Aegle marmelos Leaf Extract Phytochemical Analysis, Cytotoxicity, In Vitro Antioxidant and Antidiabetic Activities
Abstract
:1. Introduction
2. Results
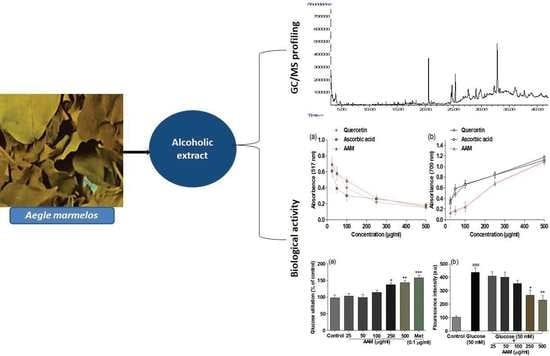
2.1. Chemical Profiling of A. marmelos Extract Using GC/MS
2.2. Identification of Gallic Acid and Rutin Using HPLC
2.3. Total Phenol and Flavonoid Content
2.4. DPPH Antioxidant Activity
2.5. Reducing Power of A. marmelos
2.6. In Vitro α-Amylase and α-Glucosidase Activity
2.7. Cytotoxicity and Cytoprotective Assay
2.8. Glucose Utilization Assay
2.9. Cellular Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Plant Material
4.2. Preparation of Alcoholic Extract of A. marmelos
4.3. GC/MS Analysis
4.4. Identification of Gallic Acid and Rutin in A. marmelos Using HPLC
4.5. Estimation of Total Phenol and Flavonoid Content
4.6. In Vitro DPPH Assay
4.7. Ferric-Reducing Antioxidant Power (FRAP) Assay
4.8. Inhibitory Activity of α-Amylase and α-Glucosidase
4.9. Cytotoxicity and Cytoprotective Assay
4.10. Glucose Utilization Activity
4.11. Cellular Antioxidant Capacity
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheng, Y.; Zheng, S.; Ma, T.; Zhang, C.; Ou, X.; He, X.; Xu, W.; Huang, K. Mulberry leaf alleviates streptozotocin-induced diabetic rats by attenuating NEFA signaling and modulating intestinal microflora. Sci. Rep. 2017, 7, 12041. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Pan, Y.; Gao, X.; Chen, H. Hypoglycemic and hypolipidemic effects of anthocyanins extract from black soybean seed coat in high fat diet and streptozotocin-induced diabetic mice. Food Funct. 2018, 9, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Ju, J.; Wang, K.; Gu, C.; Feng, Y. Evaluation of hypoglycemic activity of total lignans from Fructus Arctii in the spontaneously diabetic Goto-Kakizaki rats. J. Ethnopharmacol. 2014, 151, 548–555. [Google Scholar] [CrossRef]
- Emami-Riedmaier, A.; Schaeffeler, E.; Nies, A.T.; Mörike, K.; Schwab, M. Stratified medicine for the use of antidiabetic medication in treatment of type II diabetes and cancer: Where do we go from here? J. Intern. Med. 2015, 277, 235–247. [Google Scholar] [CrossRef]
- Awotedu, O.L.; Ogunbamowo, P.O.; Chukwudebe, E.P.; Ariwoola, O.S. Medicinal based plants: A call to nature. World News Nat. Sci. 2020, 31, 92–109. [Google Scholar]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [Green Version]
- Tugume, P.; Nyakoojo, C. Ethno-pharmacological survey of herbal remedies used in the treatment of paediatric diseases in Buhunga parish, Rukungiri District, Uganda. BMC Complement. Altern. Med. 2019, 19, 353. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal Plant Analysis: A Historical and Regional Discussion of Emergent Complex Techniques. Front. Pharmacol. 2020, 10, 1480. [Google Scholar] [CrossRef]
