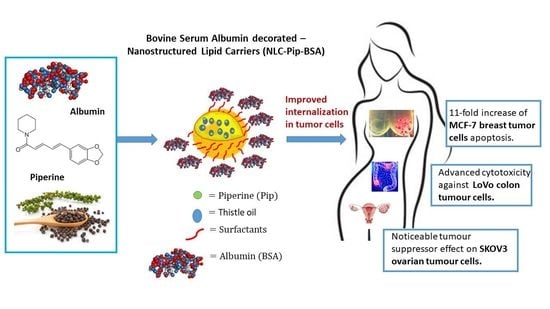
Novel Bovine Serum Albumin-Decorated–Nanostructured Lipid Carriers Able to Modulate Apoptosis and Cell-Cycle Response in Ovarian, Breast, and Colon Tumoral Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Cultures Conditions and Treatments
2.3. Preparation of Hybrid BSA-Coated Nanostructured Lipid Carriers
2.4. Characterization Methods
2.5. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Surfactants and Albumin in Obtaining Novel Biopolymer–Lipid Nanocarriers and Fluorescence Performance
3.2. Lipid Nanocarriers Coated with Bovine Serum Albumin and Loaded with Piperine
- i.
- A donor molecule absorbs energy leading to its excitement from the ground state to an excited singlet state. For the excited donor (in our case, BSA), distinct energy states are possible, i.e., spontaneous emission, and non-radiative processes;
- ii.
- If one fluorophore receiver (in our case, Pip) is nearby, the non-radiative energy transfer between the donor and the acceptor can occur. This transfer involves a resonance between the electronic transitions of the two fluorophores, generated by the transition dipole moment for the BSA absorption and a relation of the transition dipole moment to the donor’s emission [44].
3.3. The Antitumoral Functionality of NLC-Pip versus Hybrid BSA–Lipid Nanocarriers Loaded with Piperine
- A.
- In vitro cytotoxicity determined for ovarian, breast, and colon tumoral cell lines (MTS and RTCA assays)
- B.
- The effect of the NLC-Pip and NLC-Pip–BSA on the apoptosis process of colon, breast and ovarian tumor cells
- C.
- The effect of the Piperine-loaded nanocarriers on the cell cycle of normal and tumor cells.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, T.; Bae, M.; Lee, J.-Y.; Luo, Y. Solid lipid-polymer hybrid nanoparticles prepared with natural biomaterials: A new platform for oral delivery of lipophilic bioactives. Food Hydrocoll. 2018, 84, 581–592. [Google Scholar] [CrossRef]
- Truong, T.H.; Alcantara, K.P.; Bulatao, B.P.I.; Sorasitthiyanukarn, F.N.; Muangnoi, C.; Nalinratana, N.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-coated nanostructured lipid carriers for transdermal delivery of tetrahydrocurcumin for breast cancer therapy. Carbohydr. Polym. 2022, 288, 11940. [Google Scholar] [CrossRef]
- Basim, P.; Gorityala, S.; Kurakula, M. Advances in functionalized hybrid biopolymer augmented lipid-based systems: A spotlight on their role in design of gastro retentive delivery systems. Arch. Gastroenterol. Res. 2021, 2, 35–47. [Google Scholar]
- Yang, X.; Xie, Y. Recent advances in polymeric core–shell nanocarriers for targeted delivery of chemotherapeutic drugs. Int. J. Pharm. 2021, 608, 121094. [Google Scholar] [CrossRef]
- Shehata, E.M.; Gowayed, M.A.; El-Ganainy, S.O.; Sheta, E.; Elnaggar, Y.S.; Abdallah, O.Y. Pectin coated nanostructured lipid carriers for targeted piperine delivery to hepatocellular carcinoma. Int. J. Pharm. 2022, 619, 121712. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zoulikha, M.; Qiu, M.; Teng, C.; Lin, C.; Li, X.; Sallam, M.A.; Xu, Q.; He, W. The effects of protein corona on in vivo fate of nanocarriers. Adv. Drug Deliv. Rev. 2022, 186, 114356. [Google Scholar] [CrossRef]
