Oxidative Stress in Arterial Hypertension (HTN): The Nuclear Factor Erythroid Factor 2-Related Factor 2 (Nrf2) Pathway, Implications and Future Perspectives
Abstract
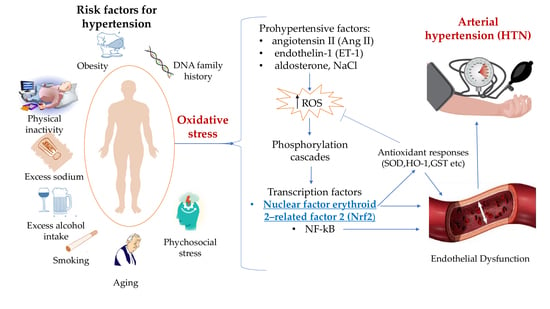
:1. Introduction
2. Oxidative Stress in Hypertension
2.1. ROS and Nitric Oxide
2.2. Mitochondria
2.3. NADPH Oxidase (Nox)
2.4. XOR
3. Nrf2
Nrf2 in Hypertension
4. Therapeutic Options in HTN
4.1. Nrf2 Activators: Bardoxolone Methyl, Sulforaphane, and Dimethyl Fumarate
4.2. Natural Nrf2 Activators
4.3. Other Therapeutic Options via Nrf2
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for Themanagement of Arterial Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Gari, A.; Elshony, N.; Shaheen, H.M.; Abubakar, M.B.; Adeyemi, S.B.; Al-kuraishy, H.M. Hypertension and its management in COVID-19 patients: The assorted view. Int. J. Cardiol. Cardiovasc. Risk Prev. 2021, 11, 200121. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Gong, Z.; Xia, H.; Wang, Y.; Wang, X.; Huang, Y.; Barajas-Martinez, H.; Hu, D. Hypertension as a sequela in patients of SARS-CoV-2 infection. PLoS ONE 2021, 16, e0250815. [Google Scholar] [CrossRef]
- Savoia, C.; Volpe, M.; Kreutz, R. Hypertension, a moving target in COVID-19: Current views and perspectives. Circ. Res. 2021, 128, 1062–1079. [Google Scholar] [CrossRef]
- Muhamad, S.A.; Ugusman, A.; Kumar, J.; Skiba, D.; Hamid, A.A.; Aminuddin, A. COVID-19 and Hypertension: The What, the Why, and the How. Front. Physiol. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Patrick, D.M.; Van Beusecum, J.P.; Kirabo, A. The role of inflammation in hypertension: Novel concepts. Curr. Opin. Physiol. 2021, 19, 92–98. [Google Scholar] [CrossRef]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative Stress and Hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef]
- Janczura, M.; Rosa, R.; Dropinski, J.; Gielicz, A.; Stanisz, A.; Kotula-Horowitz, K.; Domagala, T. The associations of perceived and oxidative stress with hypertension in a cohort of police officers. Diabetes Metab. Syndr. Obes. 2021, 14, 1783–1797. [Google Scholar] [CrossRef]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.L.; Montezano, A.C. Oxidative Stress: A Unifying Paradigm in Hypertension. Can. J. Cardiol. 2020, 36, 659–670. [Google Scholar] [CrossRef] [Green Version]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Chaudhary, P.; Pandey, A.; Azad, C.S.; Tia, N.; Singh, M.; Gambhir, I.S. Association of oxidative stress and endothelial dysfunction in hypertension. Anal. Biochem. 2020, 590, 113535. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Serra, A.J.; Pinto, J.R.; Prokić, M.D.; Arsa, G.; Vasconsuelo, A. Oxidative Stress in Muscle Diseases: Current and Future Therapy 2019. Oxid. Med. Cell. Longev. 2020, 2020, 4–7. [Google Scholar] [CrossRef]
- Sies, H. Oxidative eustress: On constant alert for redox homeostasis. Redox Biol. 2021, 41, 101867. [Google Scholar] [CrossRef]
- Qiu, D.; Wu, J.; Li, M.; Wang, L.; Zhu, X.; Chen, Y. Impaction of factors associated with oxidative stress on the pathogenesis of gestational hypertension and preeclampsia: A Chinese patients based study. Medicine 2021, 100, e23666. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 1–21. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS generation and antioxidant defense systems in normal and malignant cells. Oxid. Med. Cell. Longev. 2020, 2019, 6175804. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Ren, K.; Liu, S.H.; Li, W.M.; Huang, C.J.; Yang, X.H. MicroRNA-140-5p aggravates hypertension and oxidative stress of atherosclerosis via targeting Nrf2 and Sirt2. Int. J. Mol. Med. 2019, 43, 839–849. [Google Scholar] [CrossRef] [Green Version]
- Masi, S.; Georgiopoulos, G.; Chiriacò, M.; Grassi, G.; Seravalle, G.; Savoia, C.; Volpe, M.; Taddei, S.; Rizzoni, D.; Virdis, A. The importance of endothelial dysfunction in resistance artery remodelling and cardiovascular risk. Cardiovasc. Res. 2020, 116, 429–437. [Google Scholar] [CrossRef]
- Pinheiro, L.C.; Oliveira-Paula, G.H. Sources and Effects of Oxidative Stress in Hypertension. Curr. Hypertens. Rev. 2019, 16, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Sousa, T.; Reina-Couto, M.; Gomes, P. Role of Oxidative Stress in the Pathophysiology of Arterial Hypertension and Heart Failure. In Oxidative Stress in Heart Diseases; Chakraborti, S., Dhalla, N.S., Ganguly, N.K., Dikshit, M., Eds.; Springer: Singapore, 2019; pp. 509–537. ISBN 978-981-13-8273-4. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, Y.; Ramprasath, T.; Zou, M.H. Oxidative Stress, GTPCH1, and Endothelial Nitric Oxide Synthase Uncoupling in Hypertension. Antioxid. Redox Signal. 2021, 34, 750–764. [Google Scholar] [CrossRef] [PubMed]
