Polymeric Systems Containing Supramolecular Coordination Complexes for Drug Delivery
Abstract
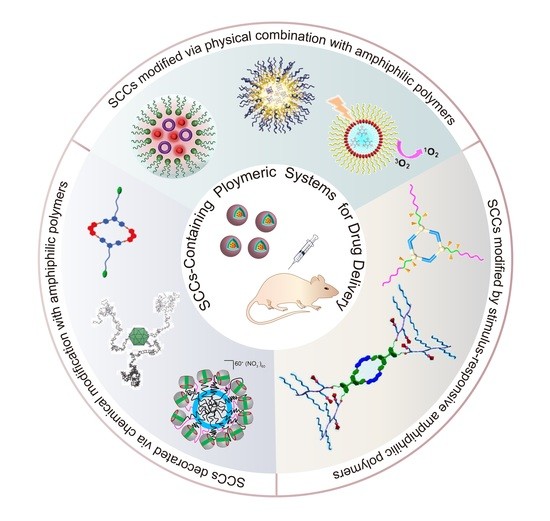
:1. Introduction
2. SCCs Modified via Physical Combination with Amphiphilic Polymers
3. SCCs Decorated via Chemical Modification with Amphiphilic Polymers
4. SCCs Modified by Stimulus-Responsive Amphiphilic Polymers
5. Perspectives and Challenges
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.-M.; Zong, Y.-N.; Cao, S.-M.; Xu, R.-H. Current Cancer Situation in China: Good or Bad News from the 2018 Global Cancer Statistics? Cancer Commun. 2019, 39, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic Therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Gu, Z.; An, H.; Chen, C.; Chen, J.; Cui, R.; Chen, S.; Chen, W.; Chen, X.; Chen, X.; et al. Precise Nanomedicine for Intelligent Therapy of Cancer. Sci. China Chem. 2018, 61, 1503–1552. [Google Scholar]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, Functionalization Strategies and Biomedical Applications of Targeted Biodegradable/Biocompatible Polymer-Based Nanocarriers for Drug Delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef]
- Fox, M.E.; Szoka, F.C.; Fréchet, J.M.J. Soluble Polymer Carriers for the Treatment of Cancer: The Importance of Molecular Architecture. Accounts Chem. Res. 2009, 42, 1141–1151. [Google Scholar] [CrossRef] [Green Version]
- Pottanam Chali, S.; Ravoo, B.J. Polymer Nanocontainers for Intracellular Delivery. Angew. Chem. Int. Ed. 2020, 59, 2962–2972. [Google Scholar] [CrossRef]
- Palivan, C.G.; Goers, R.; Najer, A.; Zhang, X.; Car, A.; Meier, W. Bioinspired Polymer Vesicles and Membranes for Biological and Medical Applications. Chem. Soc. Rev. 2016, 45, 377–411. [Google Scholar] [CrossRef] [Green Version]
- Szafraniec-Szczęsny, J.; Janik-Hazuka, M.; Odrobińska, J.; Zapotoczny, S. Polymer Capsules with Hydrophobic Liquid Cores as Functional Nanocarriers. Polymers 2020, 12, 1999. [Google Scholar] [CrossRef]
- Cook, T.R.; Stang, P.J. Recent Developments in the Preparation and Chemistry of Metallacycles and Metallacages via Coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal–Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal–Organic Materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular Coordination: Self-Assembly of Finite Two- and Three-Dimensional Ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smulders, M.M.J.; Riddell, I.A.; Browne, C.; Nitschke, J.R. Building on Architectural Principles for Three-Dimensional Metallosupramolecular Construction. Chem. Soc. Rev. 2013, 42, 1728–1754. [Google Scholar] [CrossRef] [PubMed]
- Seidel, S.R.; Stang, P.J. High-Symmetry Coordination Cages via Self-Assembly. Accounts Chem. Res. 2002, 35, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Judge, N.; Wang, L.; Ho, Y.Y.L.; Wang, Y. Molecular Engineering of Metal-Organic Cycles/Cages for Drug Delivery. Macromol. Res. 2018, 26, 1074–1084. [Google Scholar] [CrossRef]
