Electrically-Responsive Reversible Polyketone/MWCNT Network through Diels-Alder Chemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Functionalisation of Polyketone with Furan and Benzyl Groups
2.2. Functionalisation of MWCNTs with PK-Fu or B-Ma via Diels-Alder Reaction
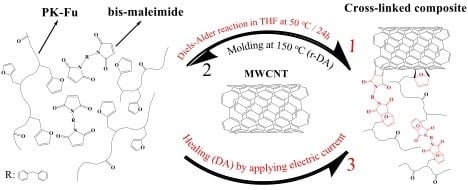
2.3. Preparation of PK-Fu/B-Ma/MWCNT Composite
2.4. Characterisation
3. Results and Discussion
3.1. PK Functionalised with Furan and Benzyl Groups via Paal-Knorr Reaction
3.2. Functionalisation of MWCNTs with PK-Fu or B-Ma via Diels-Alder Reaction and PK-Bea via Physical Interactions
3.3. PK-Fu/B-Ma/MWCNT Composite
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Leng, J.; Kin-Tak Lau, A. Multifunctional Polymer Nanocomposites; Taylor and Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2011. [Google Scholar]
- McNally, T.; Pötschke, P. Polymer-Carbon Nanotube Composites; Woodhead Publishing Limited: Philadelphia, PA, USA, 2011. [Google Scholar]
- Kasaliwal, G.R.; Villmow, T.; Pegel, S.; Pötschke, P. Influence of material and processing parameters on carbon nanotube despersion in polymer melts. In Polymer-Carbon Nanotube Composites; McNally, T., Pötschke, P., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2011; p. 92. [Google Scholar]
- Xie, X.; Mai, Y.; Zhou, X. Dispersion and Alignment of Carbon Nanotubes in Polymer Matrix: A Review. Mater. Sci. Eng. R-Rep. 2005, 49, 89–112. [Google Scholar] [CrossRef]
- Imtiaz, S.; Siddiq, M.; Kausar, A.; Muntha, S.T.; Ambreen, J.; Bibi, I. A Review Featuring Fabrication, Properties and Applications of Carbon Nanotubes (CNTs) Reinforced Polymer and Epoxy Nanocomposites. Chin. J. Polym. Sci. 2018, 36, 445–461. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, G.; Chen, J.; Su, D.S. Reinforcing Epoxy Resin with Nitrogen Doped Carbon Nanotube: A Potential Lightweight Structure Material. J. Mater. Sci. Technol. 2018, 34, 2205–2211. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, L.; Pan, C.; Li, D.; Gai, G. Thermally Sensitive, Adhesive, Injectable, Multiwalled Carbon Nanotube Covalently Reinforced Polymer Conductors with Self-Healing Capabilities. J. Mater. Chem. C 2018, 6, 1746–1752. [Google Scholar] [CrossRef]
- Guadagno, L.; De Vivo, B.; Di Bartolomeo, A.; Lamberti, P.; Sorrentino, A.; Tucci, V.; Vertuccio, L.; Vittoria, V. Effect of Functionalization on the Thermo-Mechanical and Electrical Behavior of Multi-Wall Carbon Nanotube/Epoxy Composites. Carbon 2011, 49, 1919–1930. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, X.; Mai, Y. Functionalization of Carbon Nanotubes for Polymer Nanocomposites. In Polymer–Carbon Nanotube Composites; Woodhead Publishing: Sawston/Cambridge, UK, 2011; pp. 55–91. [Google Scholar]
- Irzhak, T.F.; Irzhak, V.I. Epoxy Nanocomposites. Polym. Sci. Ser. A 2017, 59, 791–825. [Google Scholar] [CrossRef]
- Kulakov, V.; Aniskevich, A.; Ivanov, S.; Poltimae, T.; Starkova, O. Effective Electrical Conductivity of Carbon Nanotube-Epoxy Nanocomposites. J. Compos. Mater. 2017, 51, 2979–2988. [Google Scholar] [CrossRef]
- Ma, S.; Webster, D.C. Degradable Thermosets Based on Labile Bonds or Linkages: A Review. Prog. Polym. Sci. 2018, 76, 65–110. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, X.; Urban, M.W. Chemical and Physical Aspects of Self-Healing Materials. Prog. Polym. Sci. 2015, 49–50, 34–59. [Google Scholar] [CrossRef]
- Chen, J.; Ober, C.; Poliks, M. Characterization of Thermally Reworkable Thermosets: Materials for Environmentally Friendly Processing and Reuse. Polymer 2002, 43, 131–139. [Google Scholar] [CrossRef]