- Rudyk, O.; Aaronson, P.I. Redox Regulation, Oxidative Stress, and Inflammation in Group 3 Pulmonary Hypertension. Adv. Exp. Med. Biol. 2021, 1303, 209–241. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Narasimhan, M.; Rajasekaran, N.S. Exercise, Nrf2 and antioxidant signaling in cardiac aging. Front. Physiol. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, X.; Huang, Y.; Liao, B.; Cheng, L.; Ren, B. Reactive Oxygen Species in Pathogen Clearance: The Killing Mechanisms, the Adaption Response, and the Side Effects. Front. Microbiol. 2021, 11, 3610. [Google Scholar] [CrossRef]
- Maldonado, E.; Rojas, D.A.; Morales, S.; Miralles, V.; Solari, A. Dual and Opposite Roles of Reactive Oxygen Species (ROS) in Chagas Disease: Beneficial on the Pathogen and Harmful on the Host. Oxid. Med. Cell. Longev. 2020, 2020, 8867701. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxid. Med. Cell. Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef]
- Casas, A.I.; Dao, V.T.-V.; Daiber, A.; Maghzal, G.J.; Di Lisa, F.; Kaludercic, N.; Leach, S.; Cuadrado, A.; Jaquet, V.; Seredenina, T.; et al. Reactive Oxygen-Related Diseases: Therapeutic Targets and Emerging Clinical Indications. Antioxid. Redox Signal. 2015, 23, 1171–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.; Michel, L.Y.M.; Balligand, J.-L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef] [PubMed]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef]
- Kosutova, M.; Pechanova, O.; Barta, A.; Franova, S.; Cebova, M. Different adaptive NO-dependent Mechanisms in Normal and Hypertensive Conditions. Molecules 2019, 24, 1682. [Google Scholar] [CrossRef] [Green Version]
- Costa, E.D.; Rezende, B.A.; Cortes, S.F.; Lemos, V.S. Neuronal nitric oxide synthase in vascular physiology and diseases. Front. Physiol. 2016, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bondonno, C.P.; Croft, K.D.; Hodgson, J.M. Dietary Nitrate, Nitric Oxide, and Cardiovascular Health. Crit. Rev. Food Sci. Nutr. 2016, 56, 2036–2052. [Google Scholar] [CrossRef] [Green Version]
- Mónica, F.Z.; Bian, K.; Murad, F. Chapter One—The Endothelium-Dependent Nitric Oxide–cGMP Pathway. In Endothelium; Khalil, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 77, pp. 1–27. ISBN 1054-3589. [Google Scholar] [CrossRef]
- Gao, L.; Siu, K.L.; Chalupsky, K.; Nguyen, A.; Chen, P.; Weintraub, N.L.; Galis, Z.; Cai, H. Role of Uncoupled eNOS in Abdominal Aortic Aneurysm Formation: Treatment with Folic Acid. Hypertension 2012, 59, 158–166. [Google Scholar] [CrossRef]
- Fleming, I. Chapter 23—NO Signaling Defects in Hypertension. In Nitric Oxide: Biology and Pathobiology, 3rd ed.; Ignarro, L.J., Freeman, B.A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 301–311. ISBN 978-0-12-804273-1. [Google Scholar] [CrossRef]
- Higashi, Y.; Sasaki, S.; Nakagawa, K.; Fukuda, Y.; Matsuura, H.; Oshima, T.; Chayama, K. Tetrahydrobiopterin enhances forearm vascular response to acetylcholine in both normotensive and hypertensive individuals. Am. J. Hypertens. 2002, 15, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wong, H.S. Are mitochondria the main contributor of reactive oxygen species in cells. J. Exp. Biol. 2021, 224, jeb221606. [Google Scholar] [CrossRef]
- Xin, T.; Lv, W.; Liu, D.; Jing, Y.; Hu, F. Opa1 Reduces Hypoxia-Induced Cardiomyocyte Death by Improving Mitochondrial Quality Control. Front. Cell Dev. Biol. 2020, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Dikalova, A.E. Contribution of mitochondrial oxidative stress to hypertension. Curr. Opin. Nephrol. Hypertens. 2016, 25, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Togliatto, G.; Lombardo, G.; Brizzi, M.F. The future challenge of reactive oxygen species (ROS) in hypertension: From bench to bed side. Int. J. Mol. Sci. 2017, 18, 1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikalov, S.I.; Nazarewicz, R.R. Angiotensin II-induced production of mitochondrial reactive oxygen species: Potential mechanisms and relevance for cardiovascular disease. Antioxid. Redox Signal. 2013, 19, 1085–1094. [Google Scholar] [CrossRef]
- García-Navas, R.; Liceras-Boillos, P.; Gómez, C.; Baltanás, F.C.; Calzada, N.; Nuevo-Tapioles, C.; Cuezva, J.M.; Santos, E. Critical requirement of SOS1 RAS-GEF function for mitochondrial dynamics, metabolism, and redox homeostasis. Oncogene 2021, 40, 4538–4551. [Google Scholar] [CrossRef]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef] [Green Version]
- Emmerson, A.; Trevelin, S.C.; Mongue-Din, H.; Becker, P.D.; Ortiz, C.; Smyth, L.A.; Peng, Q.; Elgueta, R.; Sawyer, G.; Ivetic, A.; et al. Nox2 in regulatory T cells promotes angiotensin II–induced cardiovascular remodeling. J. Clin. Investig. 2018, 128, 3088–3101. [Google Scholar] [CrossRef] [Green Version]