- Sepehrpour, H.; Fu, W.; Sun, Y.; Stang, P.J. Biomedically Relevant Self-Assembled Metallacycles and Metallacages. J. Am. Chem. Soc. 2019, 141, 14005–14020. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, C.; Stang, P.J. Soft Materials with Diverse Suprastructures via the Self-Assembly of Metal–Organic Complexes. Accounts Chem. Res. 2019, 52, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; He, T.; Fan, Y.; Yuan, X.; Qiu, H.; Yin, S. Recent Developments in the Construction of Metallacycle/Metallacage-Cored Supramolecular Polymers via Hierarchical Self-Assembly. Chem. Commun. 2019, 55, 8036–8059. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Li, H.; Fan, Y.; He, T.; Qiu, H.; Yin, S. Metallacycle/Metallacage-Cored Fluorescent Supramolecular Assemblies with Aggregation-Induced Emission Properties. Adv. Opt. Mater. 2020, 8, 1902190. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Klosterman, J.K.; Fujita, M. Functional Molecular Flasks: New Properties and Reactions within Discrete, Self-Assembled Hosts. Angew. Chem. Int. Ed. 2009, 48, 3418–3438. [Google Scholar] [CrossRef]
- Vardhan, H.; Yusubov, M.; Verpoort, F. Self-Assembled Metal–Organic Polyhedra: An Overview of Various Applications. Coord. Chem. Rev. 2016, 306, 171–194. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, D.; Zhang, J.; Ni, R.; Xu, L.; He, T.; Lin, X.; Li, X.; Qiu, H.; Yin, S.; et al. Self-Healing Heterometallic Supramolecular Polymers Constructed by Hierarchical Assembly of Triply Orthogonal Interactions with Tunable Photophysical Properties. J. Am. Chem. Soc. 2019, 141, 17909–17917. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, M.; Tang, D.; Yan, X.; Zhang, Z.; Zhou, Z.; Song, B.; Wang, H.; Li, X.; Yin, S.; et al. Fluorescent Metallacage-Core Supramolecular Polymer Gel Formed by Orthogonal Metal Coordination and Host–Guest Interactions. J. Am. Chem. Soc. 2018, 140, 7674–7680. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shen, X.; Zhou, Z.; He, T.; Zhang, J.; Qiu, H.; Saha, M.L.; Yin, S.; Stang, P.J. Metallacycle-Cored Supramolecular Polymers: Fluorescence Tuning by Variation of Substituents. J. Am. Chem. Soc. 2018, 140, 16920–16924. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Younus, H.A.; Chughtai, A.H.; Verpoort, F. Metal–Organic Molecular Cages: Applications of Biochemical Implications. Chem. Soc. Rev. 2015, 44, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Woods, B.; Wenzel, M. The Promise of Self-Assembled 3D Supramolecular Coordination Complexes for Biomedical Applications. Inorg. Chem. 2017, 56, 14715–14729. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yan, X.; Huang, F.; Sepehrpour, H.; Stang, P.J. Near-Infrared Emissive Discrete Platinum(II) Metallacycles: Synthesis and Application in Ammonia Detection. Org. Lett. 2017, 19, 5728–5731. [Google Scholar] [CrossRef]
- Xu, W.-Q.; Fan, Y.-Z.; Wang, H.-P.; Teng, J.; Li, Y.-H.; Chen, C.-X.; Fenske, D.; Jiang, J.-J.; Su, C.-Y. Investigation of Binding Behavior between Drug Molecule 5-Fluoracil and M4L4-Type Tetrahedral Cages: Selectivity, Capture, and Release. Chem. Eur. J. 2017, 23, 3542–3547. [Google Scholar] [CrossRef]
- Kaiser, F.; Schmidt, A.; Heydenreuter, W.; Altmann, P.J.; Casini, A.; Sieber, S.A.; Kühn, F.E. Self-Assembled Palladium and Platinum Coordination Cages: Photophysical Studies and Anticancer Activity. Eur. J. Inorg. Chem. 2016, 2016, 5189–5196. [Google Scholar] [CrossRef] [Green Version]
- Preston, D.; Lewis, J.E.M.; Crowley, J.D. Multicavity [PdnL4]2n+ Cages with Controlled Segregated Binding of Different Guests. J. Am. Chem. Soc. 2017, 139, 2379–2386. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.-R.; Suntharalingam, K.; Johnstone, T.C.; Lippard, S.J. Encapsulation of Pt(IV) Prodrugs within a Pt(II) Cage for Drug Delivery. Chem. Sci. 2015, 6, 1189–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, A.; Jeong, Y.J.; Jo, J.H.; Woo, S.; Kim, D.H.; Kim, H.; Kang, S.C.; Stang, P.J.; Chi, K.-W. Anticancer Activity and Autophagy Involvement of Self-Assembled Arene–Ruthenium Metallacycles. Organometallics 2015, 34, 4507–4514. [Google Scholar] [CrossRef]
