Conductivity and Density of States of New Polyphenylquinoline
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Polymer
2.2. Characterization
2.3. Electrical Measurements
2.4. Cyclic Voltammetry (CV)
3. Results and Discussion
3.1. Temperature Dependence of Dark Conductivity
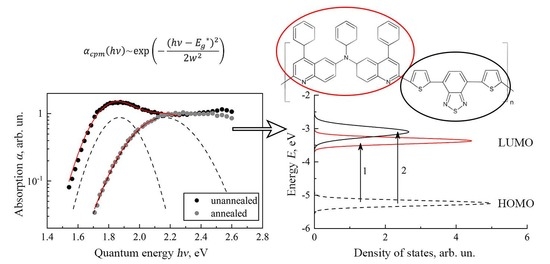
3.2. Spectral Dependences of Photoconductivity and Optical Absorption
3.3. Density of States
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wolfe, J.F.; Stille, J.K. The synthesis and solution properties of aromatic polymers containing 2,4-quinoline units in the main chain. Macromolecules 1976, 9, 489–496. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Jenekhe, S.A. Synthesis and processing of heterocyclic polymers as electronic, optoelectronic, and nonlinear optical materials. 2. New series of conjugated rigid-rod polyquinolines and polyanthrazolines. Macromolecules 1993, 26, 895–905. [Google Scholar] [CrossRef]
- Kim, J.L.; Kim, J.K.; Cho, H.N.; Kim, D.Y.; Kim, C.Y.; Hong, S.I. New polyquinoline copolymers: Synthesis, optical, luminescent, and hole-blocking/electron-transporting properties. Macromolecules 2000, 33, 5880–5885. [Google Scholar] [CrossRef]
- Jenekhe, S.A.; Lu, L.; Alam, M.M. New conjugated polymers with donor-acceptor architectures: Synthesis and photophysics of carbazole–quinoline and phenothiazine–quinolone copolymers and oligomers exhibiting large intramolecular charge transfer. Macromolecules 2001, 34, 7315–7324. [Google Scholar] [CrossRef]
- Kulkarni, A.P.; Wu, P.T.; Kwon, T.W.; Jenekhe, S.A. Phenothiazinephenylquinoline donor–acceptor molecules: Effects of structural isomerism on charge transfer photophysics and electroluminescence. J. Phys. Chem. B 2005, 109, 19584–19594. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Pak, J.S.; Yoon, K.-J.; Kim, Y.-I.; Jin, S.-H.; Kang, S.K.; Gal, Y.-S.; Kang, S.; Lee, J.Y.; Kang, J.-W.; et al. High-Efficiency Deep-Blue Light-Emitting Diodes Based on Phenylquinoline/Carbazole-Based Compound. Adv. Funct. Mater. 2008, 18, 3922–3930. [Google Scholar] [CrossRef]
- Aleksandrova, E.L.; Svetlichnyi, V.M.; Myagkova, L.A.; Matyushina, N.V.; Nekrasova, T.N.; Smyslov, R.Y.; Pautov, V.D.; Tameev, A.R.; Vannikov, A.V.; Kudryavtsev, V.V. Spectroscopic study of polyphenylquinolines—Materials with efficient intramolecular charge transfer. Opt. Spect. 2013, 114, 737–750. [Google Scholar] [CrossRef]
- Svetlichnyi, V.M.; Miagkova, L.A.; Matyushina, N.V.; Aleksandrova, E.L.; Tameev, A.R. Structural control over conductivity and conduction type in thin films of polyphenylquinones. Semiconductors 2012, 46, 491–495. [Google Scholar] [CrossRef]
- Svetlichnyi, V.M.; Aleksandrova, E.L.; Myagkova, L.A.; Matyushina, N.V.; Nekrasova, T.N.; Smyslov, R.Y.; Tameev, A.R.; Stepanenko, S.N.; Vannikov, A.V.; Kudryavtsev, V.V. Photophysical and electrical properties of polyphenylquinolines containing carbazole or indolo[3,2-b]carbazole fragments as new optoelectronic materials. Semiconductors 2011, 45, 1339–1345. [Google Scholar] [CrossRef]
- Svetlichnyi, V.M.; Nekrasova, T.N.; Smyslov, R.Y.; Miagkova, L.A.; Matyushina, N.V.; Aleksandrova, E.L. Carbazole-containing polyphenylquinolines as a basis for optoelectronic materials with white luminescence. Semiconductors 2012, 46, 496–503. [Google Scholar] [CrossRef]