- An, S.Y.; Arunbabu, D.; Noh, S.M.; Song, Y.K.; Oh, J.K. Recent Strategies to Develop Self-Healable Crosslinked Polymeric Networks. Chem. Commun. 2015, 51, 13058–13070. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A. The Furan/Maleimide Diels–Alder Reaction: A Versatile Click–unclick Tool in Macromolecular Synthesis. Prog. Polym. Sci. 2013, 38, 1–29. [Google Scholar] [CrossRef]
- Polgar, L.M.; van Duin, M.; Broekhuis, A.A.; Picchioni, F. The use of Diels-Alder Chemistry for Thermo-Reversible Cross-Linking of Rubbers: The Next Step Towards Recycling of Rubber Products? Macromolecules 2015, 48, 7096–7105. [Google Scholar] [CrossRef]
- Ursache, O.; Gaina, C.; Gaina, V.; Tudorachi, N.; Bargan, A.; Varganici, C.; Rosu, D. Studies on Diels-Alder Thermoresponsive Networks Based on Ether-Urethane Bismaleimide Functionalized Poly(Vinyl Alcohol). J. Therm. Anal. Calorim. 2014, 118, 1471–1481. [Google Scholar] [CrossRef]
- Liu, Y.; Chuo, T. Self-Healing Polymers Based on Thermally Reversible Diels-Alder Chemistry. Polym. Chem. 2013, 4, 2194–2205. [Google Scholar] [CrossRef]
- Zhang, Y.; Broekhuis, A.A.; Picchioni, F. Thermally Self-Healing Polymeric Materials: The Next Step to Recycling Thermoset Polymers? Macromolecules 2009, 42, 1906–1912. [Google Scholar] [CrossRef] [Green Version]
- Toncelli, C.; De Reus, D.C.; Picchioni, F.; Broekhuis, A.A. Properties of Reversible Diels-Alder Furan/Maleimide Polymer Networks as Function of Crosslink Density. Macromol. Chem. Phys. 2012, 213, 157–165. [Google Scholar] [CrossRef]
- Araya-Hermosilla, R.; Lima, G.; Raffa, P.; Fortunato, G.; Pucci, A.; Flores, M.E.; Moreno-Villoslada, I.; Broekhuis, A.; Picchioni, F. Intrinsic Self-Healing Thermoset through Covalent and Hydrogen Bonding Interactions. Eur. Polym. J. 2016, 81, 186–197. [Google Scholar] [CrossRef]
- Araya-Hermosilla, R.; Broekhuis, A.A.; Picchioni, F. Reversible Polymer Networks Containing Covalent and Hydrogen Bonding Interactions. Eur. Polym. J. 2014, 50, 127–134. [Google Scholar] [CrossRef]
- Zhang, Y.; Broekhuis, A.A.; Stuart, M.C.A.; Picchioni, F. Polymeric Amines by Chemical Modifications of Alternating Aliphatic Polyketones. J. Appl. Polym. Sci. 2008, 107, 262–271. [Google Scholar] [CrossRef]
- Mul, W.P.; Dirkzwager, H.; Broekhuis, A.A.; Heeres, H.J.; van der Linden, A.J.; Orpen, A.G. Highly Active, Recyclable Catalyst for the Manufacture of Viscous, Low Molecular Weight, CO-Ethene-Propene-Based Polyketone, Base Component for a New Class of Resins. Inorg. Chim. Acta 2002, 327, 147–159. [Google Scholar] [CrossRef]
- Migliore, N.; Polgar, L.M.; Araya-Hermosilla, R.; Picchioni, F.; Raffa, P.; Pucci, A. Effect of the Polyketone Aromatic Pendent Groups on the Electrical Conductivity of the Derived MWCNTs-Based Nanocomposites. Polymers 2018, 10, 618. [Google Scholar] [CrossRef]
- Araya-Hermosilla, R.; Pucci, A.; Araya-Hermosilla, E.; Pescarmona, P.; Raffa, P.; Polgar, L.; Moreno-Villoslada, I.; Flores, M.; Fortunato, G.; Broekhuis, A. An Easy Synthetic Way to Exfoliate and Stabilize MWCNTs in a Thermoplastic Pyrrole-Containing Matrix Assisted by Hydrogen Bonds. RSC Adv. 2016, 6, 85829–85837. [Google Scholar] [CrossRef]
- Chang, C.; Liu, Y. Functionalization of Multi-Walled Carbon Nanotubes with Furan and Maleimide Compounds through Diels-Alder Cycloaddition. Carbon 2009, 47, 3041–3049. [Google Scholar] [CrossRef]
- Zydziak, N.; Huebner, C.; Bruns, M.; Barner-Kowollik, C. One-Step Functionalization of Single-Walled Carbon Nanotubes (SWCNTs) with Cyclopentadienyl-Capped Macromolecules via Diels-Alder Chemistry. Macromolecules 2011, 44, 3374–3380. [Google Scholar] [CrossRef]
