Nanomedicine in Hepatocellular Carcinoma: A New Frontier in Targeted Cancer Treatment
Abstract
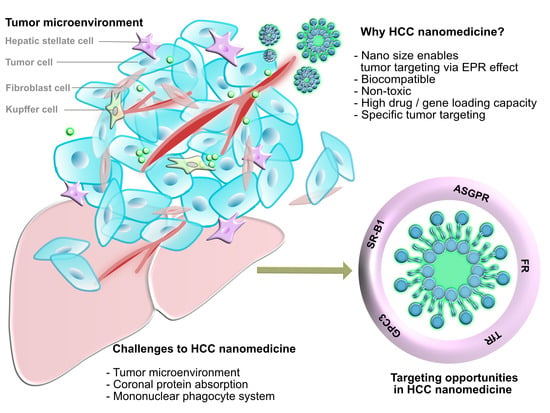
:1. Introduction
2. Progress in HCC Nanomedicine
3. Current Challenges in Treating HCC
3.1. Tumor Microenvironment
3.2. Physiological Barriers to Nanomedicine Targeting HCC Cells
3.2.1. Coronal Protein Adsorption
3.2.2. Mononuclear Phagocyte System
4. Targeting Opportunities for HCC Nanomedicine
4.1. Surface Biomarkers for Specific Targeting to HCC
4.1.1. Asialoglycoprotein Receptor (ASGPR)
4.1.2. Glypican-3 (GPC3)
4.1.3. Transferrin Receptor (TfR)
4.1.4. Folic Acid Receptor (FR)
4.1.5. Scavenger Receptor Class B Type I (SR-B1)
4.2. Emerging Use of Peptides in HCC Therapy as an Evolutionary Improvement in HCC Nanomedicine
4.3. Nanomedicine as a Vehicle for Delivery of Chemotherapy and siRNA into HCC Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wong, C.-M.; Tsang, F.H.-C.; Ng, I.O.-L. Non-coding RNAs in hepatocellular carcinoma: Molecular functions and pathological implications. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, R.; Limaye, A.; Cabrera, R. Hepatocellular carcinoma: Current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepatic Med. Evid. Res. 2012, 4, 19. [Google Scholar]
- Nault, J.-C.; Datta, S.; Imbeaud, S.; Franconi, A.; Mallet, M.; Couchy, G.; Letouzé, E.; Pilati, C.; Verret, B.; Blanc, J.-F. Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas. Nat. Genet. 2015, 47, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Kew, M.C. Aflatoxins as a cause of hepatocellular carcinoma. J. Gastrointest. Liver Dis. 2013, 22, 305–310. [Google Scholar]
- Magnussen, A.; Parsi, M.A. Aflatoxins, hepatocellular carcinoma and public health. World J. Gastroenterol. 2013, 19, 1508. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Santella, R. The role of aflatoxins in hepatocellular carcinoma. Hepat. Mon. 2012, 12, e7238. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.-Y.; Zhu, G.-Q.; Liu, T.; Zheng, J.-N.; Cheng, Z.; Zou, T.-T.; Braddock, M.; Fu, S.-W.; Zheng, M.-H. Systematic review with network meta-analysis: Antidiabetic medication and risk of hepatocellular carcinoma. Sci. Rep. 2016, 6, 33743. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Taniguchi, E.; Morita, Y.; Shirachi, M.; Tateishi, I.; Nagata, E.; Sata, M. Association of exogenous insulin or sulphonylurea treatment with an increased incidence of hepatoma in patients with hepatitis C virus infection. Liver Int. 2010, 30, 479–486. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P.P.; Singh, A.G.; Murad, M.H.; Sanchez, W. Anti-diabetic medications and the risk of hepatocellular cancer: A systematic review and meta-analysis. Off. J. Am. Coll. Gastroenterol. 2013, 108, 881–891. [Google Scholar] [CrossRef]
- Chen, H.-P.; Shieh, J.-J.; Chang, C.-C.; Chen, T.-T.; Lin, J.-T.; Wu, M.-S.; Lin, J.-H.; Wu, C.-Y. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: Population-based and in vitro studies. Gut 2013, 62, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Donadon, V.; Balbi, M.; Mas, M.D.; Casarin, P.; Zanette, G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010, 30, 750–758. [Google Scholar] [CrossRef]
