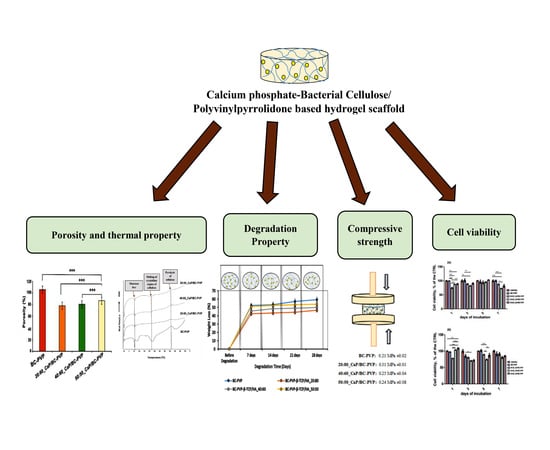
Calcium Phosphate Incorporated Bacterial Cellulose-Polyvinylpyrrolidone Based Hydrogel Scaffold: Structural Property and Cell Viability Study for Bone Regeneration Application
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. CaP/BC-PVP Hydrogel Preparation
2.2.1. Microbial Synthesis and Preparation of Homogenous Suspension of BC
2.2.2. Preparation of Hydrogel Scaffolds
2.3. Porosity and Void Fraction Study
2.4. Differential Scanning Calorimetry
2.5. Degradation Characterization
2.5.1. Weight Loss Profile Study
2.5.2. Gel Content Study
2.5.3. Gel Density Study
2.5.4. pH Change and Calcium Release Study
2.5.5. Fourier Transform Infrared Spectroscopy (FTIR) Study
2.5.6. Energy Dispersive X-ray Fluorescence Study and Morphological Characterization by Scanning Electron Microscopy
2.6. Mechanical Property
2.7. Cell Proliferation and Cytocompatibility Study
2.7.1. Cell Culture
2.7.2. Preparation of Test Samples for Cytocompatibility /Cell Viability/Proliferation Study
2.7.3. Indirect Experiments (IDE) for Cell Viability/Proliferation Study
2.7.4. Direct Experiments (DE) for Cytocompatibility Study
3. Statistical Analysis
4. Results and Discussion
4.1. Void Fraction and Porosity
4.2. Differential Scanning Calorimetry
4.3. Hydrolytic Degradation Study
4.3.1. Weight Loss Profile Study
4.3.2. Gel Content Study
4.3.3. Gel Density Study
4.3.4. pH Change and Calcium Release Study
4.3.5. Fourier Transform Infrared Spectroscopy (FTIR) Study
4.3.6. Energy Dispersive X-ray Fluorescence Study and Morphological Characterization
4.4. Mechanical Property Analysis
4.5. Cell Viability and Cytocompatibility Study
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iaquinta, M.R.; Mazzoni, E.; Manfrini, M.; D’Agostino, A.; Trevisiol, L.; Nocini, R.; Trombelli, L.; Barbanti-Brodano, G.; Martini, F.; Tognon, M. Innovative Biomaterials for Bone Regrowth. Int. J. Mol. Sci. 2019, 20, 618. [Google Scholar] [CrossRef] [PubMed]
- Chauvin-Kimoff, L.; Allard-Dansereau, C.; Colbourne, M. The medical assessment of fractures in suspected child maltreatment: Infants and young children with skeletal injury. Paediatr. Child Health 2018, 23, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Kugel, C.; May, H.; Medlej, B.; Stein, D.; Slon, V.; Hershkovitz, I.; Brosh, T. The impact velocity and bone fracture pattern: Forensic perspective. Forensic Sci. Int. 2016, 266, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Rabie, A.B.; Wong, R.W.; Hagg, U. Composite autogenous bone and demineralized bone matrices used to repair defects in the parietal bone of rabbits. Br. J. Oral. Maxillofac. Surg. 2000, 38, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P. Strategic Directions in Osteoinduction and Biomimetics. Tissue Eng. Part A 2017, 23, 1295–1296. [Google Scholar] [CrossRef]
- Hernlund, E.; Svedbom, A.; Ivergard, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef]
- Oral, A.; Kucukdeveci, A.A.; Varela, E.; Ilieva, E.M.; Valero, R.; Berteanu, M.; Christodoulou, N. Osteoporosis. The role of physical and rehabilitation medicine physicians. The European perspective based on the best evidence. A paper by the UEMS-PRM Section Professional Practice Committee. Eur. J. Phys. Rehabil. Med. 2013, 49, 565–577. [Google Scholar]