- Sreekanth, M.; Ghosh, S.; Srivastava, P. Tuning Vertical Alignment and Field Emission Properties of Multi-Walled Carbon Nanotube Bundles. Appl. Phys. A-Mater. Sci. Process. 2018, 124, 52. [Google Scholar] [CrossRef]
- Paoletti, C.; He, M.; Salvo, P.; Melai, B.; Calisi, N.; Mannini, M.; Cortigiani, B.; Bellagambi, F.G.; Swager, T.M.; Di Francesco, F.; et al. Room Temperature Amine Sensors Enabled by Sidewall Functionalization of Single-Walled Carbon Nanotubes. RSC Adv. 2018, 8, 5578–5585. [Google Scholar] [CrossRef]
- Araya-Hermosilla, R.; Fortunato, G.; Pucci, A.; Broekhuis, A.A.; Raffa, P.; Lima, G.M.R.; Pourhossein, P.; Polgar, L.; Picchioni, F. Thermally Reversible Rubber-Toughened Thermoset Networks Via Diels-Alder Chemistry. Eur. Polym. J. 2006, 74, 229–240. [Google Scholar] [CrossRef]
- Mauro, M.; Acocella, M.R.; Corcione, C.E.; Maffezzoli, A.; Guerra, G. Catalytic Activity of Graphite-Based Nanofillers on Cure Reaction of Epoxy Resins. Polymer 2014, 55, 5612–5615. [Google Scholar] [CrossRef]
- Park, J.S.; Darlington, T.; Starr, A.F.; Takahashi, K.; Riendeau, J.; Thomas Hahn, H. Multiple Healing Effect of Thermally Activated Self-Healing Composites Based on Diels–Alder Reaction. Compos. Sci. Technol. 2010, 70, 2154–2159. [Google Scholar] [CrossRef]
- Lee, J.; Stein, I.Y.; Kessler, S.S.; Wardle, B.L. Aligned Carbon Nanotube Film Enables Thermally Induced State Transformations in Layered Polymeric Materials. ACS Appl. Mater. Interfaces 2015, 7, 8900–8905. [Google Scholar] [CrossRef] [PubMed]
- Sosa, E.D.; Darlington, T.K.; Hanos, B.A.; O’Rourke, M.J.E. Multifunctional Thermally Remendable Nanocomposites. J. Compos. 2014, 2014, 12. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C.; Morrill, T.C. Spectrometric Identification of Organic Compounds; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 1991. [Google Scholar]
- Bode, S.; Enke, M.; Hernandez, M.; Bose, R.K.; Grande, A.M.; van der Zwaag, S.; Schubert, U.S.; Garcia, S.J.; Hager, M.D. Characterization of Self-Healing Polymers: From Macroscopic Healing Tests to the Molecular Mechanism. Self-Heal. Mater. 2016, 273, 113–142. [Google Scholar]
- Paramane, A.S.; Kumar, K.S. A Review on Nanocomposite Based Electrical Insulations. Trans. Electr. Electron. Mater. 2016, 17, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.; Skipa, T.; Bilotti, E.; Zhang, R.; Lellinger, D.; Mezzo, L.; Fu, Q.; Alig, I.; Peijs, T. Preparation of High-Performance Conductive Polymer Fibers through Morphological Control of Networks Formed by Nanofillers. Adv. Funct. Mater. 2010, 20, 1424–1432. [Google Scholar] [CrossRef]











| Run | CCO (%) a | η (%) b | Tg (°C) c | PDI d |
|---|---|---|---|---|
| PK-Fu | 74 | 92 | 31 | 2.3 |
| PK-Bea | 73 | 91 | 42 | 2.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araya-Hermosilla, R.; Pucci, A.; Raffa, P.; Santosa, D.; Pescarmona, P.P.; Gengler, R.Y.N.; Rudolf, P.; Moreno-Villoslada, I.; Picchioni, F. Electrically-Responsive Reversible Polyketone/MWCNT Network through Diels-Alder Chemistry. Polymers 2018, 10, 1076. https://doi.org/10.3390/polym10101076
Araya-Hermosilla R, Pucci A, Raffa P, Santosa D, Pescarmona PP, Gengler RYN, Rudolf P, Moreno-Villoslada I, Picchioni F. Electrically-Responsive Reversible Polyketone/MWCNT Network through Diels-Alder Chemistry. Polymers. 2018; 10(10):1076. https://doi.org/10.3390/polym10101076
Chicago/Turabian StyleAraya-Hermosilla, Rodrigo, Andrea Pucci, Patrizio Raffa, Dian Santosa, Paolo P. Pescarmona, Régis Y. N. Gengler, Petra Rudolf, Ignacio Moreno-Villoslada, and Francesco Picchioni. 2018. "Electrically-Responsive Reversible Polyketone/MWCNT Network through Diels-Alder Chemistry" Polymers 10, no. 10: 1076. https://doi.org/10.3390/polym10101076