Oxygen Gas Sensing Using a Hydrogel-Based Organic Electrochemical Transistor for Work Safety Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. OECT Fabrication
2.2. Electrical Characterization
2.3. Data Analysis
3. Results
3.1. PBS-vs.-Hydrogel as Gating Electrolyte for OECTs
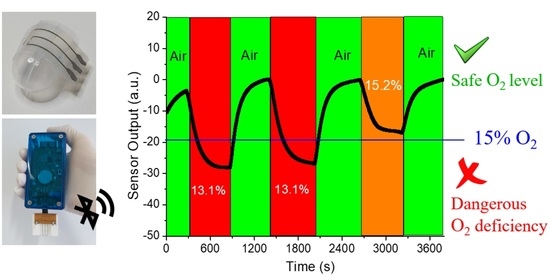
3.2. Oxygen Gas Sensing Using OECTs on Glass Substrates
3.3. Oxygen Gas Sensing Using OECTs on Flexible PEN Substrates
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Decker, H.; van Holde, K.E. Oxygen and the Evolution of Life; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 2011; pp. 1–172. [Google Scholar] [CrossRef]
- Wang, X.D.; Wolfbeis, O.S. Optical methods for sensing and imaging oxygen: Materials, spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundal, M.K.; Lilleng, P.K.; Barane, H.; Morild, I.; Vevelstad, M. Asphyxiation death caused by oxygen-depleting cargo on a ship. Forensic Sci. Int. 2017, 279, e7–e9. [Google Scholar] [CrossRef] [PubMed]
- The Importance of Oxygen Deficiency Monitors in the Work-Place. AOI. Available online: https://aoi-corp.com/articles/importance-oxygen-deficiency-monitors-work-place/ (accessed on 18 November 2021).
- Svedberg, U.; Petrini, C.; Johanson, G. Oxygen Depletion and Formation of Toxic Gases following Sea Transportation of Logs and Wood Chips. Ann. Occup. Hyg. 2009, 53, 779–787. [Google Scholar] [CrossRef] [Green Version]
- Harrison, R.J.; Retzer, K.; Kosnett, M.J.; Hodgson, M.; Jordan, T.; Ridl, S.; Kiefer, M. Sudden Deaths Among Oil and Gas Extraction Workers Resulting from Oxygen Deficiency and Inhalation of Hydrocarbon Gases and Vapors—United States, January 2010–March 2015. MMWR Morb. Mortal. Wkly. Rep. 2019, 65, 6–9. [Google Scholar] [CrossRef]
- Fatal Occupational Injuries Involving Confined Spaces. Available online: https://www.bls.gov/iif/oshwc/cfoi/confined-spaces-2011-18.htm (accessed on 18 November 2021).
- Sensori e Monitor di Ossigeno—GasLab.com. Available online: https://gaslab.com/collections/oxygen (accessed on 19 November 2021).
- O2 Portable—Portable Oxygen Monitor. Gastech. Available online: https://gastech.com/products/gas-detectors-portable/single-gas/o2-portable (accessed on 19 November 2021).
- O2 Microsensor—Unisense. Available online: https://www.unisense.com/O2/ (accessed on 12 November 2021).
- Oxygen Deficiency Monitor—Series 1300. AOI. Available online: https://aoi-corp.com/oxygen-deficiency-monitor/series-1300/ (accessed on 18 November 2021).
- Portable Detectors—Safegas. Available online: https://isafegas.com/portable-detectors/?gclid=Cj0KCQiAkNiMBhCxARIsAIDDKNWAtozHT0Hm9TadKgfcgF6LVEPvdL1QdVOwdv5GscCOHJSc6kPpMqMaAurdEALw_wcB (accessed on 19 November 2021).