- Mishra, A.; Jeong, Y.J.; Jo, J.-H.; Kang, S.C.; Lah, M.S.; Chi, K.-W. Anticancer Potency Studies of Coordination Driven Self-Assembled Arene–Ru-Based Metalla-Bowls. ChemBioChem 2014, 15, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.-S. The Development of Anticancer Ruthenium(II) Complexes: From Single Molecule Compounds to Nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Lv, F.; Yu, B.; Shen, Y.; Cong, H. Recent Advances in Ruthenium and Platinum Based Supramolecular Coordination Complexes for Antitumor Therapy. Colloids Surf. B 2019, 182, 110373. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Jana, A.; Bhowmick, S.; Kumar, S.; Singh, K.; Garg, P.; Das, N. Self-Assembly of Pt(II) Based Nanoscalar Ionic Hexagons and Their Anticancer Potencies. Inorg. Chim. Acta 2019, 484, 19–26. [Google Scholar] [CrossRef]
- McConnell, A.J.; Wood, C.S.; Neelakandan, P.P.; Nitschke, J.R. Stimuli-Responsive Metal–Ligand Assemblies. Chem. Rev. 2015, 115, 7729–7793. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Li, S.; Yan, X.; Zhou, Z.; Saha, M.L.; Wang, Y.-C.; Stang, P.J. Fluorescent Metallacycle-Cored Polymers via Covalent Linkage and Their Use as Contrast Agents for Cell Imaging. Proc. Natl. Acad. Sci. USA 2016, 113, 11100–11105. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Zhang, M.; Saha, M.L.; Mao, Z.; Chen, J.; Yao, Y.; Zhou, Z.; Liu, Y.; Gao, C.; Huang, F.; et al. Antitumor Activity of a Unique Polymer That Incorporates a Fluorescent Self-Assembled Metallacycle. J. Am. Chem. Soc. 2017, 139, 15940–15949. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Zhang, Y.; Yu, G.; Crawley, M.R.; Fulong, C.R.P.; Friedman, A.E.; Sengupta, S.; Sun, J.; Li, Q.; Huang, F.; et al. Highly Emissive Self-Assembled BODIPY-Platinum Supramolecular Triangles. J. Am. Chem. Soc. 2018, 140, 7730–7736. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jia, P.-P.; Xu, Y.-L.; Ding, F.; Yang, W.-C.; Sun, Y.; Li, X.-P.; Yin, G.-Q.; Xu, L.; Yang, G.-F. Photoacoustic Imaging-Guided Chemo-Photothermal Combinational Therapy Based on Emissive Pt(II) Metallacycle-Loaded Biomimic Melanin Dots. Sci. China Chem. 2020. [Google Scholar] [CrossRef]
- Zhu, H.; Shangguan, L.; Shi, B.; Yu, G.; Huang, F. Recent Progress in Macrocyclic Amphiphiles and Macrocyclic Host-Based Supra-Amphiphiles. Mater. Chem. Front. 2018, 2, 2152–2174. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking Cancer Nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.; McNeill, S.M.; Lewis, J.E.M.; Giles, G.I.; Crowley, J.D. Enhanced Kinetic Stability of [Pd2L4]4+ Cages through Ligand Substitution. Dalton Trans. 2016, 45, 8050–8060. [Google Scholar] [CrossRef] [Green Version]
- Petros, R.A.; DeSimone, J.M. Strategies in the Design of Nanoparticles for Therapeutic Applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Mansour, H.M.; Sohn, M.; Al-Ghananeem, A.; DeLuca, P.P. Materials for Pharmaceutical Dosage Forms: Molecular Pharmaceutics and Controlled Release Drug Delivery Aspects. Int. J. Mol. Sci. 2010, 11, 3298–3322. [Google Scholar] [CrossRef] [Green Version]