- Tian, L.; Tang, N.; Ngai, T.; Wu, C.; Ruan, Y.; Huang, L.; Qin, L. Hybrid fracture fixation systems developed for orthopaedic applications: A general review. J. Orthop. Transl. 2018, 16, 1–13. [Google Scholar] [CrossRef]
- Siallagan, S.F.; Silalahi, M.; Boediono, A.; Estuningsih, S.; Noviana, D. A Wearable Iron-Based Implant as an Intramedullary Nail in Tibial Shaft Fracture of Sheep. Int. J. Biomater. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Christensen, F.B.; Dalstra, M.; Sejling, F.; Overgaard, S.; Bunger, C. Titanium-alloy enhances bone-pedicle screw fixation: Mechanical and histomorphometrical results of titanium-alloy versus stainless steel. Eur. Spine J. 2000, 9, 97–103. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Saha, N.; Saha, P. Inorganic calcium filled bacterial cellulose based hydrogel scaffold: Novel biomaterial for bone tissue regeneration. Int. J. Polym. Mater. 2019, 68, 134–144. [Google Scholar] [CrossRef]
- Ratner, B.D. Biomaterials Science: An Introduction to Materials in Medicine; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Liu, X.; Miller II, A.L.; Park, S.; George, M.N.; Waletzki, B.E.; Xu, H.; Terzic, A.; Lu, L. Two-Dimensional Black Phosphorus and Graphene Oxide Nanosheets Synergistically Enhance Cell Proliferation and Osteogenesis on 3D Printed Scaffolds. ACS Appl. Mater. Interfaces 2019, 11, 23558–23572. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Xu, X.-M.; Liu, C.-G.; Chen, A.-Z.; Wang, S.-B. 3D-Printing of Microfibrous Porous Scaffolds Based on Hybrid Approaches for Bone Tissue Engineering. Polymers 2018, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.; Sass, F.A.; Duda, G.N.; Schmidt-Bleek, K. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge. Bone Jt. Res. 2018, 7, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.J.; Lu, S.; Gabriele, P. Commercial challenges in developing biomaterials for medical device development. Polym. Int. 2018, 67, 969–974. [Google Scholar] [CrossRef]
- Jeon, O.H.; Panicker, L.M.; Lu, Q.; Chae, J.J.; Feldman, R.A.; Elisseef, J.H. Human iPSC-derived osteoblasts and osteoclasts together promote bone regeneration in 3D biomaterials. Sci. Rep. 2016, 6, 26761. [Google Scholar] [CrossRef]
- Puppi, D.; Migone, C.; Grassi, L.; Pirosa, A.; Maisetta, G.; Batoni, G.; Chiellini, F. Integrated three-dimensional fiber/hydrogel biphasic scaffolds for periodontal bone tissue engineering. Polym. Int. 2016, 65, 631–640. [Google Scholar] [CrossRef]
- Ardeshirylajimi, A.; Dinarvand, P.; Seyedjafari, E.; Langroudi, L.; Adegani, F.J.; Soleimani, M. Enhanced reconstruction of rat calvarial defects achieved by plasma-treated electrospun scaffolds and induced pluripotent stem cells. Cell Tissue Res. 2013, 354, 849–860. [Google Scholar] [CrossRef]
- Jin, G.Z.; Kim, T.H.; Kim, J.H.; Won, J.E.; Yoo, S.Y.; Choi, S.J.; Hyun, J.K.; Kim, H.W. Bone tissue engineering of induced pluripotent stem cells cultured with macrochanneled polymer scaffold. J. Biomed. Mater. Res. A 2013, 101, 1283–1291. [Google Scholar] [CrossRef]
