Comparative Evaluation of Locally Administered 2% Gel Fabricated from Lemongrass Polymer and 10% Doxycycline Hyclate Gel as an Adjunct to Scaling and Root Planing in the Treatment of Chronic Periodontitis—A Randomized Controlled Trial
Abstract
:1. Introduction
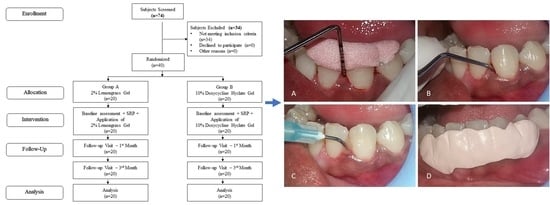
2. Materials and Methods
2.1. Study Design
2.2. Formulation of 2% Lemongrass Gel and 10% Doxycycline Hyclate Gel
2.2.1. Preparation of 2% Lemongrass Gel
2.2.2. Preparation of 10% Doxycycline Hyclate Gel
2.3. Study Procedure
2.4. Clinical Assessments
2.5. Microbiological Analysis
2.6. Study Intervention
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newman, M.G.; Takei, H.; Klokkevold, P.R.; Carranza, F.A. Newman and Carranza’s Clinical Periodontology E-Book; Elsevier Health Sciences: IOWA City, IA, USA, 2018; pp. 50–370. [Google Scholar]
- Salvi, G.E.; Mombelli, A.; Mayfield, L.; Rutar, A.; Suvan, J.; Garrett, S.; Lang, N.P. Local antimicrobial therapy after initial periodontal treatment. J. Clin. Periodontol. 2002, 29, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Slots, J. Local delivery of antimicrobial agents in the periodontal pocket. Periodontology 2000 1996, 10, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Zambon, J.J. Periodontal diseases: Microbial factors. Ann. Periodontol. 1996, 1, 879–925. [Google Scholar] [CrossRef]
- Walker, C.B.; Godowski, K.C.; Borden, L.; Lennon, J.; Nangó, S.; Stone, C.; Garrett, S. The effects of sustained release doxycycline on the anaerobic flora and antibiotic-resistant patterns in subgingival plaque and saliva. J. Periodontol. 2000, 71, 768–774. [Google Scholar] [CrossRef]
- Soskolne, W.A. Subgingival delivery of therapeutic agents in the treatment of periodontal diseases. Crit. Rev. Oral Biol. Med. 1997, 8, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Slots, J.; Rosling, B.G. Suppression of the periodontopathic microflora in localized juvenile periodontitis by systemic tetracycline. J. Clin Periodontol. 1983, 10, 465–486. [Google Scholar] [CrossRef]
- Golub, L.M.; Sorsa, T.; Lee, H.M.; Ciancio, S.; Sorbi, D.; Ramamurthy, N.S.; Gruber, B.; Salo, T.; Konttinen, Y.T. Doxycycline inhibits neutrophil (PMN)-type matrix metalloproteinases in human adult periodontitis gingiva. J. Clin. Periodontol. 1995, 22, 100–109. [Google Scholar] [CrossRef]
- Jorgensen, M.G.; Slots, J. Responsible use of antimicrobials in periodontics. J. Calif. Dent. Assoc. 2000, 28, 185–193. [Google Scholar]
- Slots, J.; Pallasch, T.J. Dentists’ role in halting antimicrobial resistance. J. Dent. Res. 1996, 75, 1338–1341. [Google Scholar] [CrossRef]
- Palombo, E.A. Traditional Medicinal Plant Extracts and Natural Products with Activity against Oral Bacteria: Potential Application in the Prevention and Treatment of Oral Diseases. Evid. Based Complement. Altern. Med. 2011, 2011, 680354. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.; Islam, B.; Akram, M.; Shakil, S.; Ahmad, A.; Ali, S.M.; Siddiqui, M.; Khan, A.U. Antimicrobial activity of five herbal extracts against multi drug resistant (MDR) strains of bacteria and fungus of clinical origin. Molecules 2009, 14, 586–597. [Google Scholar] [CrossRef]