- Ghataty, D.S.; Amer, R.I.; Amer, M.A.; Abdel Rahman, M.F.; Shamma, R.N. Green Synthesis of Highly Fluorescent Carbon Dots from Bovine Serum Albumin for Linezolid Drug Delivery as Potential Wound Healing Biomaterial: Bio-Synergistic Approach, Antibacterial Activity, and In Vitro and Ex Vivo Evaluation. Pharmaceutics 2023, 15, 234. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Tang, Q.; Yin, D.; Tang, C.; He, E.; Zou, L.; Peng, Q. The protein corona and its effects on nanoparticle-based drug delivery systems. Acta Biomater. 2021, 129, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Kudarha, R.R.; Sawant, K.K. Albumin based versatile multifunctional nanocarriers for cancer therapy: Fabrication, surface modification, multimodal therapeutics and imaging approaches. Mater. Sci. Eng. C 2017, 81, 607–626. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. An overview of biopolymer nanostructures for encapsulation of food ingredients. in Biopolymer nanostructures for food encapsulation purposes. Biopolym. Nanostructures Food Encapsulation Purp. 2019, 1, 1–35. [Google Scholar] [CrossRef]
- De Redína, I.L.; Boierob, C.; Martínez-Ohárrizc, M.C.; Agüerosa, M.; Ramosd, R.; Peñuelasd, I.; Allemandib, D.; Llabotb, J.M.; Irachea, J.M. Human serum albumin nanoparticles for ocular delivery of bevacizumab. Int. J. Pharm. 2018, 541, 214–223. [Google Scholar] [CrossRef]
- An, F.-F.; Zhang, X.-H. Strategies for preparing albumin-based nanoparticles for multifunctional bioimaging and drug delivery. Theranostics 2017, 7, 3667–3689. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhao, P.; Jiang, Y.; Tang, Y.; Jin, H.; Pan, Z.; He, H.; Yang, V.C.; Huang, Y. Blood-brain-barrier-penetrating albumin nanoparticles for biomimetic drug delivery via albumin-binding protein pathways for antiglioma therapy. ACS Nano 2016, 10, 9999–10012. [Google Scholar] [CrossRef]
- Otagiri, M.; Giam Chuang, V.T. Albumin in Medicine: Pathological and Clinical Applications; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Dayani, L.; Dehghani, M.; Aghaei, M.; Taymouri, S.; Taheri, A. Preparation and evaluation of targeted albumin lipid nanoparticles with lactobionic acid for targeted drug delivery of sorafenib in hepatocellular carcinoma. J. Drug Deliv. Sci. Technol. 2022, 69, 103142. [Google Scholar] [CrossRef]
- Miao, L.; Lin, J.; Huang, Y.; Li, L.; Delcassian, D.; Ge, Y.; Shi, Y.; Anderson, D.G. Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver. Nat. Commun. 2020, 11, 2424. [Google Scholar] [CrossRef]
- Piao, L.; Li, H.; Teng, L.; Yung, B.C.; Sugimoto, Y.; Brueggemeier, R.W.; Lee, R.J. Human serum albumin-coated lipid nanoparticles for delivery of siRNA to breast cancer. Nanomedicine 2013, 9, 122–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Xu, X.; Zhang, P.; Xiong, S.; Yuan, J.; Gao, X.; Guan, W.; Wang, F.; Li, X.; Dou, H.; et al. Lipid-coated albumin-paclitaxel nanoparticles loaded with sorcin-siRNA reverse cancer chemoresistance via restoring intracellular calcium ion homeostasis. J. Nanobiotechnol. 2022, 20, 319. [Google Scholar] [CrossRef]
- Kovács, A.N.; Katona, G.; Juhász, Á.; Balogh, G.T.; Csapó, E. Albumin-hyaluronic acid colloidal nanocarriers: Effect of human and bovine serum albumin for intestinal ibuprofen release enhancement. J. Mol. Liq. 2022, 351, 118614. [Google Scholar] [CrossRef]
- Kovács, A.N.; Varga, N.; Juhász, Á.; Csapó, E. Serum protein-hyaluronic acid complex nanocarriers: Structural characterisation and encapsulation possibilities. Carbohydr. Polym. 2021, 251, 117047. [Google Scholar] [CrossRef]
- Luo, K.; Xu, F.; Yao, T.; Zhu, J.; Yu, H.; Wang, G.; Li, J. TPGS and chondroitin sulfate dual-modified lipid-albumin nanosystem for targeted delivery of chemotherapeutic agent against multidrug-resistant cancer. Int. J. Biol. Macromol. 2021, 183, 1270–1282. [Google Scholar] [CrossRef]