- Sharma, P.; Joshi, T.; Mathpal, S.; Chandra, S.; Tamta, S. In silico identification of antidiabetic target for phytochemicals of A. marmelos and mechanistic insights by molecular dynamics simulations. J. Biomol. Struct. Dyn. 2021, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Huang, X.; Shah, M.H.; Abbasi, A.M. Evaluation of Polyphenolics Content and Antioxidant Activity in Edible Wild Fruits. BioMed Res. Int. 2019, 2019, 1381989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mujeeb, F.; Bajpai, P.; Pathak, N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. BioMed Res. Int. 2014, 2014, 497606. [Google Scholar] [CrossRef] [Green Version]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.K.; Krishnan, S.; Kumar, A.; Kumar, A. Antidiabetic phytoconstituents and their mode of action on metabolic pathways. Ther. Adv. Endocrinol. Metab. 2018, 9, 81–100. [Google Scholar] [CrossRef]
- Belury, M.A.; Cole, R.M.; Snoke, D.B.; Banh, T.; Angelotti, A. Linoleic acid, glycemic control and Type 2 diabetes. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 30–33. [Google Scholar] [CrossRef]
- Gupta, S.; Mediratta, P.K.; Singh, S.; Sharma, K.K.; Shukla, R. Antidiabetic, antihypercholesterolaemic and antioxidant effect of Ocimum sanctum (Linn) seed oil. Indian J. Exp. Biol. 2006, 44, 300–304. [Google Scholar]
- Takato, T.; Iwata, K.; Murakami, C.; Wada, Y.; Sakane, F. Chronic administration of myristic acid improves hyperglycaemia in the Nagoya-Shibata-Yasuda mouse model of congenital type 2 diabetes. Diabetologia 2017, 60, 2076–2083. [Google Scholar] [CrossRef] [Green Version]
- Kamalakkannan, N.; Prince, P.S. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin. Pharmacol. Toxicol. 2006, 98, 97–103. [Google Scholar] [CrossRef]
- Kade, I.J.; Ogunbolude, Y.; Kamdem, J.P.; Rocha, J.B. Influence of gallic acid on oxidative stress-linked streptozotocin-induced pancreatic dysfunction in diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2014, 25, 35–45. [Google Scholar] [CrossRef]
- Trendafilova, A.; Ivanova, V.; Rangelov, M.; Todorova, M.; Ozek, G.; Yur, S.; Ozek, T.; Aneva, I.; Veleva, R.; Moskova-Doumanova, V.; et al. Caffeoylquinic Acids, Cytotoxic, Antioxidant, Acetylcholinesterase and Tyrosinase Enzyme Inhibitory Activities of Six Inula Species from Bulgaria. Chem. Biodivers. 2020, 17, e2000051. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Villa-Rodriguez, J.A.; Kerimi, A.; Abranko, L.; Tumova, S.; Ford, L.; Blackburn, R.S.; Rayner, C.; Williamson, G. Acute metabolic actions of the major polyphenols in chamomile: An in vitro mechanistic study on their potential to attenuate postprandial hyperglycaemia. Sci. Rep. 2018, 8, 5471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.Y.; Yen, Y.Y.; Hung, K.C.; Hsu, S.W.; Lan, S.J.; Lin, H.C. Inhibitory effects of pu-erh tea on alpha glucosidase and alpha amylase: A systemic review. Nutr. Diabetes 2019, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Kong, K.W.; Mat-Junit, S.; Aminudin, N.; Hassan, F.A.; Ismail, A.; Abdul Aziz, A. Protective effects of the extracts of Barringtonia racemosa shoots against oxidative damage in HepG2 cells. PeerJ 2016, 4, e1628. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Chen, L.; Teng, H.; Song, H.; Wu, X.; Xu, M. Phenolic compounds ameliorate the glucose uptake in HepG2 cells’ insulin resistance via activating AMPK. J. Funct. Foods 2015, 19, 487–494. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Mohammed, M.; Aly, H.F.; Ali, S.A.; Al-Hady, D.A. Efficiency of the leaves and fruits of Aegle marmelos methanol extract (L.) Correa and their relative hepatotoxicity induced by CCL4 and identification of their active constituents by using LC/MS/MS. Toxicol. Rep. 2018, 5, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [Green Version]
