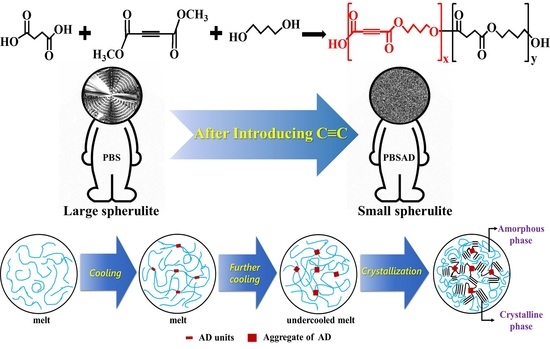
Poly(butylene succinate-co-butylene acetylenedicarboxylate): Copolyester with Novel Nucleation Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PBS and PBSAD
2.3. Characterization
3. Results
3.1. Chain Structure
3.2. Non-Isothermal and Isothermal Crystallization
3.3. Crystal Morphology and Structure
3.4. Evaluation of Melt Memory Effect
3.5. In Situ FTIR Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Guo, B.H. Poly(butylene succinate) and its copolymers: Research, development and industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Ishioka, R.; Kitakuni, E.; Ichikawa, Y. Aliphatic polyesters: “Bionolle”. In Biopolymers; Doi, Y., Steinbüchel, A., Eds.; Wiley-VCH: New York, NY, USA, 2002; Volume 4, pp. 275–297. [Google Scholar]
- Sisti, L.; Totaro, G.; Marchese, P. PBS makes its entrance into the family of biobased plastics. In Biodegradable and Biobased Polymers for Environmental and Biomedical Applications; Kalia, S., Averous, L., Eds.; Wiley: New York, NY, USA, 2016; Volume 7, pp. 225–285. [Google Scholar]
- Xu, J.; Guo, B.H. Microbial Succinic Acid, Its Polymer Poly(butylene succinate), and Applications. In Plastics from Bacteria: Natural Functions and Applications; Chen, G.Q., Ed.; Springer: Berlin, Germany, 2016; Volume 14, pp. 347–388. [Google Scholar]
- Fujimaki, T. Processability and properties of aliphatic polyesters, ‘BIONOLLE’, synthesized by polycondensation reaction. Polym. Degrad. Stabl. 1998, 59, 209–214. [Google Scholar] [CrossRef]
- Ye, H.M.; Tang, Y.R.; Xu, J.; Guo, B.H. Role of Poly(butylene fumarate) on Crystallization Behavior of Poly(butylene succinate). Ind. Eng. Chem. Res. 2013, 52, 10682–10689. [Google Scholar] [CrossRef]
- Yang, B.; Ni, H.; Huang, J.; Luo, Y. Effects of Poly(vinyl butyral) as a Macromolecular Nucleating Agent on the Nonisothermal Crystallization and Mechanical Properties of Biodegradable Poly(butylene succinate). Macromolecules 2014, 47, 284–296. [Google Scholar] [CrossRef]
- Tang, Y.R.; Lin, D.W.; Gao, Y.; Xu, J.; Guo, B.H. Prominent Nucleating Effect of Finely Dispersed Hydroxyl-Functional Hexagonal Boron Nitride on Biodegradable Poly(butylene succinate). Ind. Eng. Chem. Res. 2014, 53, 4689–4696. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, G.; Shi, Y. Isothermal crystallization and mechanical properties of poly(butylene succinate)/layered double hydroxide nanocomposites. J. Polym. Res. 2012, 19, 9930. [Google Scholar] [CrossRef]
- Yarici, T.; Kodal, M.; Ozkoc, G. Non-isothermal crystallization kinetics of Poly(Butylene succinate) (PBS) nanocomposites with different modified carbon nanotubes. Polymer 2018, 146, 361–377. [Google Scholar] [CrossRef]
- Bosq, N.; Aht-Ong, D. Isothermal and non-isothermal crystallization kinetics of poly(butylene succinate) with nanoprecipitated calcium carbonate as nucleating agent. J. Therm. Anal. Calorim. 2018, 132, 233–249. [Google Scholar] [CrossRef]
- Kim, H.S.; Yang, H.S.; Kim, H.J. Biodegradability and mechanical properties of agroflour–filled polybutylene succinate biocomposites. J. Appl. Polym. Sci. 2005, 97, 1513–1521. [Google Scholar] [CrossRef]