- Sharma, R.; Hebbal, M.; Ankola, A.V.; Murugaboopathy, V.; Shetty, S.J. Effect of two herbal mouthwashes on gingival health of school children. J. Tradit. Complement. Med. 2014, 4, 272–278. [Google Scholar] [CrossRef]
- Shah, G.; Shri, R.; Panchal, V.; Sharma, N.; Singh, B.; Mann, A.S. Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemon grass). J. Adv. Pharm. Technol. Res. 2011, 2, 3–8. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Khongkhunthian, S.; Sookkhee, S.; Okonogi, S. Antimicrobial activities against periodontopathogens of essential oil from lemon grass (Cymbopogon citratus (DC.) Stapf CMU. J. Nat. Sci. 2009, 8, 11–21. [Google Scholar]
- Susanto, S.A.; Oktavianti, T.A.; Wijaya, Y.; Wira, V.; Paramitta, V.A. Increased glutathione level in saliva of moderate gingivitis patients after lemongrass (cymbopogon citratus) essential oil gargling. Asia Pac. Dent. Stud. J. 2010, 1, 45–52. [Google Scholar]
- Anand, K.M.; Goyal, R.; SubrayaBhat, G.; Kamath, S.; Aggarwal, M.; Meghna, A.; Bhandarkar, B.S.; Sukreeth, S. A novel anti-oxidant lemon grass oil mouthwash—A clinical trial. Asian J. Exp. Biol. Sci. 2011, 2, 482–486. [Google Scholar]
- Patel, J.; Patel, B.; Banwait, H.; Parmar, K.; Patel, M. Formulation and Evaluation of Topical Aceclofenac Gel Using Different Gelling Agents. Int. J. Drug Dev. Res. 2011, 3, 156–164. [Google Scholar]
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef]
- Loe, H.; Silness, J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef]
- Silness, J.; Loe, H. Periodontal disease in pregnancy. Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- Shaddox, L.M.; Andia, D.C.; Casati, M.Z.; Nociti, F.H., Jr.; Sallum, E.A.; Gollwitzer, J.; Walker, C.B. Microbiologic changes following administration of locally delivered doxycycline in smokers: A 15-month follow-up. J. Periodontol. 2007, 78, 2143–2149. [Google Scholar] [CrossRef]
- Gu, J.M.; Robinson, J.R.; Leung, S.H. Binding of acrylic polymers to mucin/epithelial surfaces: Structure-property relationships. Crit. Rev. Ther. Drug Carr. Syst. 1988, 5, 21–67. [Google Scholar]
- Barry, B.W.; Meyer, M.C. The rheological properties of carbopol gels I. Continuous shear and creep properties of carbopol gels. Int. J. Pharm. 1979, 2, 1–25. [Google Scholar] [CrossRef]
- Davies, N.M.; Farr, S.J.; Hadgraft, J.; Kellaway, I.W. Evaluation of mucoadhesive polymers in ocular drug delivery. II. Polymer-coated vesicles. Pharm. Res. 1992, 9, 1137–1144. [Google Scholar] [CrossRef]
- Tan, Y.T.; Peh, K.K.; Hanbali, A.O. Effect of Carbopol and polyvinylpyrrolidone on the mechanical, rheological, and release properties of bioadhesive polyethylene glycol gels. AAPS PharmSciTech 2000, 1, 69–78. [Google Scholar] [CrossRef]