- Konappa, N.; Udayashankar, A.C.; Krishnamurthy, S.; Pradeep, C.K.; Chowdappa, S.; Jogaiah, S. GC–MS analysis of phytoconstituents from Amomum nilgiricum and molecular docking interactions of bioactive serverogenin acetate with target proteins. Sci. Rep. 2020, 10, 16438. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Cosmulescu, S. HPLC determination of phenolic acids, flavonoids and juglone in walnut leaves. J. Chromatogr. Sci. 2013, 51, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Ansari, M.H.; Parveen, R.; Khan, W.; Ahmad, S.; Husain, S.A. Chromatography Based Metabolomics and In Silico Screening of Gymnema sylvestre Leaf Extract for Its Antidiabetic Potential. Evid.-Based Complement. Altern. Med. 2019, 2019, 7523159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Lan, M.; Lü, J.P.; Li, J.F.; Zhang, K.Y.; Zhi, H.; Zhang, H.; Sun, J.M. Antioxidant, Anti-inflammatory and Cytotoxic Activities of Polyphenols Extracted from Chroogomphus rutilus. Chem. Biodivers. 2020, 17, e1900479. [Google Scholar] [CrossRef]
- Aras, A.; Bursal, E.; Türkan, F.; Tohma, H.; Kılıç, Ö.; Gülçin, İ.; Köksal, E. Phytochemical Content, Antidiabetic, Anticholinergic, and Antioxidant Activities of Endemic Lecokia cretica Extracts. Chem. Biodivers. 2019, 16, e1900341. [Google Scholar] [CrossRef]
- Bahuguna, A.; Khan, I.; Bajpai, V.K.; Kang, S.C. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J. Pharmacol. 2017, 12, 8–20. [Google Scholar] [CrossRef]
- Sagbo, I.J.; van de Venter, M.; Koekemoer, T.; Bradley, G. In Vitro Antidiabetic Activity and Mechanism of Action of Brachylaena elliptica (Thunb.) DC. Evid.-Based Complement. Altern. Med. 2018, 2018, 4170372. [Google Scholar] [CrossRef] [Green Version]
- Subramaniyan, S.D.; Natarajan, A.K. Citral, a Monoterpene Protect Against High Glucose Induced Oxidative Injury in HepG2 Cell In Vitro-An Experimental Study. J. Clin. Diagn. Res. 2017, 11, BC10–BC15. [Google Scholar] [CrossRef]





| S. No | Name of Metabolite | RT | % |
|---|---|---|---|
| 1. | 2-hexanone | 3.643 | 0.38 |
| 2. | PENTAN-2-ONE | 3.746 | 0.59 |
| 3. | Methyl-2-pentanone | 3.841 | 0.60 |
| 4. | 1-penten-3-ol | 3.936 | 2.92 |
| 5. | Cyclopentanol | 4.756 | 1.05 |
| 6. | p-mentha-1 (7),3-diene | 6.250 | 0.36 |
| 7. | p-cymene | 7.019 | 0.22 |
| 8. | 8-hexadecenal | 11.522 | 0.24 |
| 9. | (E)-1-(methoxymethoxy)-1-tetradecee-3-ol | 12.972 | 0.26 |
| 10. | 1-dodecanol | 14.385 | 0.47 |
| 11. | Heneicosane | 15.146 | 0.43 |
| 12. | Cyclooctasiloxane | 16.420 | 0.57 |
| 13. | Tetradecamethylcycloheptasiloxane | 16.508 | 0.42 |
| 14. | Dotriacontane | 17.687 | 0.62 |
| 15. | Cyclononasiloxane | 18.346 | 0.82 |
| 16. | Phthalic acid | 19.613 | 0.56 |
| 17. | Hexadecanoic acid | 20.572 | 7.23 |
| 18. | Cyclodecasiloxane | 21.070 | 0.91 |
| 19. | Tetradecanoic acid (myristic acid) | 21.326 | 0.70 |
