Direct Conjugation of Streptavidin to Encoded Hydrogel Microparticles for Multiplex Biomolecule Detection with Rapid Probe-Set Modification
Abstract
:1. Introduction
2. Experimental Section
2.1. Fabrication of the Polydimethylsiloxane (PDMS) Microfluidic Device
2.2. Stop Flow Lithography (SFL) Setup
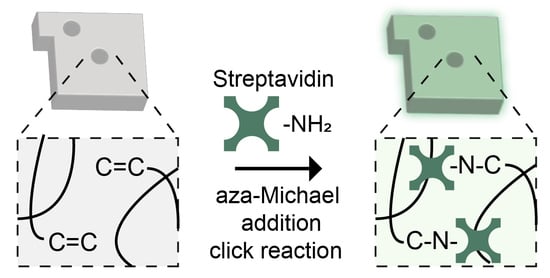
2.3. Fabrication of Streptavidin-Conjugated Encoded Hydrogel Microparticles
2.4. Characterization of Streptavidin-Conjugated Encoded Hydrogel Microparticles
2.5. Spectroscopic Analysis
2.6. DNA and Protein Detection
2.7. Detection of Cancer-Associated miRNAs
2.8. Image Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of Streptavidin-Conjugated Encoded Hydrogel Microparticles
3.2. Spectroscopic Analysis of Streptavidin-Conjugated Encoded Hydrogel Microparticles
3.3. Assay Performance and Flexibility of the Streptavidin-Conjugated Encoded Hydrogel Microparticles
3.4. Multiplex Detection of Cancer-Related miRNAs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nolan, J.P.; Sklar, L.A. Suspension array technology: Evolution of the flat-array paradigm. Trends Biotechnol. 2002, 20, 9–12. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Hu, J.; Xu, M.; Zhao, W.; Sun, L.; Zhu, C.; Xu, H.; Gu, Z. Encoded porous beads for label-free multiplex detection of tumor markers. Adv. Mater. 2009, 21, 569–572. [Google Scholar] [CrossRef]
- Kim, S.H.; Shim, J.W.; Yang, S.M. Microfluidic multicolor encoding of microspheres with nanoscopic surface complexity for multiplex immunoassays. Angew. Chem., Int. Ed. 2011, 50, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-W.; Liu, Z.-B.; Yang, H.; Nagai, K.; Zhao, Y.-H.; Gu, Z.-Z. Uniformly colorized beads for multiplex immunoassay. Chem. Mater. 2006, 18, 2443–2449. [Google Scholar] [CrossRef]
- Jun, B.-H.; Kim, J.-H.; Park, H.; Kim, J.-S.; Yu, K.-N.; Lee, S.-M.; Choi, H.; Kwak, S.-Y.; Kim, Y.-K.; Jeong, D.H. Surface-enhanced Raman spectroscopic-encoded beads for multiplex immunoassay. J. Comb. Chem. 2007, 9, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Elshal, M.F.; McCoy, J.P. Multiplex bead array assays: Performance evaluation and comparison of sensitivity to ELISA. Methods 2006, 38, 317–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunbar, S.A. Applications of Luminex® xMAP™ technology for rapid, high-throughput multiplexed nucleic acid detection. Clin. Chim. Acta 2006, 363, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Pregibon, D.C.; Doyle, P.S. Optimization of encoded hydrogel particles for nucleic acid quantification. Anal. Chem. 2009, 81, 4873–4881. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Choi, D.; Kim, J.-H.; Koh, W.-G. Suspension arrays of hydrogel microparticles prepared by photopatterning for multiplexed protein-based bioassays. Biomed. Microdevices 2008, 10, 813–822. [Google Scholar] [CrossRef]
- Roh, Y.H.; Lee, H.J.; Bong, K.W. Microfluidic Fabrication of Encoded Hydrogel Microparticles for Application in Multiplex Immunoassay. BioChip J. 2019, 13, 64–81. [Google Scholar] [CrossRef]
- Kingsmore, S.F. Multiplexed protein measurement: Technologies and applications of protein and antibody arrays. Nat. Rev. Drug Discov. 2006, 5, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meiring, J.E.; Schmid, M.J.; Grayson, S.M.; Rathsack, B.M.; Johnson, D.M.; Kirby, R.; Kannappan, R.; Manthiram, K.; Hsia, B.; Hogan, Z.L. Hydrogel biosensor array platform indexed by shape. Chem. Mater. 2004, 16, 5574–5580. [Google Scholar] [CrossRef]
