High-Temperature Resistant Polyborosilazanes with Tailored Structures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
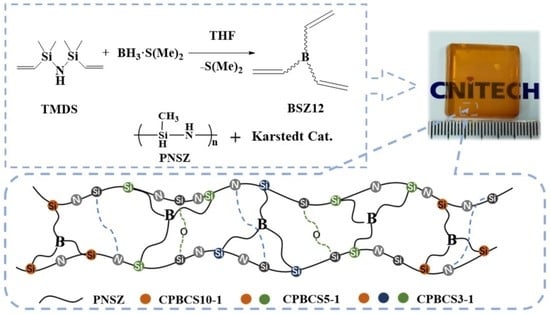
2.2. Preparation of BSZ12 Monomer
2.3. Preparation of Cross-Linked Boron-Containing Silicone Polymers (CPBCSs)
2.4. Characterization
3. Results
3.1. Synthesis of BSZ12 Monomer and CPBCSs
3.2. Synthesis of Crosslinked CPBCSs
3.3. Optical Properties and Surface Characteristics of CPBCSs
3.4. Thermal Stability of CPBCSs
3.5. Flammability of CPBCSs
3.6. Mechanical Properties of CPBCSs
3.7. An Insight into CPBCSs’ Structure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macan, J.; Brnardic, I.; Orlic, S.; Ivankovic, H.; Ivankovic, M. Thermal degradation of epoxy-silica organic-inorganic hybrid materials. Polym. Degrad. Stab. 2006, 91, 122–127. [Google Scholar] [CrossRef]
- Kanamori, K.; Aizawa, M.; Nakanishi, K.; Hanada, T. Elastic organic-inorganic hybrid aerogels and xerogels. J. Sol-Gel Sci. Technol. 2008, 48, 172–181. [Google Scholar] [CrossRef]
- Fu, R.Q.; Woo, J.J.; Seo, S.J.; Lee, J.S.; Moon, S.H. Covalent organic/inorganic hybrid proton-conductive membrane with semi-interpenetrating polymer network: Preparation and characterizations. J. Power Sources 2008, 179, 458–466. [Google Scholar] [CrossRef]
- Pandey, S.; Mishra, S.B. Sol-gel derived organic-inorganic hybrid materials: Synthesis, characterizations and applications. J. Sol-Gel Sci. Technol. 2011, 59, 73–94. [Google Scholar] [CrossRef]
- Wu, Y.H.; Wu, C.M.; Xu, T.W.; Fu, Y.X. Novel anion-exchange organic-inorganic hybrid membranes prepared through sol-gel reaction of multi-alkoxy precursors. J. Membr. Sci. 2009, 329, 236–245. [Google Scholar] [CrossRef]
- Hu, H.; Wang, L.; Wang, L.; Li, L.; Feng, S. Imine-functionalized polysiloxanes for supramolecular elastomers with tunable mechanical properties. Polym. Chem. 2020, 11, 7721–7728. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P.; Darder, M.; Ogawa, M. Hybrid and biohybrid silicate based materials: Molecular vs. block-assembling bottom-up processes. Chem. Soc. Rev. 2011, 40, 801–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundu, C.K.; Li, Z.W.; Song, L.; Hu, Y. An overview of fire retardant treatments for synthetic textiles: From traditional approaches to recent applications. Eur. Polym. J. 2020, 137. [Google Scholar] [CrossRef]
- Mosurkal, R.; Kirby, R.; Muller, W.S.; Soares, J.W.; Kumar, J. Simple green synthesis of polyborosiloxanes as environmentally-safe, non-halogenated flame retardant polymers. Green Chem. 2011, 13, 659–665. [Google Scholar] [CrossRef]
- Mosurkal, R.; Samuelson, L.A.; Parmar, V.S.; Kumar, J.; Watterson, A.C. Biocatalytic synthesis of organosiloxane copolyimide. Macromolecules 2007, 40, 7742–7744. [Google Scholar] [CrossRef]
- Mosurkal, R.; Samuelson, L.A.; Tewari, A.; Watterson, A.C.; Parmar, V.S.; Kumar, J. Crosslinking of Polydimethyl Siloxane Copolymers with Aromatic Dianhydrides: The Study of Thermal and Flame Retardant Properties. J. Macromol. Sci. Part A Pure Appl. Chem. 2009, 46, 1228–1232. [Google Scholar] [CrossRef]
- Wesolek, D.; Gasiorowski, R.; Rojewski, S.; Walentowska, J.; Wojcik, R. New Flexible Flame Retardant Coatings Based on Siloxane Resin and Ethylene-Vinyl Chloride Copolymer. Polymers 2016, 8, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.; Guo, S.; Liu, Y.; Chen, R.; Wang, Q. Organic-inorganic hybrid strategy based on ternary copolymerization to prepare flame retardant poly(methyl methacrylate) with high performance. Compos. Part B Eng. 2020, 203, 108437. [Google Scholar] [CrossRef]
