Low-Mass Liquid Crystalline Materials Blended in Recycled Thermoplastic Polyester Elastomer for Corrosion Inhibitor Application
Abstract
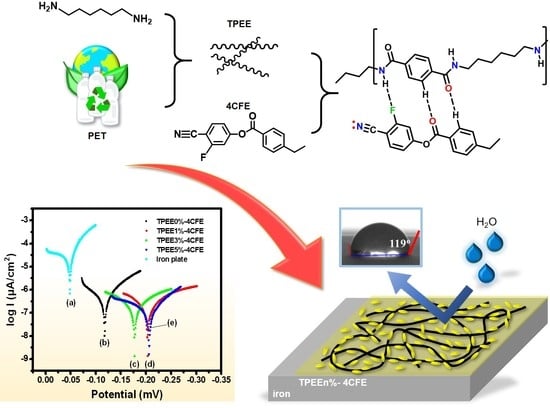
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bagherzadeh, M.; Ghahfarokhi, Z.S.; Yazdi, E.G. Electrochemical and Surface Evaluation of the Anti-Corrosion Properties of Reduced Graphene Oxide. RSC Adv. 2016, 6, 22007–22015. [Google Scholar] [CrossRef]
- Koenig, S.P.; Boddeti, N.G.; Dunn, M.L.; Bunch, J.S. Ultrastrong Adhesion of Graphene Membranes. Nat. Nanotechnol. 2011, 6, 543–546. [Google Scholar] [CrossRef]
- Mayavan, S.; Siva, T.; Sathiyanarayanan, S. Graphene Ink as a Corrosion Inhibiting Blanket for Iron in an Aggressive Chloride Environment. RSC Adv. 2013, 3, 24868–24871. [Google Scholar] [CrossRef]
- Pushpavanam, M.; Raman, V.; Shenoi, B.A. Rhodium—Electrodeposition and Applications. Surf. Technol. 1981, 12, 351–360. [Google Scholar] [CrossRef]
- Ishizaki, T.; Chiba, S.; Watanabe, K.; Suzuki, H. Corrosion Resistance of Mg–Al Layered Double Hydroxide Container-Containing Magnesium Hydroxide Films Formed Directly on Magnesium Alloy by Chemical-Free Steam Coating. J. Mater. Chem. A 2013, 1, 8968–8977. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Huang, W.-J.; Wu, S.-H.; Lee, M.; Yeh, J.-M.; Chen, H.-H. Excellent Superhydrophobic Surface and Anti-Corrosion Performance by Nanostructure of Discotic Columnar Liquid Crystals. Corros. Sci. 2018, 138, 1–7. [Google Scholar] [CrossRef]
- Chen, H.-H.; Chang, K.-C.; Chu, C.-M.; Hung, H.-H.; Hsu, M.-H.; Hung, Y.-C.; Wang, C.-S.; Yeh, J.-M. Discotic Liquid Crystals as Novel Corrosion-Resistant Coatings. Chem. Commun. 2015, 51, 921–924. [Google Scholar] [CrossRef]
- Coneski, P.N.; Weise, N.K.; Fulmer, P.A.; Wynne, J.H. Development and Evaluation of Self-Polishing Urethane Coatings with Tethered Quaternary Ammonium Biocides. Prog. Org. Coat. 2013, 76, 1376–1386. [Google Scholar] [CrossRef]
- Bandeira, R.M.; van Drunen, J.; Tremiliosi-Filho, G.; dos Santos, J.R.; de Matos, J.M.E. Polyaniline/Polyvinyl Chloride Blended Coatings for the Corrosion Protection of Carbon Steel. Prog. Org. Coat. 2017, 106, 50–59. [Google Scholar] [CrossRef]
- Ansari, R.; Alikhani, A.H. Application of Polyaniline/Nylon Composites Coating for Corrosion Protection of Steel. J. Coat. Technol. Res. 2009, 6, 221–227. [Google Scholar] [CrossRef]
- Awaja, F.; Pavel, D. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Shen, L.; Worrell, E.; Patel, M.K. Open-Loop Recycling: A LCA Case Study of PET Bottle-to-Fibre Recycling. Resour. Conserv. Recycl. 2010, 55, 34–52. [Google Scholar] [CrossRef]
- Duarte, L.T. Production and Characterization of Thermally Sprayed Polyethylene Terephthalate Coatings. Surf. Coat. Technol. 2004, 182, 261–267. [Google Scholar] [CrossRef]