- Aleksandrova, E.L.; Svetlychnyi, V.M.; Miagkova, L.A.; Nekrasova, T.N.; Tameev, A.R.; Vannikov, A.V.; Kudryavtsev, V.V. Photoelectric and electrical properties of soluble polyphenylquinolines containing an oxygen or phenylamine bridge group between quinoline moieties. Semiconductors 2009, 43, 359–364. [Google Scholar] [CrossRef]
- Aleksandrova, E.L.; Svetlichnyi, V.M.; Nekrasova, T.N.; Smyslov, R.Y.; Myagkova, L.A.; Matyushina, N.V.; Kudryavtsev, V.V. Copolymers of carbazole-and indolocarbazole-containing phenylquinolines as new materials for electroluminescent devices. Semiconductors 2013, 47, 1058–1067. [Google Scholar] [CrossRef]
- Svetlichnyi, V.M.; Alexandrova, E.L.; Miagkova, L.A.; Matushina, N.V.; Nekrasova, T.N.; Tameev, A.R.; Stepanenko, S.N.; Vannikov, A.V.; Kudryavtsev, V.V. Photophysical properties of indolo[3,2-b]carbazoles as a promising class of optoelectronic materials. Semiconductors 2010, 44, 1581–1587. [Google Scholar] [CrossRef]
- Boudrealt, P.-L.T.; Wakim, S.; Bliou, N.; Simard, M.; Tessier, C.; Tao, Y.; Leclerc, M. Synthesis, Characterization, and Application of Indolo[3,2-b]carbazole Semiconductors. Am. J. Chem. Soc. 2007, 129, 9125–9136. [Google Scholar] [CrossRef]
- Blouin, N.; Machaud, A.; Wakim, S.; Boudreault, P.T.; Leclerc, M.; Vercelli, B.; Zecchin, S.; Zotti, G. Optical, Electrochemical, Magnetic, and Conductive Properties of New Polyindolocarbazoles and Polydiindolocarbazoles. Macromol. Chem. Phys. 2006, 207, 166–174. [Google Scholar] [CrossRef]
- Belletête, M.; Blouin, N.; Boudreault, P.T.; Leclerc, M.; Durocher, G. Optical and Photophysical Properties of Indolocarbazole Derivatives. J. Phys. Chem. A 2006, 110, 13696–13704. [Google Scholar] [CrossRef]
- Suman; Bagui, A.; Gupta, V.; Maurya, K.K.; Singh, S.P. High-Performance Non-Fullerene Acceptor Derived from Diathiafulvalene Wings for Solution-Processed Organic Photovoltaics. J. Phys. Chem. C 2016, 120, 24615–24622. [Google Scholar] [CrossRef]
- Kimyonok, A.; Wang, X.-Y.; Weck, M. Electroluminescent Poly(quinoline)s and Metalloquinolates. J. Macromol. Sci. C 2006, 46, 47–77. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Lapis, A.A.M.; da Silva, E.N., Jr.; Dupont, J. 2,1,3-Benzothiadiazole and Derivatives: Synthesis, Properties, Reactions, and Applications in Light Technology of Small Molecules. Eur. J. Org. Chem. 2013, 2, 228–255. [Google Scholar] [CrossRef]
- Aleksandrova, E.L.; Svetlichnyi, V.M.; Matyushina, N.V.; Myagkova, L.A.; Kudryavtsev, V.V.; Tameev, A.R. Sensitization of the Photoelectric Effect in Carbazole- and Indolocarbazole-Containing Poly(Phenylquinoline)s by Benzothiadiazole Acceptor Molecules. Semiconductors 2014, 48, 1481–1484. [Google Scholar] [CrossRef]
- Mróz, W.; Villafiorita-Monteleone, F.; Pasini, M.; Grisci, G.; Paolino, M.; Razzano, V.; Cappelli, A.; Botta, C. π-Stacked polybenzofulvene derivatives as hosts for yellow and red emitting OLEDs. Mat. Let. 2005, 142, 197–200. [Google Scholar] [CrossRef]
- Cappelli, A.; Villafiorita-Monteleone, F.; Grisci, G.; Paolino, M.; Razzano, V.; Fabio, G.; Giuliani, G.; Donati, A.; Mendichi, R.; Boccia, A.C.; et al. Highly emissive supramolecular assemblies based on π-stacked polybenzofulvene hosts and a benzothiadiazole guest. J. Mater. Chem. C 2014, 2, 7897–7905. [Google Scholar] [CrossRef]
