Sodium p-Toluenesulfinate Enhances the Bonding Durability of Universal Adhesives on Deproteinized Eroded Dentin
Abstract
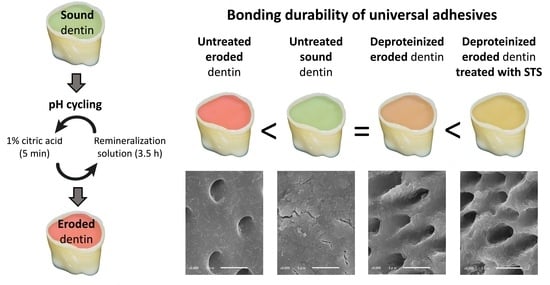
:1. Introduction
2. Materials and Methods
3. Results
3.1. µTBS
3.2. Failure Mode Analysis
3.3. Morphological Analysis of the Dentin Surfaces
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlueter, N.; Luka, B. Erosive tooth wear—A review on global prevalence and on its prevalence in risk groups. Br. Dent. J. 2018, 224, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Marthaler, T.M. Changes in dental caries 1953–2003. Caries Res. 2004, 38, 173–181. [Google Scholar] [CrossRef]
- Jaeggi, T.; Lussi, A. Prevalence, incidence and distribution of erosion. Monogr. Oral Sci. 2014, 25, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Myklebust, S.; Espelid, I.; Svalestad, S.; Tveit, A.B. Dental health behavior, gastroesophageal disorders and dietary habits among Norwegian recruits in 1990 and 1999. Acta Odontol. Scand. 2003, 61, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, T.; Gruninger, A.; Lussi, A. Restorative therapy of erosion. Monogr. Oral Sci. 2006, 20, 200–214. [Google Scholar] [CrossRef]
- Lussi, A.; Schlueter, N.; Rakhmatullina, E.; Ganss, C. Dental erosion—An overview with emphasis on chemical and histopathological aspects. Caries Res. 2011, 45 (Suppl. 1), 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ganss, C.; Lussi, A. Diagnosis of erosive tooth wear. Monogr. Oral Sci. 2014, 25, 22–31. [Google Scholar] [CrossRef]
- Loomans, B.; Opdam, N.; Attin, T.; Bartlett, D.; Edelhoff, D.; Frankenberger, R.; Benic, G.; Ramseyer, S.; Wetselaar, P.; Sterenborg, B.; et al. Severe Tooth Wear: European Consensus Statement on Management Guidelines. J. Adhes. Dent. 2017, 19, 111–119. [Google Scholar] [CrossRef]
- Belmar da Costa, M.; Delgado, A.H.S.; Pinheiro de Melo, T.; Amorim, T.; Mano Azul, A. Analysis of laboratory adhesion studies in eroded enamel and dentin: A scoping review. Biomater. Investig. Dent. 2021, 8, 24–38. [Google Scholar] [CrossRef]
- De Rossi, G.R.C.; Pasquantonio, G.; Özcan, M.; Maziero Volpato, C.A. Adhesion of resin-based materials to eroded dentin: A systematic review and meta-analysis. J. Adhes. Sci. Technol. 2021, 2021, 1–30. [Google Scholar] [CrossRef]
- Zimmerli, B.; De Munck, J.; Lussi, A.; Lambrechts, P.; Van Meerbeek, B. Long-term bonding to eroded dentin requires superficial bur preparation. Clin. Oral Investig. 2012, 16, 1451–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdigão, J.; Reis, A.; Loguercio, A.D. Dentin Adhesion and MMPs: A Comprehensive Review. J. Esthet. Restor. Dent. 2013, 25, 219–241. [Google Scholar] [CrossRef] [PubMed]
- Shellis, R.P.; Ganss, C.; Ren, Y.; Zero, D.T.; Lussi, A. Methodology and Models in Erosion Research: Discussion and Conclusions. Caries Res. 2011, 45 (Suppl. 1), 69–77. [Google Scholar] [CrossRef] [PubMed]
- Deari, S.; Wegehaupt, F.J.; Tauböck, T.T.; Attin, T. Influence of Different Pretreatments on the Microtensile Bond Strength to Eroded Dentin. J. Adhes. Dent. 2017, 19, 147–155. [Google Scholar] [CrossRef]
- Ramos, T.M.; Ramos-Oliveira, T.M.; de Freitas, P.M.; Azambuja, N., Jr.; Esteves-Oliveira, M.; Gutknecht, N.; de Paula Eduardo, C. Effects of Er:YAG and Er,Cr:YSGG laser irradiation on the adhesion to eroded dentin. Lasers Med. Sci. 2015, 30, 17–26. [Google Scholar] [CrossRef]