- Bouquey, M.; Brochon, C.; Bruzaud, S.; Mingotaud, A.F.; Schappacher, M.; Soum, A. Ring-opening polymerization of nitrogen-containing cyclic organosilicon monomers. J. Organomet. Chem. 1996, 521, 21–27. [Google Scholar] [CrossRef]
- Brook, M.A. Silicon in Organic, Organometallic, and Polymer Chemistry; John Wiley & Sons: New York, NY, USA, 2000; Volume 123. [Google Scholar]
- Olejarka, J.; Lacz, A.; Olejniczak, Z.; Hasik, M. Non-porous and porous materials prepared by cross-linking of polyhydromethylsiloxane with silazane compounds. Eur. Polym. J. 2018, 99, 150–164. [Google Scholar] [CrossRef]
- Sun, J.T.; Huang, Y.D.; Cao, H.L.; Gong, G.F. Effects of ambient-temperature curing agents on the thermal stability of poly(methylphenylsiloxane). Polym. Degrad. Stab. 2004, 85, 725–731. [Google Scholar] [CrossRef]
- Kroke, E.; Li, Y.L.; Konetschny, C.; Lecomte, E.; Fasel, C.; Riedel, R. Silazane derived ceramics and related materials. Mater. Sci. Eng. R Rep. 2000, 26, 97–199. [Google Scholar] [CrossRef]
- Zhang, J.D.; Teng, Y.D.; Li, X.R.; Wang, S.L. Progress in synthesis and application of silazane. Chin. J. Org. Chem. 2007, 27, 1358–1365. [Google Scholar] [CrossRef]
- Krüger, C.R.; Rochow, E.G. Polyorganosilazanes. J. Polym. Sci. Part A Gen. Pap. 1964, 2, 3179–3189. [Google Scholar] [CrossRef]
- Seyferth, D. Synthesis of Some Organosilicon Polymers and Their Pyrolytic Conversion to Ceramics. In Silicon-Based Polymer Science; American Chemical Society: Washington, DC, USA, 1989; Volume 224, pp. 565–591. [Google Scholar]
- Ahmad, F.; Zulkurnain, E.S.B.; Ullah, S.; Al-Sehemi, A.G.; Raza, M.R. Improved fire resistance of boron nitride/epoxy intumescent coating upon minor addition of nano-alumina. Mater. Chem. Phys. 2020, 256. [Google Scholar] [CrossRef]
- Arslan, F.; Dilsiz, N. Flame resistant properties of LDPE/PLA blends containing halogen-free flame retardant. J. Appl. Polym. Sci. 2020, 137. [Google Scholar] [CrossRef]
- Donmez Cavdar, A.; Mengeloğlu, F.; Karakus, K. Effect of boric acid and borax on mechanical, fire and thermal properties of wood flour filled high density polyethylene composites. Measurement 2015, 60, 6–12. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, Y.; Liang, D.; Liu, S.; Chi, Z.; Xu, J. Influence of zinc borate on the flame retardancy and thermal stability of intumescent flame retardant polypropylene composites. J. Anal. Appl. Pyrolysis 2015, 115, 224–232. [Google Scholar] [CrossRef]
- Green, J. Mechanisms for Flame Retardancy and Smoke suppression—A Review. J. Fire Sci. 2016, 14, 426–442. [Google Scholar] [CrossRef]
- Riensch, N.A.; Deniz, A.; Kuhl, S.; Muller, L.; Adams, A.; Pich, A.; Helten, H. Borazine-based inorganic-organic hybrid cyclomatrix microspheres by silicon/boron exchange precipitation polycondensation. Polym. Chem. 2017, 8, 5264–5268. [Google Scholar] [CrossRef]
- Abdalla, M.O.; Ludwick, A.; Mitchell, T. Boron-modified phenolic resins for high performance applications. Polymer 2003, 44, 7353–7359. [Google Scholar] [CrossRef]
- Ma, C.; Ma, Z.; Gao, L.H.; Liu, Y.B.; Wu, T.T.; Wang, F.C.; Ishida, H. Ablation behavior of boron-modified phenolic resin irradiated by high-energy continuous-wave laser and its evolution of carbon structure. Mater. Des. 2019, 180, 107954. [Google Scholar] [CrossRef]