- Kramer, J.R.; Natarajan, Y.; Dai, J.; Yu, X.; Li, L.; El-Serag, H.; Kanwal, F. Effect of diabetes medications and glycemic control on risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Hepatology 2021, 1–9. [Google Scholar] [CrossRef]
- Chang, C.H.; Lin, J.W.; Wu, L.C.; Lai, M.S.; Chuang, L.M.; Chan, K.A. Association of thiazolidinediones with liver cancer and colorectal cancer in type 2 diabetes mellitus. Hepatology 2012, 55, 1462–1472. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, J.C.; Wong, G.L.-H.; Tse, Y.-K.; Wong, V.W.-S.; Yip, T.C.-F.; Chan, H.L.-Y. Statin and the risk of hepatocellular carcinoma and death in a hospital-based hepatitis B-infected population: A propensity score landmark analysis. J. Hepatol. 2015, 63, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, P.P.; Singh, A.G.; Murad, M.H.; Sanchez, W. Statins are associated with a reduced risk of hepatocellular cancer: A systematic review and meta-analysis. Gastroenterology 2013, 144, 323–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, T.G.; Ma, Y.; Ludvigsson, J.F.; Chong, D.Q.; Giovannucci, E.L.; Fuchs, C.S.; Meyerhardt, J.A.; Corey, K.E.; Chung, R.T.; Zhang, X. Association between aspirin use and risk of hepatocellular carcinoma. JAMA Oncol. 2018, 4, 1683–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, W.Y.; Su, C.W.; Chia-Hui Tan, E.; Lee, P.C.; Chen, P.H.; Tang, J.H.; Huang, Y.H.; Huo, T.I.; Chang, C.C.; Hou, M.C. Proton pump inhibitors and risk of hepatocellular carcinoma in patients with chronic hepatitis B or C. Hepatology 2019, 69, 1151–1164. [Google Scholar] [CrossRef]
- Lau, W.-y.; Leung, T.W.; Lai, B.-s.; Liew, C.-t.; Ho, S.K.; Yu, S.C.; Tang, A.M. Preoperative systemic chemoimmunotherapy and sequential resection for unresectable hepatocellular carcinoma. Ann. Surg. 2001, 233, 236. [Google Scholar] [CrossRef] [PubMed]
- Raoul, J.-L.; Forner, A.; Bolondi, L.; Cheung, T.T.; Kloeckner, R.; de Baere, T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat. Rev. 2019, 72, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Systemic therapy for hepatocellular carcinoma: Latest advances. Cancers 2018, 10, 412. [Google Scholar] [CrossRef] [Green Version]
- Marisi, G.; Cucchetti, A.; Ulivi, P.; Canale, M.; Cabibbo, G.; Solaini, L.; Foschi, F.G.; De Matteis, S.; Ercolani, G.; Valgiusti, M. Ten years of sorafenib in hepatocellular carcinoma: Are there any predictive and/or prognostic markers? World J. Gastroenterol. 2018, 24, 4152. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.S.; Adnane, J.; Trail, P.A.; Levy, J.; Henderson, A.; Xue, D.; Bortolon, E.; Ichetovkin, M.; Chen, C.; McNabola, A. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother. Pharmacol. 2007, 59, 561–574. [Google Scholar] [CrossRef]
- Raoul, J.-L.; Frenel, J.-S.; Raimbourg, J.; Gilabert, M. Current options and future possibilities for the systemic treatment of hepatocellular carcinoma. Hepatic Oncol. 2019, 6, HEP11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, K.; Kudo, M.; Kawazoe, S.; Osaki, Y.; Ikeda, M.; Okusaka, T.; Tamai, T.; Suzuki, T.; Hisai, T.; Hayato, S. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J. Gastroenterol. 2017, 52, 512–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, M. Lenvatinib in advanced hepatocellular carcinoma. Liver Cancer 2017, 6, 253–263. [Google Scholar] [CrossRef]