- Wolfbeis, O.S. An Overview of Nanoparticles Commonly Used in Fluorescent Bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [Green Version]
- Akash, M.S.H.; Rehman, K. Recent Progress in Biomedical Applications of Pluronic (PF127): Pharmaceutical Perspectives. J. Control. Release 2015, 209, 120–138. [Google Scholar] [CrossRef]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent Advances in Polymeric Micelles for Anti-Cancer Drug Delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef]
- Jarak, I.; Varela, C.L.; Tavares da Silva, E.; Roleira, F.F.M.; Veiga, F.; Figueiras, A. Pluronic-Based Nanovehicles: Recent Advances in Anticancer Therapeutic Applications. Eur. J. Med. Chem. 2020, 206, 112526. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Tumor-Selective Delivery of Macromolecular Drugs via the EPR Effect: Background and Future Prospects. Bioconjugate Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR Effect: Unique Features of Tumor Blood Vessels for Drug Delivery, Factors Involved, and Limitations and Augmentation of the Effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Yu, G.; Cook, T.R.; Li, Y.; Yan, X.; Wu, D.; Shao, L.; Shen, J.; Tang, G.; Huang, F.; Chen, X.; et al. Tetraphenylethene-Based Highly Emissive Metallacage as a Component of Theranostic Supramolecular Nanoparticles. Proc. Natl. Acad. Sci. USA 2016, 113, 13720–13725. [Google Scholar] [CrossRef] [Green Version]
- Yue, Z.; Wang, H.; Bowers, D.J.; Gao, M.; Stilgenbauer, M.; Nielsen, F.; Shelley, J.T.; Zheng, Y.-R. Nanoparticles of Metal–Organic Cages Designed to Encapsulate Platinum-Based Anticancer Agents. Dalton Trans. 2018, 47, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Master, A.; Livingston, M.; Sen Gupta, A. Photodynamic Nanomedicine in the Treatment of Solid Tumors: Perspectives and Challenges. J. Control. Release 2013, 168, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Xu, Q.; He, S.; Xiong, H.; Zhang, Q.; Xu, W.; Ricotta, V.; Bai, L.; Zhang, Q.; Yu, Z.; et al. Recent Advances in Delivery of Photosensitive Metal-Based Drugs. Coord. Chem. Rev. 2019, 387, 154–179. [Google Scholar] [CrossRef]
- Li, Y.; Lin, T.-y.; Luo, Y.; Liu, Q.; Xiao, W.; Guo, W.; Lac, D.; Zhang, H.; Feng, C.; Wachsmann-Hogiu, S.; et al. A Smart and Versatile Theranostic Nanomedicine Platform Based on Nanoporphyrin. Nat. Commun. 2014, 5, 4712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovell, J.F.; Liu, T.W.B.; Chen, J.; Zheng, G. Activatable Photosensitizers for Imaging and Therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.F.; Jin, C.S.; Huynh, E.; Jin, H.; Kim, C.; Rubinstein, J.L.; Chan, W.C.W.; Cao, W.; Wang, L.V.; Zheng, G. Porphysome Nanovesicles Generated by Porphyrin Bilayers for Use as Multimodal Biophotonic Contrast Agents. Nat. Mater. 2011, 10, 324–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, G.; Denoyelle-Di-Muro, E.; Mbakidi, J.-P.; Leroy-Lhez, S.; Sol, V.; Therrien, B. Delivery of Porphin to Cancer Cells by Organometallic Rh(III) and Ir(III) Metalla-Cages. J. Organomet. Chem. 2015, 787, 44–50. [Google Scholar] [CrossRef]