- Park, S.; Lee, H.J.; Koh, W.-G. Multiplex immunoassay platforms based on shape-coded poly (ethylene glycol) hydrogel microparticles incorporating acrylic acid. Sensors 2012, 12, 8426–8436. [Google Scholar] [CrossRef] [PubMed]
- Appleyard, D.C.; Chapin, S.C.; Srinivas, R.L.; Doyle, P.S. Bar-coded hydrogel microparticles for protein detection: Synthesis, assay and scanning. Nat. Protoc. 2011, 6, 1761. [Google Scholar] [CrossRef] [PubMed]
- Dendukuri, D.; Gu, S.S.; Pregibon, D.C.; Hatton, T.A.; Doyle, P.S. Stop-flow lithography in a microfluidic device. Lab Chip 2007, 7, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.H.; Sim, S.J.; Cho, I.-J.; Choi, N.; Bong, K.W. Vertically encoded tetragonal hydrogel microparticles for multiplexed detection of miRNAs associated with Alzheimer’s disease. Analyst 2016, 141, 4578–4586. [Google Scholar] [CrossRef]
- Lee, H.J.; Roh, Y.H.; Kim, H.U.; Kim, S.M.; Bong, K.W. Multiplexed immunoassay using post-synthesis functionalized hydrogel microparticles. Lab Chip 2019, 19, 111–119. [Google Scholar] [CrossRef]
- Roh, Y.H.; Lee, H.J.; Moon, H.J.; Kim, S.M.; Bong, K.W. Post-synthesis functionalized hydrogel microparticles for high performance microRNA detection. Anal. Chim. Acta 2019, 1076, 110–117. [Google Scholar] [CrossRef]
- Sharma, P.; Sahni, N.S.; Tibshirani, R.; Skaane, P.; Urdal, P.; Berghagen, H.; Jensen, M.; Kristiansen, L.; Moen, C.; Sharma, P. Early detection of breast cancer based on gene-expression patterns in peripheral blood cells. Breast Cancer Res. 2005, 7, R634. [Google Scholar] [CrossRef] [Green Version]
- Fung, K.Y.; Tabor, B.; Buckley, M.J.; Priebe, I.K.; Purins, L.; Pompeia, C.; Brierley, G.V.; Lockett, T.; Gibbs, P.; Tie, J. Blood-based protein biomarker panel for the detection of colorectal cancer. PLoS ONE 2015, 10, e0120425. [Google Scholar] [CrossRef] [Green Version]
- Srisa-Art, M.; Dyson, E.C.; deMello, A.J.; Edel, J.B. Monitoring of real-time streptavidin− biotin binding kinetics using droplet microfluidics. Anal. Chem. 2008, 80, 7063–7067. [Google Scholar] [CrossRef] [PubMed]
- González, M.n.; Argaraña, C.E.; Fidelio, G.D. Extremely high thermal stability of streptavidin and avidin upon biotin binding. Biomol. Eng. 1999, 16, 67–72. [Google Scholar] [CrossRef]
- Ellison, D.; Beynon, R.J.; Hinton, J.; Hubbard, S.J. Limited proteolysis of native proteins: The interaction between avidin and proteinase K. Protein Sci. 1995, 4, 1337–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elia, G. Biotinylation reagents for the study of cell surface proteins. Proteomics 2008, 8, 4012–4024. [Google Scholar] [CrossRef] [PubMed]
- Bong, K.W.; Kim, J.J.; Cho, H.; Lim, E.; Doyle, P.S.; Irimia, D. Synthesis of Cell-Adhesive Anisotropic Multifunctional Particles by Stop Flow Lithography and Streptavidin–Biotin Interactions. Langmuir 2015, 31, 13165–13171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, J.E.; Macdonald, M.; Nie, J.; Bowman, C.N.J.P. Structure and swelling of poly (acrylic acid) hydrogels: Effect of pH, ionic strength, and dilution on the crosslinked polymer structure. Polymers 2004, 45, 1503–1510. [Google Scholar] [CrossRef]
- Graves, H.C. The effect of surface charge on non-specific binding of rabbit immunoglobulin G in solid-phase immunoassays. J. Immunol. Methods 1988, 111, 157–166. [Google Scholar] [CrossRef]
- Cuatrecasas, P.; Parikh, I. Adsorbents for affinity chromatography. Use of N-hydroxysuccinimide esters of agarose. Biochemistry 1972, 11, 2291–2299. [Google Scholar] [CrossRef]
- Dendukuri, D.; Pregibon, D.C.; Collins, J.; Hatton, T.A.; Doyle, P.S. Continuous-flow lithography for high-throughput microparticle synthesis. Nat. Mater. 2006, 5, 365. [Google Scholar] [CrossRef]