- Li, J.; Luo, X.; Lin, X. Preparation and characterization of hollow glass microsphere reinforced poly(butylene succinate) composites. Mater. Des. 2013, 46, 902–909. [Google Scholar] [CrossRef]
- Nikolic, M.S.; Djonlagic, J. Synthesis and characterization of biodegradable poly(butylene succinate-co-butylene adipate)s. Polym. Degrad. Stabl. 2001, 74, 263–270. [Google Scholar] [CrossRef]
- Li, F.; Xu, X.; Hao, Q.; Li, Q.; Yu, J.; Cao, A. Effects of comonomer sequential structure on thermal and crystallization behaviors of biodegradable poly(butylene succinate-co-butylene terephthalate)s. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1635–1644. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, J.; Guo, B.; Xie, X. Crystallization kinetics and morphology of biodegradable poly(butylene succinatecopropylene succinate)s. J. Polym. Sci. B Polym. Phys. 2007, 45, 420–428. [Google Scholar] [CrossRef]
- Mochizuki, M.; Mukai, K.; Yamada, K.; Ichise, N.; Murase, S.; Iwaya, Y. Structural Effects upon Enzymatic Hydrolysis of Poly(butylene succinate-co-ethylene succinate)s. Macromolecules 1997, 30, 7403–7407. [Google Scholar] [CrossRef]
- Liu, X.Q.; Li, C.C.; Zhang, D.; Xiao, Y.N. Melting behaviors, crystallization kinetics, and spherulitic morphologies of poly(butylene succinate) and its copolyester modified with rosin maleopimaric acid anhydride. J. Polym. Sci. B Polym. Phys. 2006, 44, 900–913. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, Z. Crystallization kinetics and morphology of biodegradable poly(butylene succinate-co-ethylene succinate) copolyesters: Effects of comonomer composition and crystallization temperature. CrystEngComm 2011, 13, 2408–2417. [Google Scholar] [CrossRef]
- Sun, Z.; Jiang, Z.; Qiu, Z. Thermal, crystallization and mechanical properties of branched Poly(butylene succinate) copolymers with 1,2-decanediol being the comonomer. Polymer 2020, 123197. [Google Scholar]
- Wang, L.; Zhang, M.; Lawson, T.; Kanwal, A.; Miao, Z. Poly(butylene succinate-co-salicylic acid) copolymers and their effect on promoting plant growth. Roy. Soc. Open Sci. 2019, 6, 190504. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.M.; Wang, X.Y.; Wu, T. Preparation, crystallization and degradation properties of poly(butylene succinate-co-neopentyl glycol succinate) copolymer/graphite oxide composites. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Qi, Z.; Ye, H.; Xu, J.; Chen, J.; Guo, B. Improved the thermal and mechanical properties of poly(butylene succinate-co-butylene adipate) by forming nanocomposites with attapulgite. Colloids Surf. A 2013, 421, 109–117. [Google Scholar]
- Ye, H.M.; Wang, R.D.; Liu, J.; Xu, J.; Guo, B.H. Isomorphism in Poly(butylene succinate-co-butylene fumarate) and Its Application as Polymeric Nucleating Agent for Poly(butylene succinate). Macromolecules 2012, 45, 5667–5675. [Google Scholar] [CrossRef]
- Zeng, J.B.; Wu, F.; Huang, C.L.; He, Y.S.; Wang, Y.Z. Urethane Ionic Groups Induced Rapid Crystallization of Biodegradable Poly(ethylene succinate). ACS Macro Lett. 2012, 1, 965–968. [Google Scholar] [CrossRef]
- Tabata, H.; Fujii, M.; Hayashi, S.; Doi, T.; Wakabayashi, T. Raman and surface-enhanced Raman scattering of a series of size-separated polyynes. Carbon 2006, 44, 3168–3176. [Google Scholar] [CrossRef]