- Costa, C.A.G.; Passos, V.F.; Neri, J.R.; Mendonça, J.S.; Santiago, S.L. Effect of Metalloproteinase Inhibitors on Bond Strength of a Self-etching Adhesive on Erosively Demineralized Dentin. J. Adhes. Dent. 2019, 21, 337–344. [Google Scholar] [CrossRef]
- Francisconi-dos-Rios, L.F.; Casas-Apayco, L.C.; Calabria, M.P.; Francisconi, P.A.; Borges, A.F.; Wang, L. Role of chlorhexidine in bond strength to artificially eroded dentin over time. J. Adhes. Dent. 2015, 17, 133–139. [Google Scholar] [CrossRef]
- Giacomini, M.; Scaffa, P.; Chaves, L.; Vidal, C.; Machado, T.; Honório, H.; Tjäderhane, L.; Wang, L. Role of Proteolytic Enzyme Inhibitors on Carious and Eroded Dentin Associated With a Universal Bonding System. Oper. Dent. 2017, 42, E188–E196. [Google Scholar] [CrossRef]
- Augusto, M.G.; Torres, C.; Pucci, C.R.; Schlueter, N.; Borges, A.B. Bond Stability of a Universal Adhesive System to Eroded/Abraded Dentin After Deproteinization. Oper. Dent. 2018, 43, 291–300. [Google Scholar] [CrossRef]
- Siqueira, F.; Cardenas, A.; Gomes, G.; Chibinski, A.; Gomes, O.; Bandeca, M.; Loguercio, A.; Gomes, J. Three-Year Effects of Deproteinization on the In Vitro Durability of Resin/Dentin-Eroded Interfaces. Oper. Dent. 2018, 43, 60–70. [Google Scholar] [CrossRef]
- Taniguchi, G.; Nakajima, M.; Hosaka, K.; Iwamoto, N.; Ikeda, M.; Foxton, R.M.; Tagami, J. Improving the effect of NaOCl pretreatment on bonding to caries-affected dentin using self-etch adhesives. J. Dent. 2009, 37, 769–775. [Google Scholar] [CrossRef]
- Lai, S.C.; Mak, Y.F.; Cheung, G.S.; Osorio, R.; Toledano, M.; Carvalho, R.M.; Tay, F.R.; Pashley, D.H. Reversal of compromised bonding to oxidized etched dentin. J. Dent. Res. 2001, 80, 1919–1924. [Google Scholar] [CrossRef] [Green Version]
- Kunawarote, S.; Nakajima, M.; Shida, K.; Kitasako, Y.; Foxton, R.M.; Tagami, J. Effect of dentin pretreatment with mild acidic HOCl solution on microtensile bond strength and surface pH. J. Dent. 2010, 38, 261–268. [Google Scholar] [CrossRef]
- Vongphan, N.; Senawongse, P.; Somsiri, W.; Harnirattisai, C. Effects of sodium ascorbate on microtensile bond strength of total-etching adhesive system to NaOCl treated dentine. J. Dent. 2005, 33, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Prasansuttiporn, T.; Nakajima, M.; Kunawarote, S.; Foxton, R.M.; Tagami, J. Effect of reducing agents on bond strength to NaOCl-treated dentin. Dent. Mater. 2011, 27, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Prasansuttiporn, T.; Thanatvarakorn, O.; Mamanee, T.; Hosaka, K.; Tagami, J.; Foxton, R.; Nakajima, M. Effect of antioxidant/reducing agents on the initial and long-term bonding performance of a self-etch adhesive to caries-affected dentin with and without smear layer-deproteinizing. Int. J. Adhes. Adhes. 2020, 102, 102648. [Google Scholar] [CrossRef]
- Prasansuttiporn, T.; Thanatvarakorn, O.; Tagami, J.; Foxton, R.M.; Nakajima, M. Bonding Durability of a Self-etch Adhesive to Normal Versus Smear-layer Deproteinized Dentin: Effect of a Reducing Agent and Plant-extract Antioxidant. J. Adhes. Dent. 2017, 19, 253–258. [Google Scholar] [CrossRef]
- Hasegawa, M.; Tichy, A.; Hosaka, K.; Kuno, Y.; Ikeda, M.; Nozaki, K.; Chiba, A.; Nakajima, M.; Tagami, J. Degree of conversion and dentin bond strength of light-cured multi-mode adhesives pretreated or mixed with sulfinate agents. Dent. Mater. J. 2021, 40, 877–884. [Google Scholar] [CrossRef]