- Argarana, C.E.; Kuntz, I.D.; Birken, S.; Axel, R.; Cantor, C.R. Molecular cloning and nucleotide sequence of the streptavidin gene. Nucleic Acids Res. 1986, 14, 1871–1882. [Google Scholar] [CrossRef] [Green Version]
- Bosica, G.; Abdilla, R. Aza-Michael mono-addition using acidic alumina under solventless conditions. Molecules 2016, 21, 815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konuray, O.; Fernández-Francos, X.; Ramis, X.; Serra, À. State of the art in dual-curing acrylate systems. Polymers 2018, 10, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noordzij, G.; Wilsens, C. Cascade aza-Michael addition-cyclizations; towards renewable and multifunctional carboxylic acids for melt-polycondensation. Front. Chem. 2019, 7, 729. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Stuparu, M.C.; Daugaard, A.; Khan, A. Aza-Michael addition reaction: Post-polymerization modification and preparation of PEI/PEG-based polyester hydrogels from enzymatically synthesized reactive polymers. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 745–749. [Google Scholar] [CrossRef]
- Genest, A.; Binauld, S.; Pouget, E.; Ganachaud, F.; Fleury, E.; Portinha, D. Going beyond the barriers of aza-Michael reactions: Controlling the selectivity of acrylates towards primary amino-PDMS. Polym. Chem. 2017, 8, 624–630. [Google Scholar] [CrossRef]
- Chen, H.; Huang, R.; Li, Z.; Zhu, W.; Chen, J.; Zhan, Y.; Jiang, B. Selective lysine modification of native peptides via aza-Michael addition. Org. Biomol. Chem. 2017, 15, 7339–7345. [Google Scholar] [CrossRef] [Green Version]
- Furman, J.L.; Kang, M.; Choi, S.; Cao, Y.; Wold, E.D.; Sun, S.B.; Smider, V.V.; Schultz, P.G.; Kim, C.H. A genetically encoded aza-Michael acceptor for covalent cross-linking of protein–receptor complexes. J. Am. Chem. Soc. 2014, 136, 8411–8417. [Google Scholar] [CrossRef]
- Almonte, L.; Lopez-Elvira, E.; Baró, A.M. Surface-Charge Differentiation of Streptavidin and Avidin by Atomic Force Microscopy–Force Spectroscopy. ChemPhysChem 2014, 15, 2768–2773. [Google Scholar] [CrossRef]
- Echeverri, M.; Hamad, C.; Kyu, T. Highly conductive, completely amorphous polymer electrolyte membranes fabricated through photo-polymerization of poly (ethylene glycol diacrylate) in mixtures of solid plasticizer and lithium salt. Solid State Ion. 2014, 254, 92–100. [Google Scholar] [CrossRef]
- Krüger, A.; Bürkle, A.; Mangerich, A.; Hauser, K. A combined approach of surface passivation and specific immobilization to study biomolecules by ATR-FTIR spectroscopy. Biomed. Spectrosc. Imaging 2018, 7, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Tuschel, D. Practical group theory and Raman spectroscopy, part II: Application of polarization. Spectroscopy 2014, 29, 14–23. [Google Scholar]
- Kondo, T.; Hashimoto, R.; Ohrui, Y.; Sekioka, R.; Nogami, T.; Muta, F.; Seto, Y. Analysis of chemical warfare agents by portable Raman spectrometer with both 785 nm and 1064 nm excitation. Forensic Sci. Int. 2018, 291, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, N.; Chechetkin, V.; Pan’kov, S.; Somova, O.; Livshits, M.; Donnikov, M.; Turygin, A.; Barsky, V.; Zasedatelev, A. Kinetics of hybridization on surface oligonucleotide microchips: Theory, experiment, and comparison with hybridization on gel-based microchips. J. Biomol. Struct. Dyn. 2006, 24, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Tang, Y. Hydrogel based sensors for biomedical applications: An updated review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broder, G.R.; Ranasinghe, R.T.; She, J.K.; Banu, S.; Birtwell, S.W.; Cavalli, G.; Galitonov, G.S.; Holmes, D.; Martins, H.F.; MacDonald, K.F. Diffractive micro bar codes for encoding of biomolecules in multiplexed assays. Anal. Chem. 2008, 80, 1902–1909. [Google Scholar] [CrossRef]