- Li, S.; Chen, T.; Wang, Y.; Liu, L.; Lv, F.; Li, Z.; Huang, Y.; Schanze, K.S.; Wang, S. Conjugated Polymer with Intrinsic Alkyne Units for Synergistically Enhanced Raman Imaging in Living Cells. Angew. Chem. Int. Ed. 2017, 56, 13455–13458. [Google Scholar] [CrossRef]
- Wei, L.; Hu, F.; Shen, Y.; Chen, Z.; Yu, Y.; Lin, C.C.; Wang, M.C.; Min, W. Live-cell imaging of alkyne-tagged small biomolecules by stimulated Raman scattering. Nat. Methods 2014, 11, 410–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustapha, A.; Salga, M.S.; Sabo, S. Synthesis and characterisation of some mixed ligands adducts of benzoylacetone and salicylaldehyde. J. Pure Appl. Sci. 2018, 11, 168–173. [Google Scholar]
- Abood, Z.H.; Haiwal, R.T.; Kadum, I.L.; Gzar, K.O.; Radhi, S.M.; Hameem, R.K.; Abbas, S.K. Synthesis of Some New Azo Schiff Bases and Tetrazole Derivatives from 2-Amino-1,3,4-thiadiazole-5-thiol. J. Kerbala Univ. 2008, 6, 140–145. [Google Scholar]
- Tserki, V.; Matzinos, P.; Pavlidou, E.; Vachliotis, D.; Panayiotou, C. Biodegradable aliphatic polyesters. I. Properties and biodegradation of poly(butylene succinate-co-butylene adipate). Polym. Degrad. Stabl. 2006, 91, 367–376. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis, Cocrystallization, and Enzymatic Degradation of Novel Poly(butylene-co-propylene succinate) Copolymers. Biomacromolecules 2007, 8, 2437–2449. [Google Scholar] [CrossRef]
- Nagata, M.; Goto, H.; Sakai, W.; Tsutsumi, N. Synthesis and enzymatic degradation of poly(tetramethylene succinate), copolymers with terephthalic acid. Polymer 2000, 41, 4373–4376. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. I. General Theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, Phase Change, and Microstructure Kinetics of Phase Change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Dai, X.; Qiu, Z. Crystallization kinetics, morphology, and hydrolytic degradation of novel biobased poly(butylene succinate-co-decamethylene succinate) copolyesters. Polym. Degrad. Stabl. 2017, 137, 197–204. [Google Scholar] [CrossRef]
- Mao, H.I.; Chen, C.W.; Rwei, S.P. Synthesis and Nonisothermal Crystallization Kinetics of Poly(Butylene Terephthalate-co-Tetramethylene Ether Glycol) Copolyesters. Polymers 2020, 12, 1897. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wen, X.; Yang, D. Isothermal crystallization kinetics and morphology of biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate). J. Mater. Sci. 2011, 46, 1281–1288. [Google Scholar] [CrossRef]
- Wang, T.; Wang, H.; Li, H.; Gan, Z.; Yan, S. Banded spherulitic structures of poly(ethylene adipate), poly(butylene succinate) and in their blends. Phys. Chem. Chem. Phys. 2009, 11, 1619–1627. [Google Scholar] [CrossRef]
- Yoo, E.S.; Im, S.S. Melting behavior of poly(butylene succinate) during heating scan by DSC. J. Polym. Sci. B Polym. Phys. 1999, 37, 1357–1366. [Google Scholar] [CrossRef]
- Jiang, J.; Zhuravlev, E.; Hu, W.B.; Schick, C.; Zhou, D.S. The effect of self-nucleation on isothermal crystallization kinetics of poly(butylene succinate) (PBS) investigated by differential fast scanning calorimetry. Chinese J. Polym. Sci. 2017, 35, 1009–1019. [Google Scholar] [CrossRef]