- Bruix, J.; Tak, W.-Y.; Gasbarrini, A.; Santoro, A.; Colombo, M.; Lim, H.-Y.; Mazzaferro, V.; Wiest, R.; Reig, M.; Wagner, A. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open-label, phase II safety study. Eur. J. Cancer 2013, 49, 3412–3419. [Google Scholar] [CrossRef]
- Kelley, R.; Verslype, C.; Cohn, A.; Yang, T.-S.; Su, W.-C.; Burris, H.; Braiteh, F.; Vogelzang, N.; Spira, A.; Foster, P. Cabozantinib in hepatocellular carcinoma. Cabozantinib in hepatocellular carcinoma: Results of a phase 2 placebo-controlled randomized discontinuation study. Ann. Oncol. 2017, 28, 528–534. [Google Scholar] [CrossRef]
- Lee, M.; Ryoo, B.-Y.; Hsu, C.-H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.; Spahn, J.; Liu, B.; Abdullah, H. Randomised efficacy and safety results for atezolizumab (Atezo)+ bevacizumab (Bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC). Ann. Oncol. 2019, 30, v875. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, N.; Hillan, K.J.; Novotny, W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005, 333, 328–335. [Google Scholar] [CrossRef]
- Finn, R.S.; Bentley, G.; Britten, C.D.; Amado, R.; Busuttil, R.W. Targeting vascular endothelial growth factor with the monoclonal antibody bevacizumab inhibits human hepatocellular carcinoma cells growing in an orthotopic mouse model. Liver Int. 2009, 29, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Citterio, D.; Facciorusso, A.; Sposito, C.; Rota, R.; Bhoori, S.; Mazzaferro, V. Hierarchic interaction of factors associated with liver decompensation after resection for hepatocellular carcinoma. JAMA Surg. 2016, 151, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.; Postow, M.A.; Orlowski, R.J.; Mick, R.; Bengsch, B.; Manne, S.; Xu, W.; Harmon, S.; Giles, J.R.; Wenz, B. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017, 545, 60–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swartz, M.; Hirosue, S.; Hubbell, J. Engineering approaches to immunotherapy. Sci. Transl. Med. 2012, 4, 148rv9. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, X.; Hong, L.; Zhao, X.; Cui, G.; Li, A.; Liu, Y.; Zhou, L.; Sun, R.; Shen, S. Nanoparticle conjugation of ginsenoside Rg3 inhibits hepatocellular carcinoma development and metastasis. Small 2020, 16, 1905233. [Google Scholar] [CrossRef] [Green Version]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Kearney, C.J.; Mooney, D.J. Macroscale delivery systems for molecular and cellular payloads. Nat. Mater. 2013, 12, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Roche, K.C.; Tian, S.; Eblan, M.J.; McKinnon, K.P.; Caster, J.M.; Chai, S.; Herring, L.E.; Zhang, L.; Zhang, T. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol. 2017, 12, 877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pranatharthiharan, S.; Patel, M.D.; Malshe, V.C.; Pujari, V.; Gorakshakar, A.; Madkaikar, M.; Ghosh, K.; Devarajan, P.V. Asialoglycoprotein receptor targeted delivery of doxorubicin nanoparticles for hepatocellular carcinoma. Drug Deliv. 2017, 24, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, N. On the basic concept of nanotechnology. In Proceedings of the International Conference on Production Engineering, Tokyo, Japan, 26–29 August 1974. [Google Scholar]