- Levi, B.; Hyun, J.S.; Montoro, D.T.; Lo, D.D.; Chan, C.K.; Hu, S.; Sun, N.; Lee, M.; Grova, M.; Connolly, A.J.; et al. In vivo directed differentiation of pluripotent stem cells for skeletal regeneration. Proc. Natl. Acad. Sci. USA 2012, 109, 20379–20384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, X.; Tu, Q.; Zhang, J.; Ye, J.; Sommer, C.; Mostoslavsky, G.; Kaplan, D.; Yang, P.; Chen, J. Application of induced pluripotent stem (iPS) cells in periodontal tissue regeneration. J. Cell. Physiol. 2011, 226, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.Y.; Park, S.; Im, G.I. Osteogenesis from human induced pluripotent stem cells: An in vitro and in vivo comparison with mesenchymal stem cells. Stem Cells Dev. 2014, 23, 1788–1797. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.H.; Xu, Y.J.; Gao, J.; Yan, S.G.; Zhao, J.; Tu, Q.; Zhang, J.; Duan, X.J.; Sommer, C.A.; Mostoslavsky, G.; et al. Critical-size calvarial bone defects healing in a mouse model with silk scaffolds and SATB2-modified iPSCs. Biomaterials 2011, 32, 5065–5076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, D.; Zhang, X.; Wang, S.; Liu, L. Effect on Mechanical and Thermal Properties of Random Copolymer Polypropylene/Microcrystalline Cellulose Composites Using T-ZnOw as an Additive. Adv. Polym. Technol. 2019, 2019, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Schröpfer, S.B.; Bottene, M.K.; Bianchin, L.; Robinson, L.C.; de Lima, V.; Jahno, V.D.; da Silva Barud, H.; Ribeiro, S.J. Biodegradation evaluation of bacterial cellulose, vegetable cellulose and poly (3-hydroxybutyrate) in soil. Polímeros 2015, 25, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Qi, G.X.; Luo, M.T.; Huang, C.; Guo, H.J.; Chen, X.F.; Xiong, L.; Wang, B.; Lin, X.Q.; Peng, F.; Chen, X.D. Comparison of bacterial cellulose production by Gluconacetobacter xylinus on bagasse acid and enzymatic hydrolysates. J. Appl. Polym. Sci. 2017, 134, 45066. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyaia, P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Pirsa, S.; Shamusi, T.; Kia, E.M. Smart films based on bacterial cellulose nanofibers modified by conductive polypyrrole and zinc oxide nanoparticles. J. Appl. Polym. Sci. 2018, 135, 46617. [Google Scholar] [CrossRef]
- Basu, P.; Saha, N.; Alexandrova, R.; Andonova-Lilova, B.; Georgieva, M.; Miloshev, G.; Saha, P. Biocompatibility and Biological Efficiency of Inorganic Calcium Filled Bacterial Cellulose Based Hydrogel Scaffolds for Bone Bioengineering. Int. J. Mol. Sci. 2018, 19, 3980. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, T.; Zhao, Z.; Ren, H.; Zhang, Q.; Yan, Y.; Lv, G. Preparation and characterization of bacterial cellulose microfiber/goat bone apatite composites for bone repair. J. Appl. Polym. Sci. 2013, 129, 595–603. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, Y.; Wang, J.; Zhu, Y.; Zhu, Y.; Zhang, X.; Liao, J.; Li, X.; Wu, X.; Qin, Y.-X.; et al. A Composite Hydrogel with High Mechanical Strength, Fluorescence, and Degradable Behavior for Bone Tissue Engineering. Polymers 2019, 11, 1112. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, B.; Palmer, J.; Ladewig, K.; Facal Marina, P.; Stevens, G.W.; Abberton, K.; Morrison, W.A.; Blencowe, A.; Qiao, G.G. Biocompatible Porous Polyester-Ether Hydrogel Scaffolds with Cross-Linker Mediated Biodegradation and Mechanical Properties for Tissue Augmentation. Polymers 2018, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- De Mori, A.; Peña Fernández, M.; Blunn, G.; Tozzi, G.; Roldo, M. 3D Printing and Electrospinning of Composite Hydrogels for Cartilage and Bone Tissue Engineering. Polymers 2018, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.; Shah, R.; Gupta, P.; Mandal, B.B.; Alexandrova, R.; Sikiric, M.D.; Saha, P. PVP-CMC hydrogel: An excellent bioinspired and biocompatible scaffold for osseointegration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 95, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Kocen, R.; Gasik, M.; Gantar, A.; Novak, S. Viscoelastic behaviour of hydrogel-based composites for tissue engineering under mechanical load. Biomed. Mater. 