- Hashad, A.M.; Sancho, M.; Brett, S.E.; Welsh, D.G. Reactive Oxygen Species Mediate the Suppression of Arterial Smooth Muscle T-type Ca2+ Channels by Angiotensin II. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Gray, S.P.; Shah, A.M.; Smyrnias, I. NADPH oxidase 4 and its role in the cardiovascular system. Vasc. Biol. 2019, 1, H59–H66. [Google Scholar] [CrossRef] [Green Version]
- Birk, M.; Baum, E.; Zadeh, J.K.; Manicam, C.; Pfeiffer, N.; Patzak, A.; Helmstädter, J.; Steven, S.; Kuntic, M.; Daiber, A.; et al. Angiotensin ii induces oxidative stress and endothelial dysfunction in mouse ophthalmic arteries via involvement of at1 receptors and nox2. Antioxidants 2021, 10, 1238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020, 17, 170–194. [Google Scholar] [CrossRef]
- Harrison, C.B.; Trevelin, S.C.; Richards, D.A.; Santos, C.X.C.; Sawyer, G.; Markovinovic, A.; Zhang, X.; Zhang, M.; Brewer, A.C.; Yin, X.; et al. Fibroblast Nox2 (NADPH Oxidase-2) Regulates ANG II (Angiotensin II)-Induced Vascular Remodeling and Hypertension via Paracrine Signaling to Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2021, 2, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Ali Shah, S.M.; Munir, N.; Daniyal, M.; Tahir, I.M.; Mahmood, Z.; Irshad, M.; Akhlaq, M.; Sultana, S.; Zainab, R. Hexose monophosphate shunt, the role of its metabolites and associated disorders: A review. J. Cell. Physiol. 2019, 9, 14473–14482. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-Y.; Lee, D.-Y.; Chun, K.-S.; Kim, E.-H. The Role of NRF2/KEAP1 Signaling Pathway in Cancer Metabolism. Int. J. Mol. Sci. 2021, 22, 4376. [Google Scholar] [CrossRef]
- Hashimoto, R.; Gupte, S. Pentose Shunt, Glucose-6-Phosphate Dehydrogenase, NADPH Redox, and Stem Cells in Pulmonary Hypertension. Adv. Exp. Med. Biol. 2017, 967, 47–55. [Google Scholar] [CrossRef]
- Packer, M. Uric Acid Is a Biomarker of Oxidative Stress in the Failing Heart: Lessons Learned from Trials With Allopurinol and SGLT2 Inhibitors. J. Card. Fail. 2020, 26, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Mervaala, E.; Cheng, Z.; Tikkanen, I.; Lapatto, R.; Nurminen, K.; Vapaatalo, H.; Müller, D.; Fiebeler, A.; Ganten, U.; Ganten, D.; et al. Endothelial Dysfunction and Xanthine Oxidoreductase Activity in Rats With Human Renin and Angiotensinogen Genes. Hypertension 2001, 37, 414–418. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bolognesi, A. Xanthine oxidoreductase in atherosclerosis pathogenesis: Not only oxidative stress. Atherosclerosis 2014, 237, 562–567. [Google Scholar] [CrossRef] [Green Version]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA 1994, 91, 9926–9930. [Google Scholar] [CrossRef] [Green Version]
- Liou, S.F.; Nguyen, T.T.N.; Hsu, J.H.; Sulistyowati, E.; Huang, S.E.; Wu, B.N.; Lin, M.C.; Yeh, J.L. The preventive effects of xanthohumol on vascular calcification induced by vitamin D3 plus nicotine. Antioxidants 2020, 9, 956. [Google Scholar] [CrossRef] [PubMed]
- Sarutipaiboon, I.; Settasatian, N.; Komanasin, N.; Kukongwiriyapan, U.; Sawanyawisuth, K.; Intharaphet, P.; Senthong, V.; Settasatian, C. Association of Genetic Variations in NRF2, NQO1, HMOX1, and MT with Severity of Coronary Artery Disease and Related Risk Factors. Cardiovasc. Toxicol. 2020, 20, 176–189. [Google Scholar] [CrossRef]
- Yagishita, Y.; Gatbonton-schwager, T.N.; McCallum, M.L.; Kensler, T.W. Current landscape of NRF2 biomarkers in clinical trials. Antioxidants 2020, 9, 716. [Google Scholar] [CrossRef]
- Zgorzynska, E.; Dziedzic, B.; Walczewska, A. An overview of the nrf2/are pathway and its role in neurodegenerative diseases. Int. J. Mol. Sci. 2021, 22, 9592. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.; Cadenas, S. The Antioxidant Transcription Factor Nrf2 in Cardiac Ischemia-Reperfusion Injury. Int. J. Mol. Sci. 2021, 22, 11939. [Google Scholar] [CrossRef]
- Tan, X.; Jiao, P.L.; Sun, J.C.; Wang, W.; Ye, P.; Wang, Y.K.; Leng, Y.Q.; Wang, W.Z. β-Arrestin1 Reduces Oxidative Stress via Nrf2 Activation in the Rostral Ventrolateral Medulla in Hypertension. Front. Neurosci. 2021, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Lee, K.-M.; Lee, H.J.; Yun, J.H.; Nho, C.W. Physalin A regulates the Nrf2 pathway through ERK and p38 for induction of detoxifying enzymes. BMC Complement. Altern. Med. 2019, 19, 101. [Google Scholar] [CrossRef] [Green Version]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-Kinase/Akt Signaling and Redox Metabolism in Cancer. Front. Oncol. 2018, 8, 160. [Google Scholar] [CrossRef]