- Zare, Y. Recent Progress on Preparation and Properties of Nanocomposites from Recycled Polymers: A Review. Waste Manag. 2013, 33, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Saidi, N.M.; Shafaamri, A.S.; Ma, I.A.W.; Kasi, R.; Balakrishnan, V.; Subramaniam, R. Development of Anti-Corrosion Coatings Using the Disposable Waste Material. Pigm. Resin Technol. 2018, 47, 478–484. [Google Scholar] [CrossRef]
- Atta, A.M. Recycled Poly (Ethylene Terephthalate) Resins as Corrosion Protective Organic Coatings of Steel Pipelines. Recent Pat. Corros. Sci. 2011, 1, 24–32. [Google Scholar] [CrossRef]
- Visakh, P.M.; Liang, M. Poly (Ethylene Terephthalate) Based Blends, Composites and Nanocomposites, 1st ed.; William Andrew: Norwich, NY, USA, 2015. [Google Scholar]
- Dutta, D.; Fruitwala, H.; Kohli, A.; Weiss, R.A. Polymer Blends Containing Liquid Crystals: A Review. Polym. Eng. Sci. 1990, 30, 1005–1018. [Google Scholar] [CrossRef]
- Trollsås, M.; Sahlén, F.; Gedde, U.W.; Hult, A.; Hermann, D.; Rudquist, P.; Komitov, L.; Lagerwall, S.T.; Stebler, B.; Lindström, J.; et al. Novel Thermally Stable Polymer Materials for Second-Order Nonlinear Optics. Macromolecules 1996, 29, 2590–2598. [Google Scholar] [CrossRef]
- Yonezawa, J.; Martin, S.M.; Macosko, C.W.; Ward, M.D. Rheology and Morphology of Amectic Liquid Crystal/Polymer Blends. Macromolecules 2004, 37, 6424–6432. [Google Scholar] [CrossRef]
- Crawford, G.P.; SvensÏek, D.; Žumer, S. Some Aspects of Polymer Dispersed and Polymer Stabilized Chiral Liquid Crystals; Springer: New York, NY, USA, 2001. [Google Scholar]
- Zhang, T.; Cong, Y.; Zhang, B.; Zhao, W. Preparation and Characterisation: PSCLC Film Doping with FexNiy Nanoparticles. Liq. Cryst. 2015, 42, 167–173. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Chou, Y.L.; Yang, H.C.; Chen, C.W.; Rwei, S.P. Synthesis and Characterization of Thermoplastic Poly (Ester Amide)s Elastomer (TPEaE) Obtained from Recycled PET. J. Renew. Mater. 2021, 9, 867–880. [Google Scholar] [CrossRef]
- Yang, Y.G.; Chen, H.; Tang, G.; Wen, J.X. Synthesis and Mesomorphic Properties of Several Series of Fluorinated Ester Liquid Crystals. Liq. Cryst. 2002, 29, 255–261. [Google Scholar] [CrossRef]
- Modlińska, A.; Dardas, D.; Jadżyn, J.; Bauman, D. Characterization of Some Fluorinated Mesogens for Application in Liquid Crystal Displays. Mol. Cryst. Liq. Cryst. 2011, 542, 28–550. [Google Scholar] [CrossRef]
- Sasaki, M.; Takeuchi., K.; Sat, H.; Takatsu, H. Synthesis and Some Properties of 3-Fluoro-4-Cyanophenyl 4′-n-Alkylbenzoates. Mol. Cyst. Liq. Cyst. 1984, 109, 169–178. [Google Scholar] [CrossRef]
- Beving, D.E.; McDonnell, A.M.P.; Yang, W.S.; Yan, Y.S. Corrosion Resistant High-Silica-Zeolite MFI Coating-One General Solution Formulation for Aluminum Alloy AA-2024-T3, AA-5052-H32, AA-6061-T4, and AA-7075-T6. J. Electrochem. Soc. 2006, 153, B325–B329. [Google Scholar] [CrossRef]