- Schmitt, F.; Freudenreich, J.; Barry, N.P.E.; Juillerat-Jeanneret, L.; Süss-Fink, G.; Therrien, B. Organometallic Cages as Vehicles for Intracellular Release of Photosensitizers. J. Am. Chem. Soc. 2012, 134, 754–757. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Zhou, Z.; Yang, H.; Shan, C.; Yu, H.; Wojtas, L.; Zhang, M.; Mao, Z.; Wang, M.; Stang, P.J. Self-Assembly of Porphyrin-Containing Metalla-Assemblies and Cancer Photodynamic Therapy. Inorg. Chem. 2020, 59, 7380–7388. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, L.-J.; Dong, F.; Jiang, S.-T.; Yin, G.-Q.; Li, X.; Tian, Y.; Yang, H.-B. Light-Controlled Generation of Singlet Oxygen within a Discrete Dual-Stage Metallacycle for Cancer Therapy. J. Am. Chem. Soc. 2019, 141, 8943–8950. [Google Scholar] [CrossRef]
- Yu, G.; Yu, S.; Saha, M.L.; Zhou, J.; Cook, T.R.; Yung, B.C.; Chen, J.; Mao, Z.; Zhang, F.; Zhou, Z.; et al. A Discrete Organoplatinum(II) Metallacage as a Multimodality Theranostic Platform for Cancer Photochemotherapy. Nat. Commun. 2018, 9, 4335. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhu, B.; Shao, L.; Zhou, J.; Saha, M.L.; Shi, B.; Zhang, Z.; Hong, T.; Li, S.; Chen, X.; et al. Host−Guest Complexation-Mediated Codelivery of Anticancer Drug and Photosensitizer for Cancer Photochemotherapy. Proc. Natl. Acad. Sci. USA 2019, 116, 6618–6623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.-Y.; Zheng, Y.; Tan, C.-P.; Sun, J.-H.; Zhang, W.; Ji, L.-N.; Mao, Z.-W. Graphene Oxide Decorated with Ru(II)–Polyethylene Glycol Complex for Lysosome-Targeted Imaging and Photodynamic/Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 6761–6771. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yu, B.; Zhang, P.; Huang, J.; Chen, Y.; Gasser, G.; Ji, L.; Chao, H. Highly Charged Ruthenium(II) Polypyridyl Complexes as Lysosome-Localized Photosensitizers for Two-Photon Photodynamic Therapy. Angew. Chem. Int. Ed. 2015, 54, 14049–14052. [Google Scholar]
- Liu, J.; Chen, Y.; Li, G.; Zhang, P.; Jin, C.; Zeng, L.; Ji, L.; Chao, H. Ruthenium(II) Polypyridyl Complexes as Mitochondria-Targeted Two-Photon Photodynamic Anticancer Agents. Biomaterials 2015, 56, 140–153. [Google Scholar] [PubMed]
- Zhou, Z.; Liu, J.; Huang, J.; Rees, T.W.; Wang, Y.; Wang, H.; Li, X.; Chao, H.; Stang, P.J. A Self-Assembled Ru–Pt Metallacage as a Lysosome-Targeting Photosensitizer for 2-Photon Photodynamic Therapy. Proc. Natl. Acad. Sci. USA 2019, 116, 20296–20302. [Google Scholar] [PubMed] [Green Version]
- Ding, F.; Zhan, Y.; Lu, X.; Sun, Y. Recent Advances in Near-Infrared II Fluorophores for Multifunctional Biomedical Imaging. Chem. Sci. 2018, 9, 4370–4380. [Google Scholar]
- Hong, G.; Antaris, A.L.; Dai, H. Near-Infrared Fluorophores for Biomedical Imaging. Nat. Biomed. Eng. 2017, 1, 1–22. [Google Scholar]
- Miao, Q.; Pu, K. Organic Semiconducting Agents for Deep-Tissue Molecular Imaging: Second Near-Infrared Fluorescence, Self-Luminescence, and Photoacoustics. Adv. Mater. 2018, 30, 1801778. [Google Scholar]
- Lu, L.; Li, B.; Ding, S.; Fan, Y.; Wang, S.; Sun, C.; Zhao, M.; Zhao, C.-X.; Zhang, F. NIR-II Bioluminescence for In Vivo High Contrast Imaging and In Situ ATP-Mediated Metastases Tracing. Nat. Commun. 2020, 11, 4192. [Google Scholar]
- Wang, P.; Fan, Y.; Lu, L.; Liu, L.; Fan, L.; Zhao, M.; Xie, Y.; Xu, C.; Zhang, F. NIR-II Nanoprobes In-Vivo Assembly to Improve Image-Guided Surgery for Metastatic Ovarian Cancer. Nat. Commun. 2018, 9, 2898. [Google Scholar]
- Sun, Y.; Ding, F.; Zhou, Z.; Li, C.; Pu, M.; Xu, Y.; Zhan, Y.; Lu, X.; Li, H.; Yang, G.; et al. Rhomboidal Pt(II) Metallacycle-Based NIR-II Theranostic Nanoprobe for Tumor Diagnosis and Image-Guided Therapy. Proc. Natl. Acad. Sci. USA 2019, 116, 1968–1973. [Google Scholar]