2017, 12, 025004. [Google Scholar] [CrossRef] [PubMed]
- Racine, L.; Texier, I.; Auzély-Velty, R. Chitosan-based hydrogels: Recent design concepts to tailor properties and functions. Polym. Int. 2017, 66, 981–998. [Google Scholar] [CrossRef]
- Lyu, S.P.; Untereker, D. Degradability of Polymers for Implantable Biomedical Devices. Int. J. Mol. Sci. 2009, 10, 4033–4065. [Google Scholar] [CrossRef] [Green Version]
- Lodhi, B.A.; Hussain, M.A.; Sher, M.; Haseeb, M.T.; Ashraf, M.U.; Hussain, S.Z.; Hussain, I.; Bukhari, S.N.A. Polysaccharide-Based Superporous, Superabsorbent, and Stimuli Responsive Hydrogel from Sweet Basil: A Novel Material for Sustained Drug Release. Adv. Polym. Technol. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Janoušková, O. Synthetic Polymer Scaffolds for Soft Tissue Engineering. Physiother. Res. 2018, 67, S3335–S3348. [Google Scholar] [CrossRef]
- Goudouri, O.M.; Balasubramanian, P.; Boccaccini, A.R. Characterizing the degradation behavior of bioceramic scaffolds. In Characterisation and Design of Tissue Scaffolds; Tomlins, P., Ed.; Woodhead Publishing Series in Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; Chapter 6; pp. 127–147. [Google Scholar]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Kumar, P.T.S.; Srinivasan, S.; Lakshmanan, V.K.; Tamura, H.; Nair, S.V.; Jayakumar, R. β-Chitin hydrogel/nano hydroxyapatite composite scaffolds for tissue engineering applications. Carbohydr. Polym. 2011, 85, 584–591. [Google Scholar] [CrossRef]
- Will, J.; Detsch, R.; Boccaccini, A.R. Characterization of Biomaterials. In Structural and Biological Characterization of Scaffolds; Bandyopadhyay, A., Bose, S., Eds.; Academic Press: Cambridge, MA, USA, 2013; Chapter 7.1; pp. 299–310. [Google Scholar]
- Roy, N.; Saha, N.; Kitano, T.; Saha, P. Biodegradation of PVP–CMC hydrogel film: A useful food packaging material. Carbohydr. Polym. 2012, 89, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Vyroubal, R.; Fei, H.; Saha, N.; Kitano, T.; Saha, P. Preparation of bacterial cellulose based hydrogels and their viscoelastic behavior. AIP Conf. Proc. 2015, 1662, 1–7. [Google Scholar]
- LeGeros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D.; LeGeros, J.P. Biophasic calcium phosphate bioceramics: Preparation, properties and application. J. Mater. Sci. Mater. Med. 2003, 14, 201–209. [Google Scholar] [CrossRef]
- Akaraonye, E.; Filip, J.; Safarikova, M.; Salih, V.; Keshavarz, T.; Knowles, J.C.; Roy, I. Composite scaffolds for cartilage tissue engineering based on natural polymers of bacterial origin, thermoplastic poly(3-hydroxybutyrate) and micro-fibrillated bacterial cellulose. Polym. Int. 2016, 65, 780–791. [Google Scholar] [CrossRef]
- Basu, P.; Saha, N.; Saha, P. Swelling and rheological study of calcium phosphate filled bacterial cellulose based hydrogel scaffold. J. Appl. Polym. Sci. 2019, 136, 48522. [Google Scholar] [CrossRef]