- Matzinger, M.; Fischhuber, K.; Pölöske, D.; Mechtler, K.; Heiss, E.H. AMPK leads to phosphorylation of the transcription factor Nrf2, tuning transactivation of selected target genes. Redox Biol. 2020, 29, 101393. [Google Scholar] [CrossRef]
- Liu, H.; Johnston, L.J.; Wang, F.; Ma, X. Triggers for the nrf2/are signaling pathway and its nutritional regulation: Potential therapeutic applications of ulcerative colitis. Int. J. Mol. Sci. 2021, 22, 11411. [Google Scholar] [CrossRef]
- Silva-Islas, C.A.; Maldonado, P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018, 134, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lv, Y.-F.; Zhao, J.-L.; You, Q.-D.; Jiang, Z.-Y. Regulation of Nrf2 by phosphorylation: Consequences for biological function and therapeutic implications. Free Radic. Biol. Med. 2021, 168, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lu, Y.; Chen, Y.; Cheng, J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 2015, 225, R83–R99. [Google Scholar] [CrossRef] [Green Version]
- Zang, H.; Mathew, R.O.; Cui, T. The Dark Side of Nrf2 in the Heart. Front. Physiol. 2020, 11, 722. [Google Scholar] [CrossRef]
- Kannan, S.; Muthusamy, V.R.; Whitehead, K.J.; Wang, L.; Gomes, A.V.; Litwin, S.E.; Kensler, T.W.; Abel, E.D.; Hoidal, J.R.; Rajasekaran, N.S. Nrf2 deficiency prevents reductive stress-induced hypertrophic cardiomyopathy. Cardiovasc. Res. 2013, 100, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Qin, Q.; Qu, C.; Niu, T.; Zang, H.; Qi, L.; Lyu, L.; Wang, X.; Nagarkatti, M.; Nagarkatti, P.; Janicki, J.S.; et al. Nrf2-Mediated Cardiac Maladaptive Remodeling and Dysfunction in a Setting of Autophagy Insufficiency. Hypertension 2016, 67, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Ooi, B.K.; Goh, B.H.; Yap, W.H. Oxidative stress in cardiovascular diseases: Involvement of Nrf2 antioxidant redox signaling in macrophage foam cells formation. Int. J. Mol. Sci. 2017, 18, 2336. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, R.M.; Rodrigues, D.; Pereira, C.A.; Silva, J.F.; Alves, J.V.; Lobato, N.S.; Tostes, R.C. Nrf2 as a potential mediator of cardiovascular risk in metabolic diseases. Front. Pharmacol. 2019, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Karan, A.; Bhakkiyalakshmi, E.; Jayasuriya, R.; Sarada, D.V.L.; Ramkumar, K.M. The pivotal role of nuclear factor erythroid 2-related factor 2 in diabetes-induced endothelial dysfunction. Pharmacol. Res. 2020, 153, 104601. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Sehgal, A.; Sharma, E.; Kumar, A.; Grover, M.; Bungau, S. Unfolding Nrf2 in diabetes mellitus. Mol. Biol. Rep. 2021, 48, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Bayliak, M.M.; Abrat, O.B. Role of Nrf2 in Oxidative and Inflammatory Processes in Obesity and Metabolic Diseases BT—Nrf2 and its Modulation in Inflammation. In Nrf2 and Its Modulation in Inflammation; Deng, H., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 153–187. ISBN 978-3-030-44599-7. [Google Scholar] [CrossRef]
- Li, S.; Eguchi, N.; Lau, H.; Ichii, H. The Role of the Nrf2 Signaling in Obesity and Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 6973. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, L.V.; Savova, M.S.; Amirova, K.M.; Dinkova-Kostova, A.T.; Georgiev, M.I. Obesity and NRF2-mediated cytoprotection: Where is the missing link? Pharmacol. Res. 2020, 156, 104760. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; León, R.; López, M.G.; Oliva, B.; et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef] [Green Version]
- McSweeney, S.R.; Warabi, E.; Siow, R.C.M. Nrf2 as an Endothelial Mechanosensitive Transcription Factor: Going With the Flow. Hypertension 2016, 67, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Polvani, S.; Tarocchi, M.; Galli, A. PPARγ and Oxidative Stress: Conβ) Catenating NRF2 and FOXO. PPAR Res. 2012, 2012, 641087. [Google Scholar] [CrossRef] [Green Version]
- Kvandová, M.; Majzúnová, M.; Dovinová, I. The role of PPARγ in cardiovascular diseases. Physiol. Res. 2016, 65, S343–S363. [Google Scholar] [CrossRef]