- Villafiorita-Monteleone, F.; Cappelli, A.; Paolino, M.; Colombo, M.; Cariati, E.; Mura, A.; Bongiovanni, G.; Botta, C. Aggregation-Induced Förster Resonance Energy Transfer in Polybenzofulvene/Dye Nanoparticles. J. Phys. Chem. C 2015, 119, 18986–18991. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Xiao, S.; Liu, S.; You, W. Donor−Acceptor Polymers Incorporating Alkylated Dithienylbenzothiadiazole for Bulk Heterojunction Solar Cells: Pronounced Effect of Positioning Alkyl Chains. Macromolecules 2010, 43, 811–820. [Google Scholar] [CrossRef]
- Aivali, S.; Kakogianni, S.; Anastasopoulos, C.; Andreopoulou, A.; Kallitsis, J. Copolymers and Hybrids Based on Carbazole Derivatives and Their Nanomorphology Investigation. Nanomaterials 2019, 9, 133. [Google Scholar] [CrossRef]
- Bai, K.; Wang, S.; Zhao, L.; Ding, J.; Wang, L. Efficient Blue, Green, and Red Electroluminescence from Carbazole-Functionalized Poly(spirobifluorene)s. Macromolecules 2017, 50, 6945–6953. [Google Scholar] [CrossRef]
- Fu, Y.; Cha, H.; Lee, G.-Y.; Moon, B.J.; Park, C.E.; Park, T. 3,6-Carbazole Incorporated into Poly[9,9-dioctylfluorene-alt-(bisthienyl)benzothiadiazole]s Improving the Power Conversion Efficiency. Macromolecules 2012, 45, 3004–3009. [Google Scholar] [CrossRef]
- Inganäs, O.; Svensson, M.; Zhang, F.; Gadisa, A.; Persson, N.K.; Wang, X.; Andersson, M.R. Low bandgap alternating polyfluorene copolymers in plastic photodiodes and solar cells. Appl. Phys. A 2004, 79, 31–35. [Google Scholar] [CrossRef]
- Park, S.H.; Roy, A.; Beaupré, S.; Cho, S.; Coates, N.; Moon, J.S.; Moses, D.; Leclerc, M.; Lee, K.; Heeger, A.J. Bulk heterojunction solar cells with internal quantum efficiency approaching 100%. Nat. Photon. 2009, 3, 297–302. [Google Scholar] [CrossRef]
- Tan, S.E.; Sarjadi, M.S. The Recent Development of Carbazole-, Benzothiadiazole-, and Isoindigo-Based Copolymers for Solar Cells Application: A Review. Polym. Sci. Series B 2017, 59, 479–496. [Google Scholar] [CrossRef]
- Tong, H.; Sun, H.H.; Xie, Z.Y.; Wang, L.X.; Jing, X.B.; Wang, F.S. Novel polyquinolines containing phenylamine moiety. Synth. Metals 2003, 137, 1115–1116. [Google Scholar] [CrossRef]
- Svetlichnyi, V.M.; Aleksandrova, E.L.; Matyushina, N.V.; Myagkova, L.A.; Nekrasova, T.N.; Smyslov, R.Yu. Molecular design of optoelectronic structures based on carbazole and indolocarbazole-containing polyphenylquinolines. High Perform. Polym. 2017, 29, 730–749. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Jenekhe, S.A. New conjugated polyanthrazolines containing thiophene moieties in the main chain. Macromolecules 1991, 24, 6806–6808. [Google Scholar] [CrossRef]
- Shirley, D.A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709–4713. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Vaněček, M.; Kočka, J.; Stuchlik, J.; Kožišek, Z.; Štika, O.; Třiska, A. Density of the gap states in undoped and doped glow discharge a-Si:H. Sol. Energy Mater. 1983, 8, 411–423. [Google Scholar] [CrossRef]
- Armin, A.; Juska, G.; Ullah, M.; Velusamy, M.; Burn, P.L.; Meredith, P.; Pivrikas, A. Balanced carrier mobilities: Not a necessary condition for high-efficiency thin organic solar cells as determined by MIS-CELIV. Adv. Energy Mater. 2014, 4, 1300954. [Google Scholar] [CrossRef]