- Mitra, A.; Wang, Z.B.; Cao, T.G.; Wang, H.T.; Huang, L.M.; Yan, Y.S. Synthesis and Corrosion Resistance of High-Silica Zeolite MTW, BEA, and MFI Coatings on Steel and Aluminum. J. Electrochem. Soc. 2002, 149, B472–B478. [Google Scholar] [CrossRef]
- Bockris, J.; Reddy, K.N. Modern Electrochemistry; John Wiley and Sons Inc.: Hoboken, NJ, USA, 1976; p. 622. [Google Scholar]
- Kamaraj, K.; Karpakam, V.; Sathiyanarayanan, S.; Venkatachari, G. Electrosysnthesis of Poly (aniline-co-m-amino benzoic acid) for Corrosion Protection of Steel. Mater. Chem. Phys. 2010, 122, 123–128. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the Sacred Lotus, or Escape from Contamination in Biological Surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, X.; Jiang, L. Directional Adhesion of Superhydrophobic Butterfly Wings. Soft Matter. 2007, 3, 178–182. [Google Scholar] [CrossRef]










| Sample | 1st Heating | 1st Cooling | Xc a (%) | ||||
|---|---|---|---|---|---|---|---|
| Tm (°C) | ΔHm1 (J/g) | Tm2 (°C) | ΔHm2 (J/g) | Tcc (°C) | ΔHcc (J/g) | ||
| 4CFE | 81.1 | 89.1 | 13.6 | 62.5 | - | ||
| TPEE0%-4CFE | 61.9 | 7.1 | 80.1 | 14.4 | −2.1 | 2.1 | 48.8 |
| TPEE1%-4CFE | 68.6 | 14.7 | 79.2 | 9.4 | - | - | 54.6 |
| TPEE3%-4CFE | 66.0 | 11.2 | 78.8 | 10.0 | - | - | 48.2 |
| TPEE5%-4CFE | 68.3 | 8.1 | 76.5 | 4.2 | - | - | 28.0 |
| Electrochemical Corrosion Measurements a | |||||
|---|---|---|---|---|---|
| Materials | Ecorr (V vs. SCE) | Rp (Ω cm2) | Icorr (μAcm−2) | PEF (%) | Thickness (mm) |
| Iron plate | −0.90 | 615.94 | 8.78 10−5 | — | — |
| TPEE0%-4CFE | −0.71 | 3616.10 | 0.28 10−8 | >99% | 1.13 ± 0.002 |
| TPEE1%-4CFE | −0.66 | 1327.40 | 0.71 10−8 | >99% | 1.13 ± 0.009 |
| TPEE3%-4CFE | −0.68 | 1475.00 | 0.41 10−8 | >99% | 1.13 ± 0.004 |
| TPEE5%-4CFE | −0.65 | 1344.40 | 2.54 10−8 | >99% | 1.13 ± 0.009 |
| TPEE0% | TPEE1% | TPEE3% | TPEE5% | |
|---|---|---|---|---|
| Vapor permeability (gm/m2 day) | 359.7 | 320.5 | 227.1 | 256.6 |
| TPEE0%-4CFE | TPEE1%-4CFE | TPEE3%-4CFE | TPEE5%-4CFE | |
| 39.3 | 307.9 | 155.9 | 215.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-J.; Huang, B.-W.; Tseng, P.-J.; Yang, Z.-Y.; Huang, X.; Rwei, S.-P.; Chen, H.-H. Low-Mass Liquid Crystalline Materials Blended in Recycled Thermoplastic Polyester Elastomer for Corrosion Inhibitor Application. Polymers 2021, 13, 3188. https://doi.org/10.3390/polym13183188
Chen C-J, Huang B-W, Tseng P-J, Yang Z-Y, Huang X, Rwei S-P, Chen H-H. Low-Mass Liquid Crystalline Materials Blended in Recycled Thermoplastic Polyester Elastomer for Corrosion Inhibitor Application. Polymers. 2021; 13(18):3188. https://doi.org/10.3390/polym13183188
Chicago/Turabian StyleChen, Chun-Jui, Bo-Wei Huang, Po-Jung Tseng, Zhi-Yu Yang, Xiang Huang, Syang-Peng Rwei, and Hsiu-Hui Chen. 2021. "Low-Mass Liquid Crystalline Materials Blended in Recycled Thermoplastic Polyester Elastomer for Corrosion Inhibitor Application" Polymers 13, no. 18: 3188. https://doi.org/10.3390/polym13183188