- Bilensoy, E.; Sen, M.; Dogan, A.L.; Calis, S. Thermosensitive mucoadhesive gel formulation loaded with 5-Fu: Cyclodextrin complex for HPV-induced cervical cancer. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 363–370. [Google Scholar] [CrossRef]
- Patel, J.K.; Chavda, J.R. Formulation and evaluation of stomachspecific amoxicillin-loaded carbopol-934P mucoadhesive microspheres for anti-Helicobacter pylori therapy. J. Microencapsul. 2009, 2, 6365–6376. [Google Scholar]
- Sforcin, J.; Amaral, J.; Fernandes, A., Jr.; Sousa, J.; Bastos, J. Lemongrass effects on IL-1β and IL-6 production by macrophages. Nat. Prod. Res. 2009, 23, 1151–1159. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 1994, 269, 8022–8028. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, H.S.; Kim, D.J.; Noh, Y.H.; Yuk, D.Y.; Hong, J. Inhibitory effect of citral on NO production by suppression of iNOS expression and NF-κB activation in RAW264. 7 cells. Arch. Pharm. Res. 2008, 31, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Bachiega, T.F.; Sforcin, J.M. Lemongrass and citral effect on cytokines production by murine macrophages. J. Ethnopharmacol. 2011, 137, 909–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warad, S.B.; Kolar, S.S.; Kalburgi, V.; Kalburgi, N.B. Lemongrass essential oil gel as a local drug delivery agent for the treatment of periodontitis. Anc. Sci. Life 2013, 32, 205–211. [Google Scholar]
- Satthanakul, P.; Taweechaisupapong, S.; Paphangkorakit, J.; Pesee, M.; Timabut, P.; Khunkitti, W. Antimicrobial effect of lemongrass oil against oral malodour micro-organisms and the pilot study of safety and efficacy of lemongrass mouthrinse on oral malodour. J. Appl. Microbiol. 2015, 118, 11–17. [Google Scholar] [CrossRef]




| Groups | N | Mean | SD | p-Value | |
|---|---|---|---|---|---|
| GI | 2% Lemongrass Gel | 20 | 1.78 | 0.26 | 0.868 |
| 10% Doxycycline Gel | 20 | 1.76 | 0.24 | ||
| PI | 2% Lemongrass Gel | 20 | 1.82 | 0.20 | 0.814 |
| 10% Doxycycline Gel | 20 | 1.84 | 0.19 | ||
| PPD | 2% Lemongrass Gel | 20 | 5.30 | 0.47 | 0.744 |
| 10% Doxycycline Gel | 20 | 5.35 | 0.49 | ||
| CAL | 2% Lemongrass Gel | 20 | 5.50 | 0.51 | 0.759 |
| 10% Doxycycline Gel | 20 | 5.55 | 0.51 | ||
| Porphyromonas Gingivalis (103 CFU) | 2% Lemongrass Gel | 20 | 1.06 | 0.54 | 0.757 |
| 10% Doxycycline Gel | 20 | 1.00 | 0.60 | ||
| Actinomyces Naeslundi (102 CFU) | 2% Lemongrass Gel | 20 | 0.69 | 0.43 | 0.683 |
| 10% Doxycycline Gel | 20 | 0.75 | 0.51 | ||
| Prevotella Intermedia (103 CFU) | 2% Lemongrass Gel | 20 | 1.12 | 0.48 | 0.274 |
| 10% Doxycycline Gel | 20 | 0.94 | 0.52 |
| Groups | Assessment Period | Mean | SD | p-Value | |
|---|---|---|---|---|---|
| GI | 2% Lemongrass Gel | Baseline | 1.78 | 0.26 | <0.0001 * Ф |
| 1 Month | 1.34 | 0.27 | |||
| 3 Months | 1.22 | 0.28 | |||
| 10% Doxycycline Gel | Baseline | 1.76 | 0.24 | <0.0001 * Ф | |
| 1 Month | 1.34 | 0.23 | |||
| 3 Months | 1.16 | 0.28 | |||
| PI | 2% Lemongrass Gel | Baseline | 1.82 | 0.20 | 0.021 * |
| 1 Month | 1.78 | 0.25 | |||
| 3 Months | 1.73 | 0.23 | |||