- Mintz, K.J.; Leblanc, R.M. The use of nanotechnology to combat liver cancer: Progress and perspectives. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188621. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hu, L.; Liu, Y.; Xu, X.; Wu, C. Targeted delivery of paclitaxel in liver cancer using hyaluronic acid functionalized mesoporous hollow alumina nanoparticles. BioMed Res. Int. 2019, 2019, 2928507. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Dong, Y.; Ding, L.; Dong, Y.; Wu, Z.; Wang, W.; Shen, M.; Duan, Y. Local delivery of arsenic trioxide nanoparticles for hepatocellular carcinoma treatment. Signal Transduct. Target. Ther. 2019, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhou, B.; Luo, H.; Mao, J.; Huang, Y.; Zhang, K.; Mei, C.; Yan, Y.; Jin, H.; Gao, J. ZnAs@ SiO2 nanoparticles as a potential anti-tumor drug for targeting stemness and epithelial-mesenchymal transition in hepatocellular carcinoma via SHP-1/JAK2/STAT3 signaling. Theranostics 2019, 9, 4391. [Google Scholar] [CrossRef]
- Chi, X.; Zhang, R.; Zhao, T.; Gong, X.; Wei, R.; Yin, Z.; Lin, H.; Li, D.; Shan, H.; Gao, J. Targeted arsenite-loaded magnetic multifunctional nanoparticles for treatment of hepatocellular carcinoma. Nanotechnology 2019, 30, 175101. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Gao, Y.; Liu, Y.; Xu, X. Pure paclitaxel nanoparticles: Preparation, characterization, and antitumor effect for human liver cancer SMMC-7721 cells. Int. J. Nanomed. 2018, 13, 6189. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.-n.; Lu, C.-y.; Shu, G.-f.; Li, J.; Chen, M.-j.; Chen, C.-m.; Lv, X.-l.; Xu, X.-l.; Weng, W.; Weng, Q.-y. Gadolinium-loaded calcium phosphate nanoparticles for magnetic resonance imaging of orthotopic hepatocarcinoma and primary hepatocellular carcinoma. Biomater. Sci. 2020, 8, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Wang, Z.-X.; Zhang, G.; Lu, X.; Qiang, G.-H.; Hu, W.; Ji, A.-L.; Wu, J.-H.; Jiang, C.-P. Targeted co-delivery of Beclin 1 siRNA and FTY720 to hepatocellular carcinoma by calcium phosphate nanoparticles for enhanced anticancer efficacy. Int. J. Nanomed. 2018, 13, 1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, P.; Li, M.; Wang, Y.; Chen, Y.; He, C.; Zhang, X.; Yang, T.; Lu, Y.; You, J.; Lee, R.J. Enhancing anti-tumor efficiency in hepatocellular carcinoma through the autophagy inhibition by miR-375/sorafenib in lipid-coated calcium carbonate nanoparticles. Acta Biomater. 2018, 72, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wu, S.; Cheng, Y.; You, J.; Chen, Y.; Li, M.; He, C.; Zhang, X.; Yang, T.; Lu, Y. MiR-375 delivered by lipid-coated doxorubicin-calcium carbonate nanoparticles overcomes chemoresistance in hepatocellular carcinoma. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q.; Shen, Y.; Liao, M.-M.; Mao, X.; Mi, G.-J.; You, C.; Guo, Q.-Y.; Li, W.-J.; Wang, X.-Y.; Lin, N. Galactosylated chitosan triptolide nanoparticles for overcoming hepatocellular carcinoma: Enhanced therapeutic efficacy, low toxicity, and validated network regulatory mechanisms. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 86–97. [Google Scholar] [CrossRef]