- Lin, F.; Zheng, R.; Chen, J.; Su, W.; Dong, B.; Lin, C.; Huang, B.; Lu, B. Microfibrillated cellulose enhancement to mechanical and conductive properties of biocompatible hydrogels. Carbohydr. Polym. 2019, 205, 244–254. [Google Scholar] [CrossRef]
- Kumar, A.; Zhang, Y.; Terracciano, A.; Zhao, X.; Su, T.-L.; Kalyon, D.M.; Katebifar, S.; Kumbar, S.G.; Yu, X. Load-bearing biodegradable polycaprolactone-poly (lactic-co-glycolic acid)- beta tri-calcium phosphate scaffolds for bone tissue regeneration. Polym. Adv. Technol. 2019, 30, 1189–1197. [Google Scholar] [CrossRef]
- Porter, J.R.; Henson, A.; Popat, K.C. Biodegradable poly (epsilon-caprolactone) nanowires for bone tissue engineering applications. Biomaterials 2009, 30, 780–788. [Google Scholar] [CrossRef]
- Wu, L.; Ding, J. In vitro degradation of three-dimensional porous poly (D, L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 2004, 25, 5821–5830. [Google Scholar] [CrossRef] [PubMed]
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.V.; Shivakumar, H.G. Investigation of swelling and mechanical properties of pH-sensitive superporous hydrogel composite. Iran. J. Pharm. Res. 2012, 11, 481–493. [Google Scholar] [PubMed]
- Maswal, M.; Chat, O.A.; Dar, A.A. Rheological characterization of multi component hydrogel based on carboxymethyl cellulose: Insight into its encapsulation capacity and release kinetics towards ibuprofen. Colloid Polym. Sci. 2015, 293, 1723–1735. [Google Scholar] [CrossRef]
- Köse, G.T.; Kenar, H.; Hasirci, N.; Hasirci, V. Macroporous poly (3-hydroxybutyrate-co-3-hydroxyvalerate) matrices for bone tissue engineering. Biomaterials 2003, 24, 1949–1958. [Google Scholar] [CrossRef]
- Mohite, B.V.; Patil, S.V. Physical, structural, mechanical and thermal characterization of bacterial cellulose by G. hansenii NCIM 2529. Carbohydr. Polym. 2014, 106, 132–141. [Google Scholar] [CrossRef]
- Ferfera-Harrar, H.; Aouaz, N.; Dairi, N. Environmental-sensitive chitosan-g-polyacrylamide/carboxymethylcellulose superabsorbent composites for wastewater purification I: Synthesis and properties. Polym. Bull. 2016, 73, 815–840. [Google Scholar] [CrossRef]
- Sun, S.; Cao, H.; Su, H.; Tan, T. Preparation and characterization of novel injectable in situ cross-linked hydrogel. Polym. Bull. 2009, 62, 699–711. [Google Scholar] [CrossRef]
- Mao, J.S.; Zhao, L.G.; Yin, Y.J.; Yao, K.D. Structure and properties of bilayer chitosan–gelatin scaffolds. Biomaterials 2003, 24, 1067–1074. [Google Scholar] [CrossRef]
- Sheenoy, A.V. Rheology of Filled Polymer Systems; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Pandit, V.; Pai, R.S.; Devi, K.; Suresh, S. In vitro-in vivo evaluation of fast-dissolving tablets containing solid dispersion of pioglitazone hydrochloride. J. Adv. Pharm. Technol. Res. 2012, 3, 160–170. [Google Scholar] [PubMed]
- Maghraby, G.M.; Ghanem, S.F. Preparation and Evaluation of Rapidly Dissolving Tablets of Raloxifene Hydrochloride by Ternary System Formation. Int. J. Pharm. Pharm. Sci. 2016, 8, 127–136. [Google Scholar]
- Oliveira, R.L.; Vieira, J.G.; Barud, H.S.; Assunção, R.M.N.; Filho, G.R.; Ribeiro, S.J.L.; Messadeqq, Y. Synthesis and Characterization of Methylcellulose Produced from Bacterial Cellulose under Heterogeneous Condition. J. Braz. Chem. Soc. 2015, 26, 1861–1870. [Google Scholar] [CrossRef]