| 10% Doxycycline Gel | Baseline | 1.84 | 0.19 | 0.015 * Ф | |
| 1 Month | 1.72 | 0.26 | |||
| 3 Months | 1.70 | 0.28 | |||
| PPD | 2% Lemongrass Gel | Baseline | 5.30 | 0.47 | <0.0001 * Ф |
| 1 Month | 3.30 | 0.47 | |||
| 3 Months | 3.25 | 0.44 | |||
| 10% Doxycycline Gel | Baseline | 5.35 | 0.49 | <0.0001 * Ф | |
| 1 Month | 3.40 | 0.50 | |||
| 3 Months | 3.15 | 0.37 | |||
| CAL | 2% Lemongrass Gel | Baseline | 5.50 | 0.51 | <0.0001 * Ф |
| 1 Month | 3.50 | 0.61 | |||
| 3 Months | 3.45 | 0.60 | |||
| 10% Doxycycline Gel | Baseline | 5.55 | 0.51 | <0.0001 * Ф | |
| 1 Month | 3.60 | 0.50 | |||
| 3 Months | 3.35 | 0.49 |
| Groups | Assessment Period | Mean | SD | p-Value | |
|---|---|---|---|---|---|
| Porphyromonas Gingivalis (103 CFU) | 2% Lemongrass Gel | Baseline | 1.06 | 0.54 | <0.0001 * Ф |
| 1 Month | 0.31 | 0.34 | |||
| 3 Months | 0.28 | 0.31 | |||
| 10% Doxycycline Gel | Baseline | 1.00 | 0.60 | <0.0001 * Ф | |
| 1 Month | 0.37 | 0.33 | |||
| 3 Months | 0.38 | 0.35 | |||
| Actinomyces Naeslundi (102 CFU) | 2% Lemongrass Gel | Baseline | 0.69 | 0.43 | <0.0001 * Ф |
| 1 Month | 0.33 | 0.34 | |||
| 3 Months | 0.31 | 0.30 | |||
| 10% Doxycycline Gel | Baseline | 0.75 | 0.51 | <0.0001 * Ф | |
| 1 Month | 0.33 | 0.31 | |||
| 3 Months | 0.32 | 0.31 | |||
| Prevotella Intermedia (103 CFU) | 2% Lemongrass Gel | Baseline | 1.12 | 0.48 | <0.0001 * Ф |
| 1 Month | 0.70 | 0.36 | |||
| 3 Months | 0.67 | 0.35 | |||
| 10% Doxycycline Gel | Baseline | 0.94 | 0.52 | <0.0001 * Ф | |
| 1 Month | 0.51 | 0.41 | |||
| 3 Months | 0.52 | 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mittal, P.; Gokhale, S.T.; Manjunath, S.; Al-Qahtani, S.M.; Magbol, M.A.; Nagate, R.R.; Tikare, S.; Chaturvedi, S.; Agarwal, A.; Venkataram, V. Comparative Evaluation of Locally Administered 2% Gel Fabricated from Lemongrass Polymer and 10% Doxycycline Hyclate Gel as an Adjunct to Scaling and Root Planing in the Treatment of Chronic Periodontitis—A Randomized Controlled Trial. Polymers 2022, 14, 2766. https://doi.org/10.3390/polym14142766
Mittal P, Gokhale ST, Manjunath S, Al-Qahtani SM, Magbol MA, Nagate RR, Tikare S, Chaturvedi S, Agarwal A, Venkataram V. Comparative Evaluation of Locally Administered 2% Gel Fabricated from Lemongrass Polymer and 10% Doxycycline Hyclate Gel as an Adjunct to Scaling and Root Planing in the Treatment of Chronic Periodontitis—A Randomized Controlled Trial. Polymers. 2022; 14(14):2766. https://doi.org/10.3390/polym14142766
Chicago/Turabian StyleMittal, Pooja, Shankar T. Gokhale, Shiva Manjunath, Saad M. Al-Qahtani, Mohammad Al. Magbol, Raghavendra Reddy Nagate, Shreyas Tikare, Saurabh Chaturvedi, Ashish Agarwal, and Vatsala Venkataram. 2022. "Comparative Evaluation of Locally Administered 2% Gel Fabricated from Lemongrass Polymer and 10% Doxycycline Hyclate Gel as an Adjunct to Scaling and Root Planing in the Treatment of Chronic Periodontitis—A Randomized Controlled Trial" Polymers 14, no. 14: 2766. https://doi.org/10.3390/polym14142766