- Tang, P.; Sun, Q.; Yang, H.; Tang, B.; Pu, H.; Li, H. Honokiol nanoparticles based on epigallocatechin gallate functionalized chitin to enhance therapeutic effects against liver cancer. Int. J. Pharm. 2018, 545, 74–83. [Google Scholar] [CrossRef]
- Cheng, M.; Zhu, W.; Li, Q.; Dai, D.; Hou, Y. Anti-cancer efficacy of biotinylated chitosan nanoparticles in liver cancer. Oncotarget 2017, 8, 59068. [Google Scholar] [CrossRef] [Green Version]
- Kou, C.H.; Han, J.; Han, X.L.; Zhuang, H.J.; Zhao, Z.M. Preparation and characterization of the Adriamycin-loaded amphiphilic chitosan nanoparticles and their application in the treatment of liver cancer. Oncol. Lett. 2017, 14, 7833–7841. [Google Scholar] [CrossRef] [Green Version]
- Loutfy, S.A.; El-Din, H.M.A.; Elberry, M.H.; Allam, N.G.; Hasanin, M.; Abdellah, A.M. Synthesis, characterization and cytotoxic evaluation of chitosan nanoparticles: In vitro liver cancer model. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035008. [Google Scholar] [CrossRef]
- Choi, K.; Joo, H. Assessment of gold nanoparticles-inhibited cytochrome P450 3A4 activity and molecular mechanisms underlying its cellular toxicity in human hepatocellular carcinoma cell line C3A. Nanoscale Res. Lett. 2018, 13, 279. [Google Scholar] [CrossRef] [Green Version]
- Salem, D.S.; Sliem, M.A.; El-Sesy, M.; Shouman, S.A.; Badr, Y. Improved chemo-photothermal therapy of hepatocellular carcinoma using chitosan-coated gold nanoparticles. J. Photochem. Photobiol. B Biol. 2018, 182, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Khandanlou, R.; Murthy, V.; Saranath, D.; Damani, H. Synthesis and characterization of gold-conjugated Backhousia citriodora nanoparticles and their anticancer activity against MCF-7 breast and HepG2 liver cancer cell lines. J. Mater. Sci. 2018, 53, 3106–3118. [Google Scholar] [CrossRef]
- Guo, M.; Sun, Y.; Zhang, X.-D. Enhanced radiation therapy of gold nanoparticles in liver cancer. Appl. Sci. 2017, 7, 232. [Google Scholar] [CrossRef] [Green Version]
- Xue, H.-Y.; Liu, Y.; Liao, J.-Z.; Lin, J.-S.; Li, B.; Yuan, W.-G.; Lee, R.J.; Li, L.; Xu, C.-R.; He, X.-X. Gold nanoparticles delivered miR-375 for treatment of hepatocellular carcinoma. Oncotarget 2016, 7, 86675. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Ramadori, F.; Quarta, S.; Biasiolo, A.; Fabris, E.; Baldan, P.; Guarino, G.; Ruvoletto, M.; Villano, G.; Turato, C. Binding and uptake into human hepatocellular carcinoma cells of peptide-functionalized gold nanoparticles. Bioconjugate Chem. 2017, 28, 222–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajeshkumar, S. Anticancer activity of eco-friendly gold nanoparticles against lung and liver cancer cells. J. Genet. Eng. Biotechnol. 2016, 14, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaat, H.; Mostafa, A.; Moustafa, M.; Gamal-Eldeen, A.; Emam, A.; El-Hussieny, E.; Elhefnawi, M. Modified gold nanoparticles for intracellular delivery of anti-liver cancer siRNA. Int. J. Pharm. 2016, 504, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Hui, H.; Jin, Y.; Dong, D.; Liang, X.; Yang, X.; Tan, K.; Dai, Z.; Cheng, Z.; Tian, J. Enhanced immunotherapy of SM5-1 in hepatocellular carcinoma by conjugating with gold nanoparticles and its in vivo bioluminescence tomographic evaluation. Biomaterials 2016, 87, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Mocan, L.; Matea, C.; Tabaran, F.A.; Mosteanu, O.; Pop, T.; Mocan, T.; Iancu, C. Photothermal treatment of liver cancer with albumin-conjugated gold nanoparticles initiates Golgi Apparatus–ER dysfunction and caspase-3 apoptotic pathway activation by selective targeting of Gp60 receptor. Int. J. Nanomed. 2015, 10, 5435. [Google Scholar]