- Zhi, Z.-l.; Morita, Y.; Yamamura, S.; Tamiya, E. Microfabrication of encoded microparticle array for multiplexed DNA hybridization detection. Chem. Commun. 2005, 2448–2450. [Google Scholar] [CrossRef]
- Jung, K.-H.; Baek, H.; Shin, D.; Lee, G.; Park, S.; Lee, S.; Choi, D.; Kim, W.; Bae, H. Protective effects of intratracheally-administered bee venom phospholipase A2 on ovalbumin-induced allergic asthma in mice. Toxins 2016, 8, 269. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.-L.; Ren, H.-L.; Li, Y.-S.; Hu, P.; Zhou, Y.; Liu, Z.-S.; Yan, D.-M.; Hui, Q.; Liu, D.; Lin, C. A magnetic particles-based chemiluminescence enzyme immunoassay for rapid detection of ovalbumin. Anal. Biochem. 2014, 459, 12–17. [Google Scholar] [CrossRef]
- Thomas, M.L.; Marcato, P. Epigenetic modifications as biomarkers of tumor development, therapy response, and recurrence across the cancer care continuum. Cancers 2018, 10, 101. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Gu, J.; Zhang, Z.; Wang, Y.; Gu, C. MiR-451 promotes cell proliferation and metastasis in pancreatic cancer through targeting CAB39. BioMed Res. Int. 2017, 2017, 23811482. [Google Scholar] [CrossRef] [Green Version]
- Raimondi, S.; Maisonneuve, P.; Lowenfels, A.B. Epidemiology of pancreatic cancer: An overview. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ding, F.; Chen, H.; Ding, W.; Zhang, W.; Chou, S.Y. Enhancement of immunoassay’s fluorescence and detection sensitivity using three-dimensional plasmonic nano-antenna-dots array. Anal. Chem. 2012, 84, 4489–4495. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.G.; Yoo, H.; Lee, H.; Choi, Y.K.; Lee, M.; Ahn, D.J.; Hong, S. High-Speed Lateral Flow Strategy for a Fast Biosensing with an Improved Selectivity and Binding Affinity. Sensors 2018, 18, 1507. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Park, J.H.; Back, S.H.; Feng, Y.; Cui, C.; Jin, L.Y.; Ahn, D.J. Mercury ion–DNA specificity triggers a distinctive photoluminescence depression in organic semiconductor probes guided with a thymine-rich oligonucleotide sequence. Nanoscale 2018, 10, 17540–17545. [Google Scholar] [CrossRef] [PubMed]
- Chapin, S.C.; Appleyard, D.C.; Pregibon, D.C.; Doyle, P.S. Rapid microRNA profiling on encoded gel microparticles. Angew. Chem. Int. Ed. 2011, 50, 2289–2293. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 395–463. [Google Scholar]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roh, Y.H.; Kim, J.Y.; Mun, S.J.; Lee, H.S.; Hwang, C.; Park, K.H.; Bong, K.W. Direct Conjugation of Streptavidin to Encoded Hydrogel Microparticles for Multiplex Biomolecule Detection with Rapid Probe-Set Modification. Polymers 2020, 12, 546. https://doi.org/10.3390/polym12030546
Roh YH, Kim JY, Mun SJ, Lee HS, Hwang C, Park KH, Bong KW. Direct Conjugation of Streptavidin to Encoded Hydrogel Microparticles for Multiplex Biomolecule Detection with Rapid Probe-Set Modification. Polymers. 2020; 12(3):546. https://doi.org/10.3390/polym12030546
Chicago/Turabian StyleRoh, Yoon Ho, Ju Yeon Kim, Seok Joon Mun, Hye Sun Lee, Changhyun Hwang, Kyong Hwa Park, and Ki Wan Bong. 2020. "Direct Conjugation of Streptavidin to Encoded Hydrogel Microparticles for Multiplex Biomolecule Detection with Rapid Probe-Set Modification" Polymers 12, no. 3: 546. https://doi.org/10.3390/polym12030546