- Gabbott, P. Principles and Applications of Thermal Analysis; Blackwell Publishing: Hoboken, NJ, USA, 2008; p. 464. [Google Scholar]
- Matraszek, A.; Radominska, E. The revised phase diagram of the Ca3(PO4)2–YPO4 system. The temperature and concentration range of solid-solution phase fields. J. Therm. Anal. Calorim. 2014, 117, 101. [Google Scholar] [CrossRef]
- Klemm, D.; Ahrem, H.; Kramer, F.; Fried, W.; Wippermann, J.; Kinne, R.W. Bacterial Nanocellulose Hydrogels Designed as Bioartificial Medical Implants. In Bacterial NanoCellulose a Sophisticated Multifunctional Material; Gama, M., Gatenholm, P., Klemm, D., Eds.; CRC Press: Boca Raton, FL, USA, 2012; Chapter 9; pp. 175–196. [Google Scholar]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar] [PubMed]
- Gajjar, C.R.; King, M.W. Degradation Process. In Resorbable Fiber-Forming Polymers for Biotextile Applications, Briefs in Materials; Gajjar, C.R., King, M.W., Eds.; Springer: Berlin, Germany, 2014. [Google Scholar]
- Akagi, Y.; Gosho, S.; Anraku, Y.; Sakuma, I. Control of Degradation Properties of Polymer Gel. In Proceedings of the ECS Meeting Abstract (Abstract MA2018-03 88), Poster Paper Presented at First International Conference on 4D Materials and Systems, Yonezawa, Japan, 26–30 August 2018. [Google Scholar]
- Houmard, M.; Fu, Q.; Genet, M.; Saiz, E.; Tomsia, A.P. On the structural, mechanical, and biodegradation properties of HA/β-TCP robocast scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1233–1242. [Google Scholar] [CrossRef]
- Sala, G. Composite degradation due to fluid absorption. Compos. Part B 2000, 31, 357–373. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Yacob, N.; Hashim, K. Morphological effect on swelling behaviour of hydrogel. AIP Conf. Proc. 2014, 1584, 153. [Google Scholar]
- Nie, L.; Suo, J.; Zou, P.; Feng, S. Preparation and properties of biphasic calcium phosphate scaffolds multiply coated with HA/PLLA nanocomposites for bone tissue engineering applications. J. Nanomater. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Kumar, A.; Sinha, J. Chapter 8 Electrochemical Transistors for Applications in Chemical and Biological Sensing. In Organic Semiconductors in Sensor Applications; Bernards, D.A., Owens, R.M., Malliaras, G.G., Eds.; Springer Series in Materials Science; Springer: Berlin, Germany, 2008; Volume 107, pp. 245–261. [Google Scholar]
- Grover, C.; Shetty, N. Evaluation of calcium ion release and change in pH on combining calcium hydroxide with different vehicles. Contemp. Clin. Dent. 2014, 5, 434–439. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Q.; Tu, Z.; Tu, W.; Wan, Z.; Pan, M.; Zhang, H. Degradation of silicone rubbers with different hardness in various aqueous solutions. Polym. Degrad. Stabil. 2014, 109, 122–128. [Google Scholar] [CrossRef]
- Bartošová, A.; Soldán, M.; Sirotiak, M.; Blinová, L.; Michaliková, A. Application of Ftir-Atr Spectroscopy for Determination of Glucose in Hydrolysates of Selected Starches. J. Slovak Univ. Technol. 2013, 21, 116–121. [Google Scholar] [CrossRef]
- Fengyan, L.; Fu, H. Effect of Alkaline Degumming on Structure and Properties of Lotus Fibers at Different Growth Period. J. Eng. Fibers Fabr. 2015, 10, 135–139. [Google Scholar]