- Tomuleasa, C.; Soritau, O.; Orza, A.; Dudea, M.; Petrushev, B.; Mosteanu, O.; Susman, S.; Florea, A.; Pall, E.; Aldea, M. Gold nanoparticles conjugated with cisplatin/doxorubicin/capecitabine lower the chemoresistance of hepatocellular carcinoma-derived cancer cells. J. Gastrointest. Liver Dis. 2012, 21, 186–196. [Google Scholar]
- Pracht, M.; Chajon, E.; de Baere, T.; Nguyen, F.; Bronowicki, J.-P.; Vendrely, V.; Baumann, A.-S.; Croise-Laurent, V.; Rio, E.; Rolland, Y. Hepatocellular carcinoma and liver metastasis treated by hafnium oxide nanoparticles activated by stereotactic body radiation therapy. Ann. Oncol. 2018, 29, viii240. [Google Scholar] [CrossRef]
- Lunov, O.; Uzhytchak, M.; Smolková, B.; Lunova, M.; Jirsa, M.; Dempsey, N.M.; Dias, A.L.; Bonfim, M.; Hof, M.; Jurkiewicz, P. Remote actuation of apoptosis in liver cancer cells via magneto-mechanical modulation of iron oxide nanoparticles. Cancers 2019, 11, 1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Duan, J.; Wang, J.; Liu, Q.; Shang, R.; Yang, X.; Lu, P.; Xia, C.; Wang, L.; Dou, K. Superparamagnetic iron oxide nanoparticles modified with polyethylenimine and galactose for siRNA targeted delivery in hepatocellular carcinoma therapy. Int. J. Nanomed. 2018, 13, 1851. [Google Scholar] [CrossRef] [Green Version]
- Kandasamy, G.; Sudame, A.; Luthra, T.; Saini, K.; Maity, D. Functionalized hydrophilic superparamagnetic iron oxide nanoparticles for magnetic fluid hyperthermia application in liver cancer treatment. Am. Chem. Soc. Omega 2018, 3, 3991–4005. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, X.; Miao, Y.; Li, J.; Gan, Y. Lipid-coated iron oxide nanoparticles for dual-modal imaging of hepatocellular carcinoma. Int. J. Nanomed. 2017, 12, 2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depalo, N.; Iacobazzi, R.M.; Valente, G.; Arduino, I.; Villa, S.; Canepa, F.; Laquintana, V.; Fanizza, E.; Striccoli, M.; Cutrignelli, A. Sorafenib delivery nanoplatform based on superparamagnetic iron oxide nanoparticles magnetically targets hepatocellular carcinoma. Nano Res. 2017, 10, 2431–2448. [Google Scholar] [CrossRef]
- Maeng, J.H.; Lee, D.-H.; Jung, K.H.; Bae, Y.-H.; Park, I.-S.; Jeong, S.; Jeon, Y.-S.; Shim, C.-K.; Kim, W.; Kim, J. Multifunctional doxorubicin loaded superparamagnetic iron oxide nanoparticles for chemotherapy and magnetic resonance imaging in liver cancer. Biomaterials 2010, 31, 4995–5006. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, M.; Zhu, L.; Tian, Y.; Wu, M.; Li, Y.; Deng, L.; Jiang, W.; Shen, W.; Wang, Z. Cell-penetrating peptide-modified targeted drug-loaded phase-transformation lipid nanoparticles combined with low-intensity focused ultrasound for precision theranostics against hepatocellular carcinoma. Theranostics 2018, 8, 1892. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Lan, C.-H.; Wu, K.-L.; Wu, Y.M.; Jane, W.-N.; Hsiao, M.; Wu, H.