- Ding, F.; Chen, Z.; Kim, W.Y.; Sharma, A.; Li, C.; Ouyang, Q.; Zhu, H.; Yang, G.; Sun, Y.; Kim, J.S. A Nano-Cocktail of an NIR-II Emissive Fluorophore and Organoplatinum(II) Metallacycle for Efficient Cancer Imaging and Therapy. Chem. Sci. 2019, 10, 7023–7028. [Google Scholar]
- Fan, Q.; Cheng, K.; Hu, X.; Ma, X.; Zhang, R.; Yang, M.; Lu, X.; Xing, L.; Huang, W.; Gambhir, S.S.; et al. Transferring Biomarker into Molecular Probe: Melanin Nanoparticle as a Naturally Active Platform for Multimodality Imaging. J. Am. Chem. Soc. 2014, 136, 15185–15194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, B.; Yang, X.; Li, X.; Lv, S.; Zhang, H.; Sun, J.; Li, L.; Wang, L.; Qu, B.; Peng, X.; et al. Photoacoustic-Imaging-Guided Therapy of Functionalized Melanin Nanoparticles: Combination of Photothermal Ablation and Gene Therapy against Laryngeal Squamous Cell Carcinoma. Nanoscale 2019, 11, 6285–6296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Fan, Q.; Yang, M.; Cheng, K.; Lu, X.; Zhang, L.; Huang, W.; Cheng, Z. Engineering Melanin Nanoparticles as an Efficient Drug-Delivery System for Imaging-Guided Chemotherapy. Adv. Mater. 2015, 27, 5063–5069. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Ding, F.; Chen, Z.; Zhang, R.; Li, C.; Xu, Y.; Zhang, Y.; Ni, R.; Li, X.; Yang, G.; et al. Melanin-Dot-Mediated Delivery of Metallacycle for NIR-II/Photoacoustic Dual-Modal Imaging-Guided Chemo-Photothermal Synergistic Therapy. Proc. Natl. Acad. Sci. USA 2019, 116, 16729–16735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, E.-K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for Theranostics: Recent Advances and Future Challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhao, R.; Shi, Y.; Cai, Y.; Chen, J.; Sun, S.; Zhang, W.; Tang, R. 2D Amphiphilic Organoplatinum(II) Metallacycles: Their Syntheses, Self-Assembly in Water and Potential Application in Photodynamic therapy. Chem. Commun. 2018, 54, 8068–8071. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, X.; Yu, J.; Fan, Y.; He, T.; Lu, S.; Li, X.; Qiu, H.; Yin, S. Amphiphilic Rhomboidal Organoplatinum(II) Metallacycles with Encapsulated Doxorubicin for Synergistic Cancer Therapy. ACS Appl. Bio Mater. 2020, 3, 8061–8068. [Google Scholar] [CrossRef]
- Zhao, D.; Tan, S.; Yuan, D.; Lu, W.; Rezenom, Y.H.; Jiang, H.; Wang, L.-Q.; Zhou, H.-C. Surface Functionalization of Porous Coordination Nanocages Via Click Chemistry and Their Application in Drug Delivery. Adv. Mater. 2011, 23, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Kim, K.; Ogoshi, T.; Yao, W.; Gibb, B.C. The Aqueous Supramolecular Chemistry of Cucurbit[n]urils, Pillar[n]arenes and Deep-Cavity Cavitands. Chem. Soc. Rev. 2017, 46, 2479–2496. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Samal, S.; Selvapalam, N.; Kim, H.-J.; Kim, K. Cucurbituril Homologues and Derivatives: New Opportunities in Supramolecular Chemistry. Accounts Chem. Res. 2003, 36, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, I.-S.; Kim, S.-Y.; Lee, E.; Kang, J.-K.; Sakamoto, S.; Yamaguchi, K.; Kim, K. New Cucurbituril Homologues: Syntheses, Isolation, Characterization, and X-ray Crystal Structures of Cucurbit[n]uril (n = 5, 7, and 8). J. Am. Chem. Soc. 2000, 122, 540–541. [Google Scholar] [CrossRef]
