-valerolactone | - | - | 43 | 55 | 2 | 110 | 3.8 | 148.9 | 281 | −24.4 | 7.9 | [43] |
| C. necator USMAA2-4, oleic acid + γ-valerolactone | - | - | 71 | 27 | 2 | 570 | 2.9 | 147.8 | 275 | −13.2 | 19.7 | [43] |
| C. necator USMAA2-4, oleic acid + γ-valerolactone | - | - | 90 | 9 | 1 | 280 | 3.4 | 145.2 | 263 | −12.8 | 20.8 | [43] |
| C. necator ATCC 17699, glycerol + levulinic acid (3-L fermenter, fed-batch) | 58 | 80 | 79 | 18.5 | 2.5 | 810 | 2.2 | 79 161 175 | - | −1.3 | 42 | [44] |
| C. necator ATCC 17699, levulinic acid | - | - | 38 | 55.3 | 6.7 | 1060 | 2.2 | 60 | - | −8.3 | 34 | [44] |
| R. eutropha H16, glucose + levulinic acid (2-L fermenter) | 15.5 | 81.2 | 46.1 | 53.9 | - | - | - | - | - | - | - | [45] |
| R. eutropha H16, glucose + levulinic acid | - | - | 84 | 16 | - | - | - | 150.2 | 298.3 | 50.3 | [45] | |
| R. eutropha H16, glucose + levulinic acid | - | - | 47 | 53 | - | - | - | 101.9 | 284.4 | 51.9 | [45] | |
| Cupriavidus sp. L7L, levulinic acid | 3.9 | 55 | 45.1 | 50.1 | 4.8 | 4032 | 4.1 | 71.8 | 256.6 | −7.87 | - | [46] |
| Cupriavidus sp. L7L, levulinic acid | 3.5 | 60 | 64.2 | 33.7 | 2.1 | 2825 | 3.6 | 92.0 | 250.5 | −0.62 | - | [46] |
| C. necator H16 CCM 3726, fructose + levulinic acid | 7.3 | 47.8 | 84.2 | 15.8 | - | 668 | 1.05 | 167.2 | - | - | - | [47] |
| Burkholderia sacchari DSM 17165, xylose + levulinic acid | 3.3 | 45 | 57 | 53 | - | 3910 | 2.98 | 148.3 157.7 | - | 2 −13 | [48] | |
| Burkholderia sacchari DSM 17165, xylose + levulinic acid | 3.1 | 32 | 35 | 65 | - | 2774 | 2.78 | 99.2 | - | −4 −14 | [48] | |
| Burkholderia sacchari DSM 17165, xylose + glucose + levulinic acid | 5.3 | 49 | 88 | 12 | - | 2542 | 2.48 | 168.7 | 3 | [48] | ||
| Burkholderia sacchari DSM 17165, xylose + glucose + levulinic acid | 2.7 | 30 | 24 | 76 | - | 2930 | 2.91 | 103.4 | 3 −14 | [48] |
| N | Composition of Monomers, mol.% | Mn, kDa | Mw, kDa | Ð | Cx, % | Tmelt, °C | Tdegr, °C | Tg, °C | Tcryst °C | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3HB | 3HV | 4HV | |||||||||
| 1 | 89.9 | 7.3 | 2.8 | 45 ± 1 | 212 ± 9 | 4.7 ± 0.1 | 49 | 145.0/ 168.0 | 284.1 | 0.5 | 54.0 |
| 2 | 88.3 | 9.4 | 2.3 | 51 ± 2 | 248 ± 29 | 4.9 ± 0.7 | 46 | 147.1/ 162.9 | 280.4 | 0.3 | 71.2 55.9 |
| 3 | 83.7 | 14.4 | 1.9 | 87 ± 6 | 242 ± 10 | 2.8 ± 0.1 | 43 | 151.0 164.5 | 270.6 | 1.1 | 55.7 58.2 |