- Sun, X.; Yao, F.; Li, J. Nanocomposite hydrogel-based strain and pressure sensors: A review. J. Mater. Chem. A 2020, 8, 18605–18623. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, K.; Xu, H.; Li, T.; Jin, Q.; Cui, D. Recent developments in sensors for wearable device applications. Anal. Bioanal. Chem. 2021, 413, 6037–6057. [Google Scholar] [CrossRef] [PubMed]
- Afsarimanesh, N.; Nag, A.; Sarkar, S.; Sabet, G.S.; Han, T.; Mukhopadhyay, S.C. A review on fabrication, characterization and implementation of wearable strain sensors. Sens. Actuators A Phys. 2020, 315, 112355. [Google Scholar] [CrossRef]
- Bag, A.; Lee, N.E. Recent Advancements in Development of Wearable Gas Sensors. Adv. Mater. Technol. 2021, 6, 2000883. [Google Scholar] [CrossRef]
- Harito, C.; Utari, L.; Putra, B.R.; Yuliarto, B.; Purwanto, S.; Zaidi, S.Z.J.; Bavykin, D.V.; Marken, F.; Walsh, F.C. Review—The Development of Wearable Polymer-Based Sensors: Perspectives. J. Electrochem. Soc. 2020, 167, 037566. [Google Scholar] [CrossRef]
- Mokhtar, S.M.A.; de Eulate, E.A.; Yamada, M.; Prow, T.W.; Evans, D.R. Conducting polymers in wearable devices. Med. Devices Sens. 2021, 4, e10160. [Google Scholar] [CrossRef]
- Gualandi, I.; Tessarolo, M.; Mariani, F.; Possanzini, L.; Scavetta, E.; Fraboni, B. Textile Chemical Sensors Based on Conductive Polymers for the Analysis of Sweat. Polymers 2021, 13, 894. [Google Scholar] [CrossRef] [PubMed]
- Decataldo, F.; Gualandi, I.; Tessarolo, M.; Scavetta, E.; Fraboni, B. Transient-doped organic electrochemical transistors working in current-enhancing mode as sensing devices for low concentration of oxygen dissolved in solution. APL Mater. 2020, 8, 091103. [Google Scholar] [CrossRef]
- Wang, L.; Yue, X.; Sun, Q.; Zhang, L.; Ren, G.; Lu, G.; Yu, H.-D.; Huang, W. Flexible organic electrochemical transistors for chemical and biological sensing. Nano Res. 2021, 15, 2433–2464. [Google Scholar] [CrossRef]
- Burtscher, B.; Allison, P.; Urbina, M.; Diacci, C.; Borghi, S.; Pinti, M.; Cossarizza, A.; Salvarani, C.; Berggren, M.; Biscarini, F.; et al. Sensing Inflammation Biomarkers with Electrolyte-Gated Organic Electronic Transistors. Adv. Healthc. Mater. 2021, 10, 2100955. [Google Scholar] [CrossRef] [PubMed]
- Picca, R.A.; Manoli, K.; Macchia, E.; Sarcina, L.; Di Franco, C.; Cioffi, N.; Blasi, D.; Österbacka, R.; Torricelli, F.; Scamarcio, G.; et al. Ultimately Sensitive Organic Bioelectronic Transistor Sensors by Materials and Device Structure Design. Adv. Funct. Mater. 2020, 30, 1904513. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, N.; Yang, A.; Ka-wai Law, H.; Li, L.; Yan, F.; Fu, Y.; Wang, N.; Yang, A.; Yan, F.; et al. Highly Sensitive Detection of Protein Biomarkers with Organic Electrochemical Transistors. Adv. Mater. 2017, 29, 1703787. [Google Scholar] [CrossRef]
- Keene, S.T.; Fogarty, D.; Cooke, R.; Casadevall, C.D.; Salleo, A.; Parlak, O. Wearable Organic Electrochemical Transistor Patch for Multiplexed Sensing of Calcium and Ammonium Ions from Human Perspiration. Adv. Healthc. Mater. 2019, 8, 1901321. [Google Scholar] [CrossRef]