- Wang, F.; Huang, Z.; Guo, Z. Bionic boron/silicon-modified phenolic resin system with multifunctional groups: Synthesis, thermal properties and ablation mechanism. Biosurf. Biotribol. 2018, 4, 85–93. [Google Scholar] [CrossRef]
- Yajima, S.; Hayashi, J.; Okamura, K. Pyrolysis of a Polyborodiphenylsiloxane. Nature 1977, 266, 521–522. [Google Scholar] [CrossRef]
- Sundar, R.A.; Keller, T.M. Synthesis and characterization of linear boron-silicon-diacetylene copolymers. Macromolecules 1996, 29, 3647–3650. [Google Scholar] [CrossRef]
- Zhou, Q.; Feng, X.; Ni, L.Z.; Chen, J.D. Novel heat resistant methyl-tri(phenylethynyl)silane resin: Synthesis, characterization and thermal properties. J. Appl. Polym. Sci. 2006, 102, 2488–2492. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, Q.; Zhou, Q.; Ni, L.Z.; Chen, Q. Highly heat resistant and thermo-oxidatively stable borosilane alkynyl hybrid polymers. RSC Adv. 2015, 5, 12161–12167. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Z.H.; Jia, D.C.; Chen, Q.Q.; Zhou, Y. Synthesis and structural evolution of dual-boron-source-modified polysilazane derived SiBCN ceramics. New J. Chem. 2016, 40, 7034–7042. [Google Scholar] [CrossRef]
- Haberecht, J.; Krumeich, F.; Grutzmacher, H.; Nesper, R. High-yield molecular borazine precursors for Si-B-N-C ceramics. Chem. Mater. 2004, 16, 418–423. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 2011, 7, 1564–1583. [Google Scholar] [CrossRef]
- Viard, A.; Fonblanc, D.; Lopez-Ferber, D.; Schmidt, M.; Lale, A.; Durif, C.; Balestrat, M.; Rossignol, F.; Weinmann, M.; Riedel, R.; et al. Polymer Derived Si-B-C-N Ceramics: 30 Years of Research. Adv. Eng. Mater. 2018, 20. [Google Scholar] [CrossRef]
- Liu, T.; Zeng, X.; Lai, X.; Li, H.; Wang, Y. Remarkable improvement of organic-to-inorganic conversion of silicone rubber at elevated temperature through platinum-nitrogen catalytic system. Polym. Degrad. Stab. 2020, 171, 109026. [Google Scholar] [CrossRef]
- Maeda, K.; Motokura, K. Recent Advances on Heterogeneous Metal Catalysts for Hydrosilylation of Olefins. J. Jpn. Pet. Inst. 2020, 63, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Troegel, D.; Stohrer, J. Recent advances and actual challenges in late transition metal catalyzed hydrosilylation of olefins from an industrial point of view. Coord. Chem. Rev. 2011, 255, 1440–1459. [Google Scholar] [CrossRef]
- Hofmann, R.J.; Vlatkovic, M.; Wiesbrock, F. Fifty Years of Hydrosilylation in Polymer Science: A Review of Current Trends of Low-Cost Transition-Metal and Metal-Free Catalysts, Non-Thermally Triggered Hydrosilylation Reactions, and Industrial Applications. Polymers 2017, 9, 534. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.Y.; Schneider, A.F.; Lu, G.H.; Chen, Y.; Brook, M.A. Autoxidation: Catalyst-free route to silicone rubbers by crosslinking Si-H functional groups. Green Chem. 2019, 21, 6483–6490. [Google Scholar] [CrossRef]
- Zheng, P.; McCarthy, T.J. Rediscovering silicones: Molecularly smooth, low surface energy, unfilled, UV/vis-transparent, extremely cross-linked, thermally stable, hard, elastic PDMS. Langmuir 2010, 26, 18585–18590. [Google Scholar] [CrossRef] [PubMed]
- Demirci, A.; Yamamoto, S.; Matsui, J.; Miyashita, T.; Mitsuishi, M. Facile synthesis of cyclosiloxane-based polymers for hybrid film formation. Polym. Chem. 2015, 6, 2695–2706. [Google Scholar] [CrossRef]