- Nishihira, V.S.K.; Rubim, A.M.; Brondani, M.; Dos Santos, J.T.; Pohl, A.R.; Friedrich, J.F.; Dotto de Lara, J.; Nunes, C.M.; Feksa, L.R.; Simão, E.; et al. In vitro and in silico protein corona formation evaluation of curcumin and capsaicin loaded-solid lipid nanoparticles. Toxicol. In Vitro 2019, 61, 104598. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, Z.; Li, Y.; Wang, W.; Shi, J.; Fu, F.; Huang, Y.; Pan, X.; Wu, C. Impact of particle size and pH on protein corona formation of solid lipid nanoparticles: A proof-of-concept study. Acta Pharm. Sin. B 2021, 11, 1030–1046. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Khan, M.R.; Khan, A.; Ahmed, B. Preparation, characterization, in vivo and biochemical evaluation of brain targeted piperine solid lipid nanoparticles in an experimentally induced Alzheimer’s disease model. J. Drug Target. 2013, 21, 300–311. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine-the bioactive compound of black pepper: From isolation to medicinal formulations. Compr. Rev. Food Sci. Food 2016, 16, 17–40. [Google Scholar] [CrossRef]
- Sedeky, A.S.; Khalil, I.A.; Hefnawy, A.; El-Sherbiny, I.M. Development of core-shell nanocarrier system for augmenting piperine cytotoxic activity against human brain cancer cell line. Eur. J. Pharm. Sci. 2018, 118, 103–112. [Google Scholar] [CrossRef]
- Pachauri, M.; Gupta, E.D.; Ghosh, P.C. Piperine loaded PEG-PLGA nanoparticles: Preparation, characterization and targeted delivery for adjuvant breast cancer chemotherapy. J. Drug Deliv. Sci. Technol. 2015, 29, 269–282. [Google Scholar] [CrossRef]
- Bolat, Z.B.; Islek, Z.; Demir, B.N.; Yilmaz, E.N.; Sahin, F.; Ucisik, M.H. Curcumin- and piperine-loaded emulsions as combinational treatment approach enhance the anticancer activity of curcumin on HCT116 colorectal cancer model. Front. Bioeng. Biotechnol. 2020, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Lacatusu, I.; Badea, N.; Badea, G.; Mihaila, M.; Ott, C.; Stan, R.; Meghea, A. Advanced bioactive lipid nanocarriers loaded with natural and synthetic anti-inflammatory actives. Chem. Eng. Sci. 2019, 200, 113–126. [Google Scholar] [CrossRef]
- Ott, C.; Lacatusu, I.; Badea, G.; Grafu, I.A.; Istrati, D.; Babeanu, N.; Stan, R.; Badea, N.; Meghea, A. Exploitation of amaranth oil fractions enriched in squalene for dual delivery of hydrophilic and lipophilic actives. Ind. Crops Prod. 2015, 77, 342–352. [Google Scholar] [CrossRef]
- Lacatusu, I.; Iordache, T.A.; Mihaila, M.; Mihaiescu, D.E.; Pop, A.L.; Badea, N. Multifaced role of dual herbal principles loaded-lipid nanocarriers in providing high therapeutic efficacity. Pharmaceutics 2021, 13, 1511. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, N.; Udeanu, D.; Coc, L.; Pop, A.; Cioates Negut, C.; Tanase, C.; Stan, R.; Meghea, A. Improved anti-obesity effect of herbal active and endogenous lipids co-loaded lipid nanocarriers: Preparation, in vitro and in vivo evaluation. Mat. Sci. Eng. C 2019, 99, 12–24. [Google Scholar] [CrossRef]
- Stecoza, C.E.; Nitulescu, G.M.; Draghici, C.; Caproiu, M.T.; Olaru, O.T.; Bostan, M.; Mihaila, M. Synthesis and anticancer evaluation of new 1,3,4-oxadiazole derivatives. Pharmaceuticals 2021, 14, 438–453. [Google Scholar] [CrossRef]
- Mihaila, M.; Hotnog, C.M.; Bostan, M.; Munteanu, A.C.; Vacaroiu, I.A.; Brasoveanu, L.I.; Uivarosi, V. Anticancer activity of some ruthenium (III) complexes with quinolone antibiotics: In vitro cytotoxicity, cell cycle modulation, and apoptosis-inducing properties in LoVo colon cancer cell line. Appl. Sci. 2021, 11, 8594–8613. [Google Scholar] [CrossRef]