- Sakano, W.; Nakajima, M.; Prasansuttiporn, T.; Foxton, R.M.; Tagami, J. Polymerization behavior within adhesive layer of one- and two-step self-etch adhesives: A micro-Raman spectroscopic study. Dent. Mater. J. 2013, 32, 992–998. [Google Scholar] [CrossRef] [Green Version]
- Kuno, Y.; Hosaka, K.; Nakajima, M.; Ikeda, M.; Klein Junior, C.A.; Foxton, R.M.; Tagami, J. Incorporation of a hydrophilic amide monomer into a one-step self-etch adhesive to increase dentin bond strength: Effect of application time. Dent. Mater. J. 2019, 38, 892–899. [Google Scholar] [CrossRef] [Green Version]
- Tichy, A.; Hosaka, K.; Abdou, A.; Nakajima, M.; Tagami, J. Degree of Conversion Contributes to Dentin Bonding Durability of Contemporary Universal Adhesives. Oper. Dent. 2020, 45, 556–566. [Google Scholar] [CrossRef]
- Walter, R.; Swift, E.J., Jr.; Nagaoka, H.; Chung, Y.; Bartholomew, W.; Braswell, K.M.; Pereira, P.N. Two-year bond strengths of “all-in-one” adhesives to dentine. J. Dent. 2012, 40, 549–555. [Google Scholar] [CrossRef]
- Kenshima, S.; Francci, C.; Reis, A.; Loguercio, A.D.; Filho, L.E. Conditioning effect on dentin, resin tags and hybrid layer of different acidity self-etch adhesives applied to thick and thin smear layer. J. Dent. 2006, 34, 775–783. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Peumans, M.; Poitevin, A.; Mine, A.; Van Ende, A.; Neves, A.; De Munck, J. Relationship between bond-strength tests and clinical outcomes. Dent. Mater. 2010, 26, e100–e121. [Google Scholar] [CrossRef]
- Kirschner, J.; Szillat, F.; Bouzrati-Zerelli, M.; Becht, J.-M.; Klee, J.E.; Lalevée, J. Sulfinates and sulfonates as high performance co-initiators in CQ based systems: Towards aromatic amine-free systems for dental restorative materials. Dent. Mater. 2020, 36, 187–196. [Google Scholar] [CrossRef]
- Suyama, Y.; Luhrs, A.K.; De Munck, J.; Mine, A.; Poitevin, A.; Yamada, T.; Van Meerbeek, B.; Cardoso, M.V. Potential smear layer interference with bonding of self-etching adhesives to dentin. J. Adhes. Dent. 2013, 15, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Mine, A.; De Munck, J.; Cardoso, M.V.; Van Landuyt, K.L.; Poitevin, A.; Van Ende, A.; Matsumoto, M.; Yoshida, Y.; Kuboki, T.; Yatani, H.; et al. Dentin-smear remains at self-etch adhesive interface. Dent. Mater. 2014, 30, 1147–1153. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H. Aggressiveness of contemporary self-etching systems. I: Depth of penetration beyond dentin smear layers. Dent. Mater. 2001, 17, 296–308. [Google Scholar] [CrossRef]
- Yoshihara, K.; Nagaoka, N.; Okihara, T.; Kuroboshi, M.; Hayakawa, S.; Maruo, Y.; Nishigawa, G.; De Munck, J.; Yoshida, Y.; Van Meerbeek, B. Functional monomer impurity affects adhesive performance. Dent. Mater. 2015, 31, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Snauwaert, J.; Peumans, M.; De Munck, J.; Lambrechts, P.; Van Meerbeek, B. The role of HEMA in one-step self-etch adhesives. Dent. Mater. 2008, 24, 1412–1419. [Google Scholar] [CrossRef]