- Mróz, W.; Bombalska, A.; Budne, B.; Burdynska, S.; Jedynski, M.; Prokopiuk, A.; Menaszek, E.; Scisłowska-Czarnecka, A.; Niedzielska, A.; Niedzielski, K. Comparative study of hydroxyapatite and octacalcium phosphate coatings deposited on metallic implants by PLD method. Appl. Phys. A 2010, 101, 713–716. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.Z.; Boccaccini, A.R. Bioactive Materials and scaffolds for Tissue Engineering. In Encyclopedia of Biomaterials and Biomedical Engineering, 2nd ed.; Wnek, G.E., Bowlin, G.L., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 142–151. [Google Scholar]
- Deshmukh, M.; Singh, Y.; Gunaseelan, S.; Gao, D.; Stein, S.; Sinko, P.J. Biodegradable poly (ethylene glycol) hydrogels based on a self-elimination degradation mechanism. Biomaterials 2010, 31, 6675–6684. [Google Scholar] [CrossRef] [PubMed]
- Rodel, M.; Meininger, S.; Groll, J.; Gbureck, U. Bioceramics as drug delivery systems. In Fundamental Biomaterials: Ceramics; Thomas, S., Balakrishnan, P., Sreekala, M.S., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2018; Chapter 7; pp. 153–194. [Google Scholar]
- Odelius, K.; Höglund, A.; Kumar, S.; Hakkarainen, M.; Ghosh, A.K.; Bhatnagar, N.; Albertsson, A.-C. Porosity and Pore Size Regulate the Degradation Product Profile of Polylactide. Biomacromolecules 2011, 12, 1250–1258. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Kocagöz, S.; Larson, J.C.; Brey, E.M. Evaluation of Physical and Mechanical Properties of Porous Poly (Ethylene Glycol)-co-(L-Lactic Acid) Hydrogels during Degradation. PLoS ONE 2013, 8, e60728. [Google Scholar] [CrossRef]
- Gerhardt, L.-C.; Boccaccini, A.R. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [Green Version]
- Misch, C.E.; Zhimin, Q.; Bidez, M.W.J. Mechanical properties of trabecular bone in the human mandible: Implications for dental implant treatment planning and surgical placement. J. Oral. Maxillofac. Surg. 1999, 57, 700–706. [Google Scholar] [CrossRef]










| Sample Index | BC-PVP Solution (mL) | β-TCP (g) | HA (g) |
|---|---|---|---|
| BC-PVP | 100 | 0.0 | 0.0 |
| 20:80_CaP/BC-PVP | 99 | 0.2 | 0.8 |
| 40:60_CaP/BC-PVP | 99 | 0.4 | 0.6 |
| 50:50_CaP/BC-PVP | 99 | 0.5 | 0.5 |
| BC-PVP Based Hydrogel Scaffolds | Compressive Strength (MPa) | |
|---|---|---|
| BC-PVP based scaffold without CaP | BC-PVP | 0.21 ± 0.02 |
| BC-PVP based scaffold with CaP | 20:80_CaP/BC-PVP | 0.31 ± 0.01 |
| 40:60_CaP/BC-PVP | 0.25 ± 0.04 | |
| 50:50_CaP/BC-PVP | 0.24 ± 0.08 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basu, P.; Saha, N.; Alexandrova, R.; Saha, P. Calcium Phosphate Incorporated Bacterial Cellulose-Polyvinylpyrrolidone Based Hydrogel Scaffold: Structural Property and Cell Viability Study for Bone Regeneration Application. Polymers 2019, 11, 1821. https://doi.org/10.3390/polym11111821
Basu P, Saha N, Alexandrova R, Saha P. Calcium Phosphate Incorporated Bacterial Cellulose-Polyvinylpyrrolidone Based Hydrogel Scaffold: Structural Property and Cell Viability Study for Bone Regeneration Application. Polymers. 2019; 11(11):1821. https://doi.org/10.3390/polym11111821
Chicago/Turabian StyleBasu, Probal, Nabanita Saha, Radostina Alexandrova, and Petr Saha. 2019. "Calcium Phosphate Incorporated Bacterial Cellulose-Polyvinylpyrrolidone Based Hydrogel Scaffold: Structural Property and Cell Viability Study for Bone Regeneration Application" Polymers 11, no. 11: 1821. https://doi.org/10.3390/polym11111821