| 20. | Methyl linolelaidate | 24.628 | 1.86 |
| 21. | Oleic acid | 24.767 | 3.52 |
| 22. | 9,17-octadecadienal | 24.906 | 0.99 |
| 23. | Octadecanoic acid | 25.360 | 4.87 |
| 24. | Hentriacontane | 26.334 | 2.26 |
| 25. | 9,12,15-Octadecatrienoic acid, methyl ester (Linolenic acid, methyl ester) | 26.788 | 2.72 |
| 26. | Octadecanoic acid (Stearic acid) | 27.059 | 1.05 |
| 27. | Alpha-Neodene | 27.147 | 0.80 |
| 28. | Sulfurous acid | 27.191 | 0.82 |
| 29. | Phenol | 27.660 | 4.09 |
| 30. | Tetracosamethyl-cyclododecasilaxane | 27.835 | 2.42 |
| 31. | Oleyl alcohol | 28.567 | 2.37 |
| 32. | 2-propenoic acid, 3-(4-hydroxy-3-methoxyphenyl)-, methyl ester (cinnamic acid, 4-hydroxy-3-methoxy-, methyl ester) | 28.692 | 0.87 |
| 33. | cis-13-eicosenoic acid | 29.073 | 3.20 |
| 34. | Nonahexacontanoic acid | 29.607 | 1.49 |
| 35. | Nonacosane | 29.878 | 5.93 |
| 36. | Benzoic acid | 31.035 | 1.36 |
| 37. | Vitamin-E | 31.247 | 0.21 |
| 38. | Gamma-tocopheryl | 31.504 | 0.30 |
| 39. | 1-heptadec-1-ynyl-cyclopentanol | 32.316 | 2.65 |
| 40. | 7-pentadecyne | 32.514 | 0.90 |
| 41. | 13-docosenoic acid | 32.851 | 14.58 |
| 42. | Docosanoic acid | 33.334 | 2.53 |
| 43. | Z,Z-10,12-hexadecadien-1-ol acetat | 34.220 | 0.71 |
| 44. | n-triacontane | 36.080 | 5.67 |
| 45. | Tetracosamethyl-cyclododecasiloxane | 36.299 | 2.53 |
| 46. | (+)-(9.beta.H)-labda-8(17),13(E)-diene-5-ol | 36.973 | 0.84 |
| 47. | 15-tetracosenoic acid | 37.698 | 2.68 |
| 48. | Benchequiol | 38.452 | 1.16 |
| 49. | Retinoic acid | 38.979 | 3.33 |
| 50. | Cyclodecasiloxane | 40.722 | 1.38 |
| 51. | Farnesyl acetone | 41.601 | 0.82 |
| Parameter | Value | · |
|---|---|---|
| Mobile phase | Solvent A (0.5% formic acid in water) and solvent B (acetonitrile) | · |
| Stationary phase | C18 column (150 × 4.6 mm, particle size 5.0 µm, Phenomenex, Torrance, CA, USA) | · |
| Wavelength | 278 nm | · |
| Solvent flow rate | 1.0 mL/min | · |
| Gradient | Time (min) | Gradient ratio (Solvent A:Solvent B) |
| · | Initially | 10:90 |
| · | 0–5 | 20:80 |
| · | 5–7 | 25:75 |
| · | 7–10 | 30:70 |
| · | 10–15 | 60:40 |
| · | 15–18 | 20:80 |
| · | 18–25 | 10:90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, W.; Amir, M.; Ahmad, A.; Ali, A.; Ali, A.; Wahab, S.; Barkat, H.A.; Ansari, M.A.; Sarafroz, M.; Ahmad, A.; et al. Aegle marmelos Leaf Extract Phytochemical Analysis, Cytotoxicity, In Vitro Antioxidant and Antidiabetic Activities. Plants 2021, 10, 2573. https://doi.org/10.3390/plants10122573
Ahmad W, Amir M, Ahmad A, Ali A, Ali A, Wahab S, Barkat HA, Ansari MA, Sarafroz M, Ahmad A, et al. Aegle marmelos Leaf Extract Phytochemical Analysis, Cytotoxicity, In Vitro Antioxidant and Antidiabetic Activities. Plants. 2021; 10(12):2573. https://doi.org/10.3390/plants10122573
Chicago/Turabian StyleAhmad, Wasim, Mohd Amir, Adil Ahmad, Abuzer Ali, Amena Ali, Shadma Wahab, Harshita Abul Barkat, Mohammad Azam Ansari, Mohammad Sarafroz, Ayaz Ahmad, and et al. 2021. "Aegle marmelos Leaf Extract Phytochemical Analysis, Cytotoxicity, In Vitro Antioxidant and Antidiabetic Activities" Plants 10, no. 12: 2573. https://doi.org/10.3390/plants10122573