- Botezatu, A.; Iancu, I.V.; Plesa, A.; Manda, D.; Popa, O.; Bostan, M.; Mihaila, M.; Albulescu, A.; Fudulu, A.; Vladoiu, S.V.; et al. Methylation of tumour suppressor genes associated with thyroid cancer. Cancer Biomark. 2019, 25, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Bostan, M.; Petrica-Matei, G.G.; Ion, G.; Radu, N.; Mihaila, M.; Hainarosie, R.; Brasoveanu, L.I.; Roman, V.; Constantin, C.; Neagu, M.T. Cisplatin effect on head and neck squamous cell carcinoma cells is modulated by ERK1/2 protein kinases. Exp. Ther. Med. 2019, 18, 5041–5051. [Google Scholar] [CrossRef] [Green Version]
- Naik, P.; Nandibewoor, S.T.; Chimatadar, S.A. Non-covalent binding analysis of sulfamethoxazole to Human serum albumin: Fluorescence spectroscopy, UV-vis, FT-IR, voltammetric and molecular modelling. J. Pharm. Anal. 2015, 358, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Maiti, T.K.; Ghosh, K.S.; Debnath, J.; Dasgupta, S. Binding of all-trans retinoic acid to human serum albumin: Fluorescence, FT-IR and circular dichroism studies. Int. J. Biol. Macromol. 2006, 38, 5197–5202. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Wu, P. Investigation on the conformations of AOT in water-in-oil microemulsions using 2D-ATR-FTIR correlation spectroscopy. J. Mol. Struct. 2008, 883–884, 236–240. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Zhou, B.; Liu, Y.-X.; Zhou, C.-X.; Ding, X.-L.; Liu, Y. Fluorescence study on the interaction of Bovine B. Serum Albumin with P-Aminoazobenzene. J. Fluoresc. 2008, 18, 109–118. [Google Scholar] [CrossRef]
- Fonseca, D.P.; Khalil, N.M.; Mainardes, R.M. Bovine serum albumin-based nanoparticles containing resveratrol: Characterization and antioxidant activity. J. Drug Deliv. Sci. Technol. 2017, 39, 147–155. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Zeng, B.; Kang, Q.; Dai, L. Study on the binding of chloroamphenicol with bovine serum albumin by fluorescence and UV–vis spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 105, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Mishra, J. Understanding the intrinsic fluorescence of piperine in microheterogeneous media: Partitioning and loading studies. New J. Chem. 2020, 44, 8317–8324. [Google Scholar] [CrossRef]
- Kaur, A.; Kaur, P.; Ahuja, S. Forster resonance energy transfer (FRET) and applications thereof. Anal. Methods 2020, 12, 5532–5550. [Google Scholar] [CrossRef]
- De Barros Dragana, P.C.; Reed, P.; Alves, M.; Santos, R.; Oliva, A. Biocompatibility and antimicrobial activity of nanostructured lipid carriers for topical applications are affected by type of oils used in their composition. Pharmaceutics 2021, 13, 1950. [Google Scholar] [CrossRef] [PubMed]
- Lacatusu, I.; Badea, N.; Badea, G.; Brasoveanu, L.; Stan, R.; Ott, C.; Oprea, O.; Meghea, A. Ivy leaves extract based–lipid nanocarriers and their bioefficacy on antioxidant and antitumor activities. RSC Adv. 2016, 6, 77243–77255. [Google Scholar] [CrossRef]
- Manayi, A.; Nabavi, S.M.; Setzer, W.N.; Jafari, S. Piperine as a potential anti-cancer agent: A review on preclinical studies. Curr. Med. Chem. 2018, 25, 4918–4928. [Google Scholar] [CrossRef]
- Doktorovová, S.; Kovačević, A.B.; Garcia, M.L.; Souto, E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef]
- Mihaila, M.; Bostan, M.; Hotnog, D.; Ferdes, M.; Brasoveanu, L.I. Real-time analysis of quercetin, resveratrol and /or doxorubicin effects in MCF-7 cells. Rom. Biotechnol. Lett. 2013, 18, 8106–8114. [Google Scholar]