- Dovinova, I.; Kvandova, M.; Balis, P.; Gresova, L.; Majzunova, M.; Horakova, L.; Chan, J.Y.H.; Barancik, M. The Role of Nrf2 and PPARγ in the Improvement of Oxidative Stress in Hypertension and Cardiovascular Diseases. Physiol. Res. 2020, 69, S541–S553. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [Green Version]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, R.; Khanna, G.; Kaushik, D.; Kuhad, A. Divulging the Intricacies of Crosstalk Between NF-Kb and Nrf2-Keap1 Pathway in Neurological Complications of COVID-19. Mol. Neurobiol. 2021, 58, 3347–3361. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, Z.; Mohammad, R.S.; Lokhandwala, M.F.; Banday, A.A. Nrf2 inhibition induces oxidative stress, renal inflammation and hypertension in mice. Clin. Exp. Hypertens. 2021, 43, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Luo, Z.; Carter, G.; Wellstein, A.; Jose, P.A.; Tomlinson, J.; Leiper, J.; Welch, W.J.; Wilcox, C.S.; Wang, D. NRF2 prevents hypertension, increased ADMA, microvascular oxidative stress, and dysfunction in mice with two weeks of ANG II infusion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R399–R406. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.A.; Neves, K.B.; Tostes, R.C.; Montezano, A.C.; Touyz, R.M. Downregulation of Nuclear Factor Erythroid 2-Related Factor and Associated Antioxidant Genes Contributes to Redox-Sensitive Vascular Dysfunction in Hypertension. Hypertension 2015, 66, 1240–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banday, A.A.; Lokhandwala, M.F. Transcription factor Nrf2 protects renal dopamine D1 receptor function during oxidative stress. Hypertension 2013, 62, 512–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Grotegut, C.A.; Wisler, J.W.; Li, T.; Mao, L.; Chen, M.; Chen, W.; Rosenberg, P.B.; Rockman, H.A.; Lefkowitz, R.J. β-arrestin 1 regulates β2-adrenergic receptor-mediated skeletal muscle hypertrophy and contractility. Skelet. Muscle 2018, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Pugh, D.; Gallacher, P.J.; Dhaun, N. Management of Hypertension in Chronic Kidney Disease. Drugs 2019, 79, 365–379. [Google Scholar] [CrossRef] [Green Version]
- Gray, Z.; Tu, W.; Chertow, G.M.; Bhalla, V. Aldosterone sensitivity: An opportunity to explore the pathogenesis of hypertension. Am. J. Physiol. Physiol. 2021, 320, F325–F335. [Google Scholar] [CrossRef]
- Inoue, K.; Goldwater, D.; Allison, M.; Seeman, T.; Kestenbaum, B.R.; Watson, K.E. Serum Aldosterone Concentration, Blood Pressure, and Coronary Artery Calcium. Hypertension 2020, 76, 113–120. [Google Scholar] [CrossRef]
- Ames, M.K.; Atkins, C.E.; Pitt, B. The renin-angiotensin-aldosterone system and its suppression. J. Vet. Intern. Med. 2019, 33, 363–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, L.; Gao, L.; Lazartigues, E.; Zucker, I.H. Brain-selective overexpression of angiotensin-converting enzyme 2 attenuates sympathetic nerve activity and enhances baroreflex function in chronic heart failure. Hypertension 2011, 58, 1057–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, A.; Gao, L.; Wafi, A.M.; Yu, L.; Rudebush, T.; Zhou, W.; Zucker, I.H. Overexpression of central ACE2 (angiotensin-converting enzyme 2) attenuates the pressor response to chronic central infusion of ang II (angiotensin II): A potential role for Nrf2 (nuclear factor [erythroid-derived 2]-like 2). Hypertension 2020, 2, 1514–1525. [Google Scholar] [CrossRef] [PubMed]
- Satta, S.; Mahmoud, A.M.; Wilkinson, F.L.; Yvonne Alexander, M.; White, S.J. The Role of Nrf2 in Cardiovascular Function and Disease. Oxid. Med. Cell. Longev. 2017, 2017, 9237263. [Google Scholar] [CrossRef] [PubMed]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid. Med. Cell. Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef]
- Kopacz, A.; Kloska, D.; Forman, H.J.; Jozkowicz, A.; Grochot-Przeczek, A. Beyond repression of Nrf2: An update on Keap1. Free Radic. Biol. Med. 2020, 157, 63–74. [Google Scholar] [CrossRef]
- Tkachev, V.O.; Menshchikova, E.B.; Zenkov, N.K. Mechanism of the Nrf2/Keap1/ARE signaling system. Biochemistry 2011, 76, 407–422. [Google Scholar] [CrossRef]
- Chin, M.P.; Bakris, G.L.; Block, G.A.; Chertow, G.M.; Goldsberry, A.; Inker, L.A.; Heerspink, H.J.L.; O’Grady, M.; Pergola, P.E.; Wanner, C.; et al. Bardoxolone Methyl Improves Kidney Function in Patients with Chronic Kidney Disease Stage 4 and Type 2 Diabetes: Post-Hoc Analyses from Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes Study. Am. J. Nephrol. 2018, 47, 40–47. [Google Scholar] [CrossRef]
- Sun, Q.; Ye, F.; Liang, H.; Liu, H.; Li, C.; Lu, R.; Huang, B.; Zhao, L.; Tan, W.; Lai, L. Bardoxolone and bardoxolone methyl, two Nrf2 activators in clinical trials, inhibit SARS-CoV-2 replication and its 3C-like protease. Signal Transduct. Target. Ther. 2021, 6, 2020–2022. [Google Scholar] [CrossRef]
- Nio, Y.; Sasai, M.; Akahori, Y.; Okamura, H.; Hasegawa, H.; Oshima, M.; Watashi, K.; Wakita, T.; Ryo, A.; Tanaka, Y.; et al. Bardoxolone methyl as a novel potent antiviral agent against hepatitis B and C viruses in human hepatocyte cell culture systems. Antivir. Res. 2019, 169, 104537. [Google Scholar] [CrossRef]