- Serafini, M.; Mariani, F.; Gualandi, I.; Decataldo, F.; Possanzini, L.; Tessarolo, M.; Fraboni, B.; Tonelli, D.; Scavetta, E. A Wearable Electrochemical Gas Sensor for Ammonia Detection. Sensors 2021, 21, 7905. [Google Scholar] [CrossRef]
- Decataldo, F.; Druet, V.; Pappa, A.M.; Tan, E.; Savva, A.; Pitsalidis, C.; Inal, S.; Kim, J.S.; Fraboni, B.; Owens, R.M.; et al. BMP-2 functionalized PEDOT:PSS-based OECTs for stem cell osteogenic differentiation monitoring. Flex. Print. Electron. 2019, 4, 044006. [Google Scholar] [CrossRef] [Green Version]
- Gualandi, I.; Tessarolo, M.; Mariani, F.; Tonelli, D.; Fraboni, B.; Scavetta, E. Organic Electrochemical Transistors as Versatile Analytical Potentiometric Sensors. Front. Bioeng. Biotechnol. 2019, 7, 354. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Crispin, X.; Zozoulenko, I.V. Oxygen Reduction Reaction in Conducting Polymer PEDOT: Density Functional Theory Study. J. Phys. Chem. C 2017, 121, 12270–12277. [Google Scholar] [CrossRef] [Green Version]
- Charlot, B.; Sassine, G.; Garraud, A.; Sorli, B.; Giani, A.; Combette, P. Micropatterning PEDOT:PSS layers. Microsyst. Technol. 2013, 19, 895–903. [Google Scholar] [CrossRef]
- Bernards, D.A.; Malliaras, G.G. Steady-State and Transient Behavior of Organic Electrochemical Transistors. Adv. Funct. Mater. 2007, 17, 3538–3544. [Google Scholar] [CrossRef]
- Jia, H.; Huang, Z.; Li, P.; Zhang, S.; Wang, Y.; Wang, J.Y.; Gu, X.; Lei, T. Engineering donor–acceptor conjugated polymers for high-performance and fast-response organic electrochemical transistors. J. Mater. Chem. C 2021, 9, 4927–4934. [Google Scholar] [CrossRef]





| PBS | H-Thick | H-Thin | |
|---|---|---|---|
| τ [ms] | 1.005 ± 0.006 | 1.12 ± 0.03 | 198.21 ± 0.13 |
| [µA] | −0.31 ± 0.06 | −0.27 ± 0.17 | −0.0258 ± 0.0012 |
| [µA] | −0.20 ± 0.07 | −0.14 ± 0.10 | −0.0165 ± 0.0013 |
| [µA] | −46.69 ± 0.08 | −58.27 ± 0.10 | −30.818 ± 0.007 |
| [µA] | −25.34 ± 0.07 | −41.90 ± 0.10 | −18.297 ± 0.004 |
| Gain = / (0 V) | 150 | 220 | 1195 |
| Gain = / (0.3 V) | 130 | 300 | 1110 |
| Gm peak [µS] | 207 | 174 | 241 |
| Sample | Sensitivity [µA/dec] | Error [µA/dec] |
|---|---|---|
| 1 | −13.6 | 1.3 |
| 2 | −12.5 | 1.2 |
| 3 | −11.2 | 0.9 |
| Sample | Sensitivity [µA/dec] | Error [µA/dec] |
|---|---|---|
| 1 | −15.0 | 0.9 |
| 2 | −14.9 | 0.8 |
| 3 | −16.1 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decataldo, F.; Bonafè, F.; Mariani, F.; Serafini, M.; Tessarolo, M.; Gualandi, I.; Scavetta, E.; Fraboni, B. Oxygen Gas Sensing Using a Hydrogel-Based Organic Electrochemical Transistor for Work Safety Applications. Polymers 2022, 14, 1022. https://doi.org/10.3390/polym14051022
Decataldo F, Bonafè F, Mariani F, Serafini M, Tessarolo M, Gualandi I, Scavetta E, Fraboni B. Oxygen Gas Sensing Using a Hydrogel-Based Organic Electrochemical Transistor for Work Safety Applications. Polymers. 2022; 14(5):1022. https://doi.org/10.3390/polym14051022
Chicago/Turabian StyleDecataldo, Francesco, Filippo Bonafè, Federica Mariani, Martina Serafini, Marta Tessarolo, Isacco Gualandi, Erika Scavetta, and Beatrice Fraboni. 2022. "Oxygen Gas Sensing Using a Hydrogel-Based Organic Electrochemical Transistor for Work Safety Applications" Polymers 14, no. 5: 1022. https://doi.org/10.3390/polym14051022