- Chatterjee, M.; Robb, R.; Vedaie, M.; Seum, S.; Thirumoorthy, K.; Palanichamy, K.; Harbrecht, M.; Chakravarti, A.; Terence, M.W. Caveolae-mediated endocytosis is critical for albumin cellular uptake and response to albumin-bound chemotherapy. Cancer Res. 2017, 77, 5925–5937. [Google Scholar] [CrossRef] [Green Version]
- Bostan, M.; Mihaila, M.; Petrica-Matei, G.G.; Radu, N.; Hainarosie, R.; Stefanescu, C.D.; Roman, V.; Diaconu, C.C. Resveratrol modulation of apoptosis and cell cycle response to cisplatin in head and neck cancer cell lines. Int. J. Mol. Sci. 2021, 22, 6322–6342. [Google Scholar] [CrossRef]
- Jo, M.; Park, M.H.; Kollipara, P.S.; An, B.J.; Song, H.S.; Han, S.B.; Kim, J.H.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors (DR) and inhibition of JAK2/STAT3 pathway. Toxicol. Appl. Pharmacol. 2012, 258, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Hotnog, D.; Mihaila, M.; Botezatu, A.; Matei, G.G.; Hotnog, C.; Anton, G.; Bostan, M.; Brasoveanu, L.I. Genistein potentiates the apoptotic effect of 5-fluorouracyl in colon cancer cell lines. Rom. Biotechnol. Lett. 2013, 18, 255–265. [Google Scholar]
- Zhao, Y.; Zhang, L.; Feng, S.; Hong, L.; Zheng, H.; Chen, L.; Zheng, X.; Ye, Y.; Zhao, M.; Wang, W.; et al. Efficient delivery of Notch1 siRNA to SKOV3 cells by cationic cholesterol derivative-based liposome. Int. J. Nanomed. 2016, 11, 5485–5496. [Google Scholar] [CrossRef] [PubMed] [Green Version]






















| (A) | |||||||
|---|---|---|---|---|---|---|---|
| NLCs Formulations | Lipid Phase | Aqueous Phase | |||||
| GSM (g) | CB (g) | TO (g) | Pip (g) | AOT (g) | PC (g) | TW20 (g) | |
| NLC-I | 3.5 | 3.5 | 3 | - | 0 | 0.3 | 1.7 |
| NLC-II | 3.5 | 3.5 | 3 | - | 0.3 | 0.7 | 1 |
| NLC-III | 3.5 | 3.5 | 3 | 1 | 0.3 | 0.7 | 1 |
| (B) | |||||||
| BSA-Coated NLCs Formulations | BSA Solutions Conc. (mg/mL) | BSA: NLC Ratio (% wt.) | |||||
| NLC-I/II/III-BSA-1 | 1 | 0.01 | |||||
| NLC-I/II/III-BSA-2 | 5 | 0.05 | |||||
| NLC-I/II/III-BSA-3 | 10 | 0.1 | |||||
| NLC-I/II/III-BSA-4 | 20 | 0.2 | |||||
| NLC Formulations and Drugs/Cell Lines | IC50 (μg/mL) | |||
|---|---|---|---|---|
| HUVEC | LoVo | MCF-7 | SKOV-3 | |
| NLC-III-Pip | 231.10 ± 2.1 | 191.76 ± 1.3 | 109.42 ± 1.6 | 177.00 ± 1.1 |
| NLC-III-Pip-BSA-3 | 292.43 ± 1.9 | 224.67 ± 1.1 | 209.13 ± 1.4 | 247.88 ± 1.2 |
| Cis-Pt | - | 144.25 ± 1.2 | - | 113.45 ± 0.9 |
| DOX | - | - | 12.11 ± 1.0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tincu, R.; Mihaila, M.; Bostan, M.; Teodorescu, F.; Istrati, D.; Badea, N.; Lacatusu, I. Novel Bovine Serum Albumin-Decorated–Nanostructured Lipid Carriers Able to Modulate Apoptosis and Cell-Cycle Response in Ovarian, Breast, and Colon Tumoral Cells. Pharmaceutics 2023, 15, 1125. https://doi.org/10.3390/pharmaceutics15041125
Tincu R, Mihaila M, Bostan M, Teodorescu F, Istrati D, Badea N, Lacatusu I. Novel Bovine Serum Albumin-Decorated–Nanostructured Lipid Carriers Able to Modulate Apoptosis and Cell-Cycle Response in Ovarian, Breast, and Colon Tumoral Cells. Pharmaceutics. 2023; 15(4):1125. https://doi.org/10.3390/pharmaceutics15041125
Chicago/Turabian StyleTincu, Robert, Mirela Mihaila, Marinela Bostan, Florina Teodorescu, Daniela Istrati, Nicoleta Badea, and Ioana Lacatusu. 2023. "Novel Bovine Serum Albumin-Decorated–Nanostructured Lipid Carriers Able to Modulate Apoptosis and Cell-Cycle Response in Ovarian, Breast, and Colon Tumoral Cells" Pharmaceutics 15, no. 4: 1125. https://doi.org/10.3390/pharmaceutics15041125