- Nagahama, K.; Utsumi, T.; Kumano, T.; Maekawa, S.; Oyama, N.; Kawakami, J. Discovery of a New Function of Curcumin Which Enhances Its Anticancer Therapeutic Potency. Sci. Rep. 2016, 6, 30962. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Misra, S.K.; Saha, M.L.; Lahiri, N.; Louie, J.; Pan, D.; Stang, P.J. Orthogonal Self-Assembly of an Organoplatinum(II) Metallacycle and Cucurbit[8]uril That Delivers Curcumin to Cancer Cells. Proc. Natl. Acad. Sci. USA 2018, 115, 8087–8092. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing Dendrimers for Biological Applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in Biomedical Applications—Reflections on the Field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- Astruc, D.; Ornelas, C.; Ruiz, J. Metallocenyl Dendrimers and Their Applications in Molecular Electronics, Sensing, and Catalysis. Accounts Chem. Res. 2008, 41, 841–856. [Google Scholar] [CrossRef]
- Chen, L.-J.; Zhao, G.-Z.; Jiang, B.; Sun, B.; Wang, M.; Xu, L.; He, J.; Abliz, Z.; Tan, H.; Li, X.; et al. Smart Stimuli-Responsive Spherical Nanostructures Constructed from Supramolecular Metallodendrimers via Hierarchical Self-Assembly. J. Am. Chem. Soc. 2014, 136, 5993–6001. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Zhang, X. Amphiphilic Building Blocks for Self-Assembly: From Amphiphiles to Supra-Amphiphiles. Accounts Chem. Res. 2012, 45, 608–618. [Google Scholar] [CrossRef]
- Chang, Y.; Jiao, Y.; Symons, H.E.; Xu, J.-F.; Faul, C.F.J.; Zhang, X. Molecular Engineering of Polymeric Supra-Amphiphiles. Chem. Soc. Rev. 2019, 48, 989–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanta, S.K.; Moncelet, D.; Briken, V.; Isaacs, L. Metal–Organic Polyhedron Capped with Cucurbit[8]uril Delivers Doxorubicin to Cancer Cells. J. Am. Chem. Soc. 2016, 138, 14488–14496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanta, S.K.; Quigley, J.; Vinciguerra, B.; Briken, V.; Isaacs, L. Cucurbit[7]uril Enables Multi-Stimuli-Responsive Release from the Self-Assembled Hydrophobic Phase of a Metal Organic Polyhedron. J. Am. Chem. Soc. 2017, 139, 9066–9074. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, Y.; Xie, Z.; Jing, X.; Bellotti, A.; Gu, Z. Stimuli-Responsive Polymersomes for Biomedical Applications. Biomacromolecules 2017, 18, 649–673. [Google Scholar] [CrossRef]
- Zhang, Q.; Re Ko, N.; Kwon Oh, J. Recent advances in stimuli-responsive degradable block copolymer micelles: Synthesis and controlled drug delivery applications. Chem. Commun. 2012, 48, 7542–7552. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, G.; Yang, J.; Shi, B.; Ye, B.; Wang, M.; Huang, F.; Stang, P.J. Polymeric Nanoparticles Integrated from Discrete Organoplatinum(II) Metallacycle by Stepwise Post-assembly Polymerization for Synergistic Cancer Therapy. Chem. Mater. 2020, 32, 4564–4573. [Google Scholar] [CrossRef]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Li, Y.; Lin, X.; Qiu, H.; Yin, S. Polymeric Systems Containing Supramolecular Coordination Complexes for Drug Delivery. Polymers 2021, 13, 370. https://doi.org/10.3390/polym13030370
Chen F, Li Y, Lin X, Qiu H, Yin S. Polymeric Systems Containing Supramolecular Coordination Complexes for Drug Delivery. Polymers. 2021; 13(3):370. https://doi.org/10.3390/polym13030370
Chicago/Turabian StyleChen, Feng, Yang Li, Xiongjie Lin, Huayu Qiu, and Shouchun Yin. 2021. "Polymeric Systems Containing Supramolecular Coordination Complexes for Drug Delivery" Polymers 13, no. 3: 370. https://doi.org/10.3390/polym13030370