- Malov, V.V.; Ghosh, T.; Nair, V.C.; Maslov, M.M.; Katin, K.P.; Narayanan Unni, K.N.; Tameev, A.R. Hole mobility in thieno[3,2-b]thiophene oligomers. Mendeleev Commun. 2019, 29, 218–219. [Google Scholar] [CrossRef]
- Gao, Y.; Pivrikas, A.; Xu, B.; Liu, Y.; Xu, W.; van Loosdrecht, P.H.M.; Tian, W. Measuring electron and hole mobilities in organic systems: Charge selective CELIV. Synth. Metals 2015, 203, 187–191. [Google Scholar] [CrossRef]
- Sandberg, O.J.; Nyman, M.; Dahlström, S.; Sandén, S.; Törngren, B.; Smâtt, J.-H.; Österbacka, R. On the validity of MIS-CELIV for mobility determination in organic thin-film devices. Appl. Phys. Lett. 2017, 110, 153504. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical considerations for determining absolute frontier orbital energy levels of conjugated polymers for solar cell applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef]
- Köhler, A.; Bässler, H. Electronic Processes in Organic Semiconductors; Wiley-VCH Verlag GmbH & Co: Weinheim, Germany, 2015; pp. 25–47. [Google Scholar]
- Vissenberg, M.C.J.M.; Matters, M. Theory of the field-effect mobility in amorphous organic transistors. Phys. Rev. B 1998, 57, 12964–12967. [Google Scholar] [CrossRef] [Green Version]
- Kattimani, J.; Sankarappa, T.; Praveenkumar, K.; Ashwajeet, J.S.; Ramanna, R.; Chandraprabha, G.B.; Sujatha, T. Structure and Temperature Dependence of Electrical Conductivity in Polythiophene Nanoparticles. IJARPS 2014, 1, 17–21. [Google Scholar]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Mott, N.F. Conduction in Non-Crystalline Materials; Clarendon Press: Oxford, UK, 1987. [Google Scholar]
- Zabrodskii, A.G.; Zinov’eva, K.N. Low-temperature conductivity and metal-insulator transition in compensate n-Ge. Sov. Phys. JETP 1984, 59, 425–433. [Google Scholar]
- Liao, H.-H.; Yang, C.-M.; Liu, C.-C.; Horng, S.-F.; Meng, H.-F.; Shy, J.-T. Dynamics and reversibility of oxygen doping and de-doping for conjugated polymer. J. Appl. Phys. 2008, 103, 104506. [Google Scholar] [CrossRef] [Green Version]
- Tauc, J. Optical Properties and Electronic Structure of Amorphous Semiconductors. In Optical Properties of Solids; Springer: Boston, MA, USA, 1969; pp. 123–136. [Google Scholar]
- Malov, V.V.; Tameev, A.R.; Novikov, S.V.; Khenkin, M.V.; Kazanskii, A.G.; Vannikov, A.V. Gaussian approximation of the spectral dependence of the absorption spectrum in polymer semiconductors. Semiconductors 2016, 50, 482–486. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saitov, S.R.; Amasev, D.V.; Tameev, A.R.; Malov, V.V.; Tedoradze, M.G.; Svetlichnyi, V.M.; Myagkova, L.A.; Popova, E.N.; Kazanskii, A.G. Conductivity and Density of States of New Polyphenylquinoline. Polymers 2019, 11, 934. https://doi.org/10.3390/polym11060934
Saitov SR, Amasev DV, Tameev AR, Malov VV, Tedoradze MG, Svetlichnyi VM, Myagkova LA, Popova EN, Kazanskii AG. Conductivity and Density of States of New Polyphenylquinoline. Polymers. 2019; 11(6):934. https://doi.org/10.3390/polym11060934
Chicago/Turabian StyleSaitov, Shamil R., Dmitriy V. Amasev, Alexey R. Tameev, Vladimir V. Malov, Marine G. Tedoradze, Valentin M. Svetlichnyi, Lyudmila A. Myagkova, Elena N. Popova, and Andrey G. Kazanskii. 2019. "Conductivity and Density of States of New Polyphenylquinoline" Polymers 11, no. 6: 934. https://doi.org/10.3390/polym11060934