- Cuadrado, A.; Pajares, M.; Benito, C.; Jiménez-Villegas, J.; Escoll, M.; Fernández-Ginés, R.; Garcia Yagüe, A.J.; Lastra, D.; Manda, G.; Rojo, A.I.; et al. Can Activation of NRF2 Be a Strategy against COVID-19? Trends Pharmacol. Sci. 2020, 41, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or sulforaphane: Is it the source or dose that matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwon, Y.; Oh, J.; Kim, J.S. Sulforaphane induces colorectal cancer cell proliferation through Nrf2 activation in a p53-dependent manner. Appl. Biol. Chem. 2020, 63, 86. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Suzuki, K. The integrative role of sulforaphane in preventing inflammation, oxidative stress and fatigue: A review of a potential protective phytochemical. Antioxidants 2020, 9, 521. [Google Scholar] [CrossRef]
- Senanayake, G.V.K.; Banigesh, A.; Wu, L.; Lee, P.; Juurlink, B.H.J. The dietary phase 2 protein inducer sulforaphane can normalize the kidney epigenome and improve blood pressure in hypertensive rats. Am. J. Hypertens. 2012, 25, 229–235. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Blair, H.A. Dimethyl Fumarate: A Review in Moderate to Severe Plaque Psoriasis. Drugs 2018, 78, 123–130. [Google Scholar] [CrossRef]
- Lipton, S.; Satoh, T. Recent advances in understanding NRF2 as a druggable target: Development of pro-electrophilic and non-covalent NRF2 activators to overcome systemic side effects of electrophilic drugs like dimethyl fumarate. F1000Research 2017, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Grzegorzewska, A.P.; Seta, F.; Han, R.; Czajka, C.A.; Makino, K.; Stawski, L.; Isenberg, J.S.; Browning, J.L.; Trojanowska, M. Dimethyl Fumarate ameliorates pulmonary arterial hypertension and lung fibrosis by targeting multiple pathways. Sci. Rep. 2017, 7, 41605. [Google Scholar] [CrossRef] [Green Version]
- Oh, C.J.; Park, S.; Kim, J.-Y.; Kim, H.-J.; Jeoung, N.H.; Choi, Y.-K.; Go, Y.; Park, K.-G.; Lee, I.-K. Dimethylfumarate attenuates restenosis after acute vascular injury by cell-specific and Nrf2-dependent mechanisms. Redox Biol. 2014, 2, 855–864. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.N.; Lin, Y.J.; Yu, H.R.; Lin, I.C.; Sheen, J.M.; Huang, L.T.; Tain, Y.L. Protection of male rat offspring against hypertension programmed by prenatal dexamethasone administration and postnatal high-fat diet with the Nrf2 activator dimethyl fumarate during pregnancy. Int. J. Mol. Sci. 2019, 20, 3957. [Google Scholar] [CrossRef] [Green Version]
- Kuang, Y.; Zhang, Y.; Xiao, Z.; Xu, L.; Wang, P.; Ma, Q. Protective effect of dimethyl fumarate on oxidative damage and signaling in cardiomyocytes. Mol. Med. Rep. 2020, 22, 2783–2790. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, F.; Jiang, H.; Xu, D.; Deng, D. Fumaric acid and succinic acid treat gestational hypertension by downregulating the expression of KCNMB1 and TET1. Exp. Ther. Med. 2021, 22, 1072. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.; Koontz, D.; Morse, C.; Roth, E.; Domsic, R.T.; Simon, M.A.; Stratton, E.; Buchholz, C.; Tobin-Finch, K.; Simms, R.; et al. A Pilot Study of Dimethyl Fumarate in Pulmonary Arterial Hypertension Associated with Systemic Sclerosis. J. Scleroderma Relat. Disord. 2021, 6, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Kourakis, S.; Timpani, C.A.; de Haan, J.B.; Gueven, N.; Fischer, D.; Rybalka, E. Dimethyl Fumarate and Its Esters: A Drug with Broad Clinical Utility? Pharmaceuticals 2020, 13, 306. [Google Scholar] [CrossRef]
- Qader, M.; Xu, J.; Yang, Y.; Liu, Y.; Cao, S. Natural nrf2 activators from juices, wines, coffee, and cocoa. Beverages 2020, 6, 68. [Google Scholar] [CrossRef]
- Sengupta, S.; Bhattacharyya, D.; Kasle, G.; Karmakar, S.; Sahu, O.; Ganguly, A.; Addya, S.; Das Sarma, J. Potential Immunomodulatory Properties of Biologically Active Components of Spices Against SARS-CoV-2 and Pan β-Coronaviruses. Front. Cell. Infect. Microbiol. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, M.J.; Shin, Y.; Jung, S.K.; Kim, Y.J. Green pepper (Piper nigrum l.) extract suppresses oxidative stress and lps-induced inflammation via regulation of JNK signaling pathways. Appl. Sci. 2020, 10, 2519. [Google Scholar] [CrossRef] [Green Version]
- Wafi, A.M.; Hong, J.; Rudebush, T.L.; Yu, L.; Hackfort, B.; Wang, H.; Schultz, H.D.; Zucker, I.H.; Gao, L. Curcumin improves exercise performance of mice with coronary artery ligation-induced HFrEF: Nrf2 and antioxidant mechanisms in skeletal muscle. J. Appl. Physiol. 2019, 126, 477–486. [Google Scholar] [CrossRef]
- Ji, K.; Fang, L.; Zhao, H.; Li, Q.; Shi, Y.; Xu, C.; Wang, Y.; Du, L.; Wang, J.; Liu, Q. Ginger Oleoresin Alleviated γ-Ray Irradiation-Induced Reactive Oxygen Species via the Nrf2 Protective Response in Human Mesenchymal Stem Cells. Oxid. Med. Cell. Longev. 2017, 2017, 1480294. [Google Scholar] [CrossRef] [Green Version]