- Takahashi, M.; Nakajima, M.; Hosaka, K.; Ikeda, M.; Foxton, R.M.; Tagami, J. Long-term evaluation of water sorption and ultimate tensile strength of HEMA-containing/-free one-step self-etch adhesives. J. Dent. 2011, 39, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Nagakane, K.; Fukuda, R.; Nakayama, Y.; Okazaki, M.; Shintani, H.; Inoue, S.; Tagawa, Y.; Suzuki, K.; De Munck, J.; et al. Comparative study on adhesive performance of functional monomers. J. Dent. Res. 2004, 83, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, M.; Yiu, C.K.Y.; Matinlinna, J.P. An overview of solvents in resin–dentin bonding. Int. J. Adhes. Adhes. 2015, 57, 22–33. [Google Scholar] [CrossRef]


| Solution | Composition |
|---|---|
| Demineralization | 1% citric acid (pH = 3.5) |
| Remineralization | 0.002 g ascorbic acid, 0.58 g NaCl, 0.17 g CaCl2, 0.16 g NH4Cl, 1.27 g KCl, 0.16 g NaSCN, 0.33 g KH2PO4, 0.34 g Na2HPO4 dissolved in 1 L of demineralized water (pH adjusted to 6.4 with HCl) |
| Material (Manufacturer) | Composition | pH | Application Procedure |
|---|---|---|---|
| Clearfil Universal Bond Quick (UBQ; Kuraray Noritake Dental, Tokyo, Japan) | 10-MDP, Bis-GMA, HEMA, hydrophilic amide monomers, colloidal silica, coupling agent, sodium fluoride, CQ, ethanol, water | 2.3 |
|
| Scotchbond Universal Adhesive (SBU; 3M, St.Paul, MN, USA) | 10-MDP, dimethacrylate resins, Bis-GMA, HEMA, Vitrebond copolymer, silane, ethanol, water, filler, initiator | 2.7 |
|
| G-Premio Bond (GPB; GC, Tokyo, Japan) | 10-MDP, 4-MET, MEPS, methacrylate monomer, acetone, water, initiator, silica | 1.5 |
|
| Accel (Sun Medical, Kyoto, Japan) | sodium p-toluenesulfinate, ethanol, water |
| |
| AD gel (Kuraray Noritake Dental, Tokyo, Japan) | 10% sodium hypochlorite, thickener |
| |
| Clearfil AP-X (shade A2; Kuraray Noritake Dental, Tokyo, Japan) | Bis-GMA, TEGDMA, silanated barium glass filler, silanated silica filler, silanated colloidal silica, CQ, initiators, accelerators, pigments |
|
| Adhesives | Dentin Pretreatment | 24 h | 10,000 Thermal Cycles |
|---|---|---|---|
| UBQ | Control | 63.1 (10.7) A,b | 50.1 (7.6) B,b |
| Eroded | 49.4 (9.7) A,c | 22.1 (5.7) B,c | |
| NaOCl | 61.0 (8.5) A,b | 44.3 (8.3) B,b | |
| NaOCl + STS | 71.0 (9.3) A,a | 64.5 (9.8) A,a | |
| SBU | Control | 57.6 (6.7) A,ab | 47.0 (5.8) B,ab |
| Eroded | 39.2 (8.7) A,c | 23.5 (5.8) B,c | |
| NaOCl | 55.9 (11.9) A,b | 43.8 (7.1) B,b | |
| NaOCl + STS | 63.9 (7.6) A,a | 58.2 (11.7) A,a | |
| GPB | Control | 53.1 (9.3) A,b | 42.9 (7.2) B,b |
| Eroded | 40.4 (8.5) A,c | 20.4 (4.1) B,c | |
| NaOCl | 60.9 (8.7) A,ab | 40.1 (10.2) B,ab | |
| NaOCl + STS | 61.3 (8.8) A,a | 58.7 (8.2) A,a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shioya, Y.; Tichy, A.; Yonekura, K.; Hasegawa, M.; Hatayama, T.; Ikeda, M.; Tagami, J.; Nakajima, M.; Hosaka, K. Sodium p-Toluenesulfinate Enhances the Bonding Durability of Universal Adhesives on Deproteinized Eroded Dentin. Polymers 2021, 13, 3901. https://doi.org/10.3390/polym13223901
Shioya Y, Tichy A, Yonekura K, Hasegawa M, Hatayama T, Ikeda M, Tagami J, Nakajima M, Hosaka K. Sodium p-Toluenesulfinate Enhances the Bonding Durability of Universal Adhesives on Deproteinized Eroded Dentin. Polymers. 2021; 13(22):3901. https://doi.org/10.3390/polym13223901
Chicago/Turabian StyleShioya, Yorichika, Antonin Tichy, Kazuhide Yonekura, Mayu Hasegawa, Takashi Hatayama, Masaomi Ikeda, Junji Tagami, Masatoshi Nakajima, and Keiichi Hosaka. 2021. "Sodium p-Toluenesulfinate Enhances the Bonding Durability of Universal Adhesives on Deproteinized Eroded Dentin" Polymers 13, no. 22: 3901. https://doi.org/10.3390/polym13223901