- Sangroniz, L.; Cavallo, D.; Müller, A.J. Self-Nucleation Effects on Polymer Crystallization. Macromolecules 2020, 53, 4581–4604. [Google Scholar] [CrossRef]
- Ye, H.M.; Liu, P.; Wang, C.X.; Meng, X.; Zhou, Q. Polymorphism regulation in Poly(hexamethylene succinate-co-hexamethylene fumarate): Altering the hydrogen bonds in crystalline lattice. Polymer 2017, 108, 272–280. [Google Scholar] [CrossRef]
- Meng, X.Y.; Li, Y.; Yao, S.F.; Wei, X.W.; Ye, H.M. Unusual Spherulitic Morphology of Poly(propylene fumarate). Chinese J. Polym. Sci. 2020. [Google Scholar] [CrossRef]
- Ye, H.M.; Wang, J.; Wang, C.S.; Li, H.F. Unique Isodimorphism of Poly(decamethylene succinate-ran-decamethylene fumarate): Large Pseudoeutectic Region and Fantastic Crystallization/Melting Behavior. Macromolecules 2019, 52, 1447–1457. [Google Scholar] [CrossRef]
- Tang, X.; Chen, W.; Li, L. The Tough Journey of Polymer Crystallization: Battling with Chain Flexibility and Connectivity. Macromolecules 2019, 52, 3575–3591. [Google Scholar] [CrossRef]










| Sample | PAD | Mw (g/mol) | Ð | Tc (°C) | ΔHc (J/g) | Tm (°C) | ΔHm (J/g) |
|---|---|---|---|---|---|---|---|
| PBS | 0 | 4.45 × 104 | 2.48 | 83.4 | 68.4 | 114.6 | 70.2 |
| PBSAD-5 | 5 mol% | 2.07 × 104 | 1.73 | 77.7 | 63.0 | 112.5 | 63.8 |
| PBSAD-7 | 7 mol% | 1.78 × 104 | 2.14 | 70.5 | 61.8 | 108.4 | 62.7 |
| PBSAD-14 | 14 mol% | 2.48 × 104 | 1.84 | 61.1 | 49.8 | 101.1 | 50.5 |
| Samples | Tc (°C) | n | k (s−n) | t1/2 (s) |
|---|---|---|---|---|
| PBS | 93 | 1.84 | 9.33 × 10−5 | 127 |
| 95 | 1.83 | 5.89 × 10−5 | 168 | |
| 97 | 1.76 | 3.89 × 10−5 | 260 | |
| 99 | 1.74 | 1.35 × 10−5 | 510 | |
| PBSAD-5 | 87 | 1.91 | 2.24 × 10−5 | 225 |
| 89 | 1.92 | 1.32 × 10−5 | 288 | |
| 91 | 2.15 | 7.76 × 10−7 | 586 | |
| 93 | 2.45 | 2.95 × 10−8 | 1020 | |
| PBSAD-7 | 85 | 1.87 | 4.90 × 10−5 | 166 |
| 87 | 1.81 | 2.51 × 10−5 | 284 | |
| 89 | 1.97 | 2.95 × 10−6 | 533 | |
| 91 | 2.15 | 3.02 × 10−7 | 909 | |
| PBSAD-14 | 76 | 1.91 | 5.37 × 10−5 | 142 |
| 78 | 1.83 | 3.31 × 10−5 | 230 | |
| 80 | 1.84 | 1.29 × 10−5 | 373 | |
| 82 | 1.98 | 2.24 × 10−6 | 593 |
| Sample | N (pcs/mm2) | d (μm) |
|---|---|---|
| PBS | ~56 | ~151 |
| PBSAD-5 | ~159 | ~89 |
| PBSAD-7 | ~1101 | ~34 |
| PBSAD-14 | ~2963.0 | ~21 |
| Sample | Domain II (°C) | Domain IIa (°C) | Domain IIb (°C) |
|---|---|---|---|
| PBS | 15.0 | 10.5 | 4.5 |
| PBSAD-5 | 11.0 | 6.5 | 4.5 |
| PBSAD-7 | 9.0 | 5.0 | 4.0 |
| PBSAD-14 | 6.5 | 2.3 | 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Huang, G.; Chen, C.; Wei, X.-W.; Dong, X.; Zhao, W.; Ye, H.-M. Poly(butylene succinate-co-butylene acetylenedicarboxylate): Copolyester with Novel Nucleation Behavior. Polymers 2021, 13, 365. https://doi.org/10.3390/polym13030365
Li Y, Huang G, Chen C, Wei X-W, Dong X, Zhao W, Ye H-M. Poly(butylene succinate-co-butylene acetylenedicarboxylate): Copolyester with Novel Nucleation Behavior. Polymers. 2021; 13(3):365. https://doi.org/10.3390/polym13030365
Chicago/Turabian StyleLi, Yi, Guoyong Huang, Cong Chen, Xue-Wei Wei, Xi Dong, Wei Zhao, and Hai-Mu Ye. 2021. "Poly(butylene succinate-co-butylene acetylenedicarboxylate): Copolyester with Novel Nucleation Behavior" Polymers 13, no. 3: 365. https://doi.org/10.3390/polym13030365