- Yuan, Q.; Yao, B.; Huang, Z.R.; Huang, Q. Developing a liquid and curable two-component precursor system for fabrication of SiC(N)-based composites. Ceram. Int. 2019, 45, 24007–24013. [Google Scholar] [CrossRef]
- Tang, B.J.; Zhang, Y.; Hu, S.J.; Feng, B. A dense amorphous SiBCN(O) ceramic prepared by simultaneous pyrolysis of organics and inorganics. Ceram. Int. 2016, 42, 5238–5244. [Google Scholar] [CrossRef]
- Guo, X.; Wang, D.; Guo, Z.; Zhang, Z.B.; Cui, M.Z.; Xu, C.H. SiBCN-precursor-derived gradient oxidation protective ceramic coating for C/C composites. Surf. Coat. Technol. 2018, 350, 101–109. [Google Scholar] [CrossRef]
- Li, D.X.; Yang, Z.H.; Jia, D.C.; Duan, X.M.; Cai, D.L.; Wang, S.J.; Yuan, J.K.; Zhou, Y.; Yu, D.L.; Tian, Y.J. High-temperature oxidation resistance of dense amorphous boron-rich SiBCN monoliths. Corros. Sci. 2019, 157, 312–323. [Google Scholar] [CrossRef]
- Morgan, A.B.; Jurs, J.L.; Tour, J.M. Synthesis, flame-retardancy testing, and preliminary mechanism studies of nonhalogenated aromatic boronic acids: A new class of condensed-phase polymer flame-retardant additives for acrylonitrile-butadiene-styrene and polycarbonate. J. Appl. Polym. Sci. 2000, 76, 1257–1268. [Google Scholar] [CrossRef]
- Lyon, R.E.; Walters, R.N. Pyrolysis combustion flow calorimetry. J. Anal. Appl. Pyrolysis 2004, 71, 27–46. [Google Scholar] [CrossRef]
- Benin, V.; Gardelle, B.; Morgan, A.B. Heat release of polyurethanes containing potential flame retardants based on boron and phosphorus chemistries. Polym. Degrad. Stab. 2014, 106, 108–121. [Google Scholar] [CrossRef]









| Sample | BSZ12 (g) | PNSZ (g) | Molar Ratio (Si-H/C=C) |
|---|---|---|---|
| CPBCS10-1 | 1.2962 | 4.000 | 10-1 |
| CPBCS5-1 | 2.5924 | 4.000 | 5-1 |
| CPBCS3-1 | 3.2192 | 3.000 | 3-1 |
| Samples | CPBCS10-1 | CPBCS5-1 | CPBCS3-1 |
|---|---|---|---|
| Si | 44.30% | 35.40% | 33.05% |
| C | 24.50% | 30.10% | 32.90% |
| O | 20.22% | 19.80% | 20.23% |
| H | 7.55% | 8.35% | 8.65% |
| N | 2.80% | 5.33% | 3.80% |
| B | 0.63% | 1.02% | 1.37% |
| Sample | pHRR1 (W/g) | Tp1 (°C) | pHRR2 (W/g) | Tp2 (°C) | pHRR3 (W/g) | Tp3 (°C) | sumHRC (J/g K) | THR (kJ/g) |
|---|---|---|---|---|---|---|---|---|
| CPBCS3-1 | 8.9 | 45.5 | 40.3 | 356.5 | - | - | 40 | 7.5 |
| CPBCS5-1 | 6.9 | 46.0 | 48.3 | 342.1 | 41.1 | 436.0 | 43 | 9 |
| CPBCS10-1 | 9.1 | 45.5 | 37.9 | 344.0 | 39.5 | 457.5 | 43 | 6.2 |
| CPBCS10-1 | CPBCS5-1 | CPBCS3-1 | |
|---|---|---|---|
| Reduced Modulus (GPa) | 2.32 ± 0.08 | 1.93 ± 0.09 | 1.40 ± 0.10 |
| Hardness (GPa) | 0.30 ± 0.02 | 0.18 ± 0.01 | 0.09 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Chen, K.; Li, T.; Sun, X.; Liu, M.; Yang, L.; Hu, X.; Xu, J.; He, L.; Huang, Q.; et al. High-Temperature Resistant Polyborosilazanes with Tailored Structures. Polymers 2021, 13, 467. https://doi.org/10.3390/polym13030467
Wang B, Chen K, Li T, Sun X, Liu M, Yang L, Hu X, Xu J, He L, Huang Q, et al. High-Temperature Resistant Polyborosilazanes with Tailored Structures. Polymers. 2021; 13(3):467. https://doi.org/10.3390/polym13030467
Chicago/Turabian StyleWang, Bijie, Ke Chen, Tianhao Li, Xun Sun, Ming Liu, Lingwei Yang, Xiao (Matthew) Hu, Jian Xu, Liu He, Qing Huang, and et al. 2021. "High-Temperature Resistant Polyborosilazanes with Tailored Structures" Polymers 13, no. 3: 467. https://doi.org/10.3390/polym13030467