| 4 | 81.2 | 16.3 | 2.5 | 59 ± 3 | 201 ± 10 | 3.4 ± 0.1 | 46 | 166.1 | 281.2 | 1.2 | 57.2 51.8 |
| 5 | 76.9 | 20.8 | 2.3 | 46 ± 2 | 215 ± 11 | 4.6 ± 0.1 | 43 | 142.0 160.0 | 275.1 | −0.7 | 68.0 |
| 6 | 71.9 | 23.4 | 4.7 | 70 ± 7 | 203 ± 15 | 2.9 ± 0.1 | 38 | 150.4 164.8 | 263.4 | −10.6 1.47 | 54.2 61.5 |
| 7 | 100 | 0 | 0 | 248 | 620 ± 30 | 2.5 ± 0.1 | 74 | 179.3 | 279.4 | 5.3 | 81.6 |
| N | Copolymer Composition, Mol.% | Porosity | Surface Roughness | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 3HB | 3HV | 4HV | Average Pore Area, µm2 | Number of Pores, Pores/1000 µm2 | Total Pores Area, µm2/1000 µm2 | Arithmetic Mean Surface Roughness, (Sa) nm | Root Mean Square Roughness, (Sq) nm | Peak-To-Valley Height, (Sz) nm | |
| P(3HB-co-3HV-co-4HV) | |||||||||
| 1 | 89.9 | 7.3 | 2.8 | 13.7 | 24.0 | 328 | 202 | 263 | 2010 |
| 2 | 88.3 | 9.4 | 2.3 | 248.4 | 2.6 | 643 | 544 | 699 | 3994 |
| 3 | 83.7 | 14.4 | 1.9 | 81.7 | 12.8 | 1047 | 388 | 491 | 2878 |
| 4 | 81.2 | 16.3 | 2.5 | 17.9 | 29.6 | 530 | 206 | 266 | 1768 |
| 5 | 76.9 | 20.8 | 2.3 | 2.6 | 133.7 | 353 | 208 | 260 | 1738 |
| 6 | 71.9 | 23.4 | 4.7 | 5.5 | 88.0 | 488 | 317 | 397 | 2407 |
| P(3HB-co-3HV) | |||||||||
| 1 | 85.0 | 15.0 | 0 | 1.0 | 260 | 260 | 373 | 471 | 3260 |
| 2 | 35.0 | 65.0 | 0 | 1.5 | 430 | 645 | 489 | 445 | 4295 |
| P(3HB) | |||||||||
| 1 | 100.0 | 0 | 0 | 0.02 | 38.0 | 0.76 | 154 | 180 | 1256 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhila, N.O.; Sapozhnikova, K.Y.; Kiselev, E.G.; Nemtsev, I.V.; Lukyanenko, A.V.; Shishatskaya, E.I.; Volova, T.G. Biosynthesis and Properties of a P(3HB-co-3HV-co-4HV) Produced by Cupriavidus necator B-10646. Polymers 2022, 14, 4226. https://doi.org/10.3390/polym14194226
Zhila NO, Sapozhnikova KY, Kiselev EG, Nemtsev IV, Lukyanenko AV, Shishatskaya EI, Volova TG. Biosynthesis and Properties of a P(3HB-co-3HV-co-4HV) Produced by Cupriavidus necator B-10646. Polymers. 2022; 14(19):4226. https://doi.org/10.3390/polym14194226
Chicago/Turabian StyleZhila, Natalia O., Kristina Yu. Sapozhnikova, Evgeniy G. Kiselev, Ivan V. Nemtsev, Anna V. Lukyanenko, Ekaterina I. Shishatskaya, and Tatiana G. Volova. 2022. "Biosynthesis and Properties of a P(3HB-co-3HV-co-4HV) Produced by Cupriavidus necator B-10646" Polymers 14, no. 19: 4226. https://doi.org/10.3390/polym14194226