-C. Hepatocellular carcinoma-targeted nanoparticles for cancer therapy. Int. J. Oncol. 2018, 52, 389–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Hu, J.; Chan, H.F.; Skibba, M.; Liang, G.; Chen, M. iRGD decorated lipid-polymer hybrid nanoparticles for targeted co-delivery of doxorubicin and sorafenib to enhance anti-hepatocellular carcinoma efficacy. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, Q.; Liu, W.; Li, Y.; Tang, H.; Liu, X.; Yang, X. Codelivery of doxorubicin and curcumin with lipid nanoparticles results in improved efficacy of chemotherapy in liver cancer. Int. J. Nanomed. 2015, 10, 257. [Google Scholar]
- Tang, X.; Chen, L.; Li, A.; Cai, S.; Zhang, Y.; Liu, X.; Jiang, Z.; Liu, X.; Liang, Y.; Ma, D. Anti-GPC3 antibody-modified sorafenib-loaded nanoparticles significantly inhibited HepG2 hepatocellular carcinoma. Drug Deliv. 2018, 25, 1484–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Zhang, X.; Liao, H.; Sun, Y.; Ding, L.; Teng, Y.; Zhu, W.H.; Zhang, Z.; Duan, Y. Multifunctional shell–core nanoparticles for treatment of multidrug resistance hepatocellular carcinoma. Adv. Funct. Mater. 2018, 28, 1706124. [Google Scholar] [CrossRef]
- Zheng, N.; Liu, W.; Li, B.; Nie, H.; Liu, J.; Cheng, Y.; Wang, J.; Dong, H.; Jia, L. Co-delivery of sorafenib and metapristone encapsulated by CXCR4-targeted PLGA-PEG nanoparticles overcomes hepatocellular carcinoma resistance to sorafenib. J. Exp. Clin. Cancer Res. 2019, 38, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhou, L.; Xie, K.; Wu, J.; Song, P.; Xie, H.; Zhou, L.; Liu, J.; Xu, X.; Shen, Y. Polylactide-tethered prodrugs in polymeric nanoparticles as reliable nanomedicines for the efficient eradication of patient-derived hepatocellular carcinoma. Theranostics 2018, 8, 3949. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.-M.; Yin, P.-H.; Li, Q.; Sa, Z.-Q.; Sheng, X.; Yang, L.; Huang, T.; Zhang, M.; Gao, K.-P.; Chen, Q.-H. Anti-tumor effects of brucine immuno-nanoparticles on hepatocellular carcinoma. Int. J. Nanomed. 2012, 7, 369. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Yang, L.; Sheng, X.; Sa, Z.; Huang, T.; Li, Q.; Gao, K.; Chen, Q.; Ma, J.; Shen, H. Antitumor effects of brucine immuno-nanoparticles on hepatocellular carcinoma in vivo. Oncol. Lett. 2018, 15, 6137–6146. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, H.; Li, J.; Liu, Z.; Xie, H.; Wei, X.; Lu, D.; Zhuang, R.; Xu, X.; Zheng, S. iRGD-decorated polymeric nanoparticles for the efficient delivery of vandetanib to hepatocellular carcinoma: Preparation and in vitro and in vivo evaluation. Am. Chem. Soc. Appl. Mater. Interfaces 2016, 8, 19228–19237. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gao, M.; Xu, H.; Guan, X.; Lv, L.; Deng, S.; Zhang, C.; Tian, Y. A promising emodin-loaded poly (lactic-co-glycolic acid)-d-α-tocopheryl polyethylene glycol 1000 succinate nanoparticles for liver cancer therapy. Pharm. Res. 2016, 33, 217–236. [Google Scholar] [CrossRef]
- Zhu, D.; Tao, W.; Zhang, H.; Liu, G.; Wang, T.; Zhang, L.; Zeng, X.; Mei, L. Docetaxel (DTX)-loaded polydopamine-modified TPGS-PLA nanoparticles as a targeted drug delivery system for the treatment of liver cancer. Acta Biomater. 2016, 30, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Z.; Liu, C.; Yu, D.; Lu, Z.; Zhang, N. Gadolinium-loaded polymeric nanoparticles modified with Anti-VEGF as multifunctional MRI contrast agents for the diagnosis of liver cancer. Biomaterials 2011, 32, 5167–5176. [Google Scholar] [CrossRef] [PubMed]