- Mimura, J.; Inose-Maruyama, A.; Taniuchi, S.; Kosaka, K.; Yoshida, H.; Yamazaki, H.; Kasai, S.; Harada, N.; Kaufman, R.J.; Oyadomari, S.; et al. Concomitant Nrf2- and ATF4-Activation by Carnosic Acid Cooperatively Induces Expression of Cytoprotective Genes. Int. J. Mol. Sci. 2019, 20, 1706. [Google Scholar] [CrossRef] [Green Version]
- Mohan Manu, T.; Anand, T.; Sharath Babu, G.R.; Patil, M.M.; Khanum, F. Bacopa monniera extract mitigates isoproterenol-induced cardiac stress via Nrf2/Keap1/NQO1 mediated pathway. Arch. Physiol. Biochem. 2019, 1–11. [Google Scholar] [CrossRef]
- He, T.; Li, X.; Wang, X.; Xu, X.; Yan, X.; Li, X.; Sun, S.; Dong, Y.; Ren, X.; Liu, X.; et al. Chemical composition and anti-oxidant potential on essential oils of Thymus quinquecostatus Celak. from Loess Plateau in China, regulating Nrf2/Keap1 signaling pathway in zebrafish. Sci. Rep. 2020, 10, 11280. [Google Scholar] [CrossRef]
- Korenori, Y.; Tanigawa, S.; Kumamoto, T.; Qin, S.; Daikoku, Y.; Miyamori, K.; Nagai, M.; Hou, D.-X. Modulation of Nrf2/Keap1 system by Wasabi 6-methylthiohexyl isothiocyanate in ARE-mediated NQO1 expression. Mol. Nutr. Food Res. 2013, 57, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Kanlaya, R.; Khamchun, S.; Kapincharanon, C.; Thongboonkerd, V. Protective effect of epigallocatechin-3-gallate (EGCG) via Nrf2 pathway against oxalate-induced epithelial mesenchymal transition (EMT) of renal tubular cells. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Chakraborty, S.; Anand, U.; Dey, S.; Nandy, S.; Ghorai, M.; Saha, S.C.; Patil, M.T.; Kandimalla, R.; Proćków, J.; et al. Withania somnifera (L.) Dunal (Ashwagandha): A comprehensive review on ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological aspects. Biomed. Pharmacother. 2021, 143, 112175. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 2020, 127, 110234. [Google Scholar] [CrossRef]
- Yang, P.M.; Chen, H.Z.; Huang, Y.T.; Hsieh, C.W.; Wung, B.S. Lycopene inhibits NF-κB activation and adhesion molecule expression through Nrf2-mediated heme oxygenase-1 in endothelial cells. Int. J. Mol. Med. 2017, 39, 1533–1540. [Google Scholar] [CrossRef]
- Ramyaa, P.; Padma, V.V. Ochratoxin-induced toxicity, oxidative stress and apoptosis ameliorated by quercetin—Modulation by Nrf2. Food Chem. Toxicol. 2013, 62, 205–216. [Google Scholar] [CrossRef]
- Moussa, Z.; Judeh, Z.; Ahmed, S. Nonenzymatic Exogenous and Endogenous Antioxidants. In Free Radical Medicine and Biology; IntechOpen: London, UK, 2019; ISBN 978-1-78985-143-4. [Google Scholar] [CrossRef] [Green Version]
- Dias, T.R.; Martin-Hidalgo, D.; Silva, B.M.; Oliveira, P.F.; Alves, M.G. Endogenous and Exogenous Antioxidants As a Tool to Ameliorate Male Infertility Induced by Reactive Oxygen Species. Antioxid. Redox Signal. 2020, 33, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhao, Z.; Zhang, W.; Liu, D.; Ma, C.; Zhang, T.; Meng, Q.; Yan, P.; Zou, L.; Zhang, M. Natural Antioxidants Improve the Vulnerability of Cardiomyocytes and Vascular Endothelial Cells under Stress Conditions: A Focus on Mitochondrial Quality Control. Oxid. Med. Cell. Longev. 2021, 2021, 6620677. [Google Scholar] [CrossRef]
- Seco-Cervera, M.; González-Cabo, P.; Pallardó, F.V.; Romá-Mateo, C.; García-Giménez, J.L. Thioredoxin and glutaredoxin systems as potential targets for the development of new treatments in Friedreich’s ataxia. Antioxidants 2020, 9, 1257. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.K.; Lekli, I.; Ray, D.; Yodoi, J.; Das, D.K. Redox regulation of cell survival by the thioredoxin superfamily: An implication of redox gene therapy in the heart. Antioxid. Redox Signal. 2009, 11, 2741–2758. [Google Scholar] [CrossRef] [Green Version]
- Suresh, S.C.; Selvaraju, V.; Thirunavukkarasu, M.; Goldman, J.W.; Husain, A.; Alexander Palesty, J.; Sanchez, J.A.; McFadden, D.W.; Maulik, N. Thioredoxin-1 (Trx1) engineered mesenchymal stem cell therapy increased pro-angiogenic factors, reduced fibrosis and improved heart function in the infarcted rat myocardium. Int. J. Cardiol. 2015, 201, 517–528. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.-L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [Green Version]
- Gupte, S.A.; Li, K.-X.; Okada, T.; Sato, K.; Oka, M. Inhibitors of pentose phosphate pathway cause vasodilation: Involvement of voltage-gated potassium channels. J. Pharmacol. Exp. Ther. 2002, 301, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Alzoubi, A.; Toba, M.; Abe, K.; O’Neill, K.D.; Rocic, P.; Fagan, K.A.; McMurtry, I.F.; Oka, M. Dehydroepiandrosterone restores right ventricular structure and function in rats with severe pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1708–H1718. [Google Scholar] [CrossRef] [Green Version]
- Ventetuolo, C.E.; Baird, G.L.; Barr, R.G.; Bluemke, D.A.; Fritz, J.S.; Hill, N.S.; Klinger, J.R.; Lima, J.A.C.; Ouyang, P.; Palevsky, H.I.; et al. Higher Estradiol and Lower Dehydroepiandrosterone-Sulfate Levels Are Associated with Pulmonary Arterial Hypertension in Men. Am. J. Respir. Crit. Care Med. 2016, 193, 1168–1175. [Google Scholar] [CrossRef]
- Dumas de La Roque, E.; Savineau, J.-P.; Metivier, A.-C.; Billes, M.-A.; Kraemer, J.-P.; Doutreleau, S.; Jougon, J.; Marthan, R.; Moore, N.; Fayon, M.; et al. Dehydroepiandrosterone (DHEA) improves pulmonary hypertension in chronic obstructive pulmonary disease (COPD): A pilot study. Ann. Endocrinol. 2012, 73, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, V.R.; Leibold, J.; Mina, M.; Mohan, P.; Berishaj, M.; Li, Z.; Miele, M.M.; Lailler, N.; Zhao, C.; de Stanchina, E.; et al. The Oncogenic Action of NRF2 Depends on De-glycation by Fructosamine-3-Kinase. Cell 2019, 178, 807–819.e21. [Google Scholar] [CrossRef]
- Beeraka, N.M.; Bovilla, V.R.; Doreswamy, S.H.; Puttalingaiah, S.; Srinivasan, A.; Madhunapantula, S. V The Taming of Nuclear Factor Erythroid-2-Related Factor-2 (Nrf2) Deglycation by Fructosamine-3-Kinase (FN3K)-Inhibitors-A Novel Strategy to Combat Cancers. Cancers 2021, 13, 281. [Google Scholar] [CrossRef]
- Sartore, G.; Ragazzi, E.; Burlina, S.; Paleari, R.; Chilelli, N.C.; Mosca, A.; Avemaria, F.; Lapolla, A. Role of fructosamine-3-kinase in protecting against the onset of microvascular and macrovascular complications in patients with T2DM. BMJ Open Diabetes Res. Care 2020, 8, e001256. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Chlopicki, S. Revisiting pharmacology of oxidative stress and endothelial dysfunction in cardiovascular disease: Evidence for redox-based therapies. Free Radic. Biol. Med. 2020, 157, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. npj Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Authors and Ref. | Natural Compound | Organic Compound | Species and/or Cells Researched | Meaningful Findings |
|---|---|---|---|---|
| Kim et al. [132] | Pepper | Methysticin | Murine cell cultures murine RAW 264.7 cell line |
|
| Wafi et al. [133] | Turmeric | Curcumin | Sixty male C57BL/6 mice 10 weeks of age |
|
| Ji et al. [134] | Ginger | 6-Dehydrogingerdione | Human mesenchymal stem cells |
|
| Mimura et al. [135] | Rosemary | Carnosic acid | U373MG cells (human glioblastoma astrocytoma cells) |
|
| Mohan Manu et al. [136] | Water hyssop (Bacopa monnieri) | Dammarane-type triterpenoid saponins | Adult male Wistar rats |
|
| He et al. [137] | Thyme | Thymol | Zebrafish |
|
| Korenori et al. [138] | Wasabi | Allyl isothiocyanate | HepG2 (human hepatoma) |
|
| Kanlaya et al. [139] | Green tea | Catechins | Madin–Darby Canine Kidney (MDCK) renal tubular cells |
|
| Paul et al. [140] | Ashwagandha | Triterpene lactones | Coronary artery occlusion in rats; myocardial infarction in rats |
|
| Farkhondeh et al. [141] | Grapes, berries, cranberries, nuts, cocoa, and dark chocolate | Resveratrol | Adult male Sprague-Dawley rats |
|
| Yang et al. [142] | Tomatoes, watermelons, red carrots, grapefruits, and papayas | Lycopene | Human umbilical vein cell line |
|
| Ramyaa et al. [143] | Apples, citrus fruits, onions, green leafy vegetables, honey | Quercetin | Vero cells (African green monkey kidney epithelial cells) |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanase, D.M.; Apostol, A.G.; Costea, C.F.; Tarniceriu, C.C.; Tudorancea, I.; Maranduca, M.A.; Floria, M.; Serban, I.L. Oxidative Stress in Arterial Hypertension (HTN): The Nuclear Factor Erythroid Factor 2-Related Factor 2 (Nrf2) Pathway, Implications and Future Perspectives. Pharmaceutics 2022, 14, 534. https://doi.org/10.3390/pharmaceutics14030534
Tanase DM, Apostol AG, Costea CF, Tarniceriu CC, Tudorancea I, Maranduca MA, Floria M, Serban IL. Oxidative Stress in Arterial Hypertension (HTN): The Nuclear Factor Erythroid Factor 2-Related Factor 2 (Nrf2) Pathway, Implications and Future Perspectives. Pharmaceutics. 2022; 14(3):534. https://doi.org/10.3390/pharmaceutics14030534
Chicago/Turabian StyleTanase, Daniela Maria, Alina Georgiana Apostol, Claudia Florida Costea, Claudia Cristina Tarniceriu, Ionut Tudorancea, Minela Aida Maranduca, Mariana Floria, and Ionela Lacramioara Serban. 2022. "Oxidative Stress in Arterial Hypertension (HTN): The Nuclear Factor Erythroid Factor 2-Related Factor 2 (Nrf2) Pathway, Implications and Future Perspectives" Pharmaceutics 14, no. 3: 534. https://doi.org/10.3390/pharmaceutics14030534