- Shoshan, M.S.; Vonderach, T.; Hattendorf, B.; Wennemers, H. Peptide-Coated Platinum Nanoparticles with Selective Toxicity against Liver Cancer Cells. Angew. Chem. Int. Ed. 2019, 58, 4901–4905. [Google Scholar] [CrossRef]
- Medhat, A.; Mansour, S.; El-Sonbaty, S.; Kandil, E.; Mahmoud, M. Evaluation of the antitumor activity of platinum nanoparticles in the treatment of hepatocellular carcinoma induced in rats. Tumor Biol. 2017, 39, 1010428317717259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S.; Zhou, J.; Li, Z.; Appelman, H.D.; Zhao, L.; Zhu, J.; Wang, T.D. Sorafenib encapsulated in nanocarrier functionalized with glypican-3 specific peptide for targeted therapy of hepatocellular carcinoma. Colloids Surf. B Biointerfaces 2019, 184, 110498. [Google Scholar] [CrossRef]
- Gao, X.; He, Z.; Ni, W.; Jian, X.; Hu, C.; Zhao, Y.; Yan, Y.; Wei, X. Layer-by-Layer Assembly of Functional Nanoparticles for Hepatocellular Carcinoma Therapy. Adv. Funct. Mater. 2019, 29, 1904246. [Google Scholar] [CrossRef]
- Song, X.; You, J.; Shao, H.; Yan, C. Effects of surface modification of As2O3-loaded PLGA nanoparticles on its anti-liver cancer ability: An in vitro and in vivo study. Colloids Surf. B Biointerfaces 2018, 169, 289–297. [Google Scholar] [CrossRef]
- Song, X.; Wang, J.; Xu, Y.; Shao, H.; Gu, J. Surface-modified PLGA nanoparticles with PEG/LA-chitosan for targeted delivery of arsenic trioxide for liver cancer treatment: Inhibition effects enhanced and side effects reduced. Colloids Surf. B Biointerfaces 2019, 180, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.-C.; Sung, Y.-C.; Ramjiawan, R.R.; Lin, T.-T.; Chang, C.-C.; Jeng, K.-S.; Chang, C.-F.; Liu, C.-H.; Gao, D.-Y. Overcoming sorafenib evasion in hepatocellular carcinoma using CXCR4-targeted nanoparticles to co-deliver MEK-inhibitors. Sci. Rep. 2017, 7, 44123. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Liang, Y.; Tan, Y.; Xie, C.; Shen, J.; Zhang, M.; Liu, X.; Yang, L.; Zhang, F.; Liu, L. Genistein-loaded nanoparticles of star-shaped diblock copolymer mannitol-core PLGA–TPGS for the treatment of liver cancer. Mater. Sci. Eng. C 2016, 59, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zong, Y.; Yang, Z.; Luo, M.; Li, G.; Yingsa, W.; Cao, Y.; Xiao, M.; Kong, T.; He, J. Dual-targeted controlled delivery based on folic acid modified pectin-based nanoparticles for combination therapy of liver cancer. Am. Chem. Soc. Sustain. Chem. Eng. 2019, 7, 3614–3623. [Google Scholar] [CrossRef]
- Huang, L.; Chaurasiya, B.; Wu, D.; Wang, H.; Du, Y.; Tu, J.; Webster, T.J.; Sun, C. Versatile redox-sensitive pullulan nanoparticles for enhanced liver targeting and efficient cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhong, J.; Zhao, M.; Tang, Y.; Han, N.; Hua, L.; Xu, T.; Wang, C.; Zhu, B. Galactose-modified selenium nanoparticles for targeted delivery of doxorubicin to hepatocellular carcinoma. Drug Deliv. 2019, 26, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Zhao, M.; Chen, Y.; Hua, L.; Xu, T.; Wang, C.; Li, Y.; Zhu, B. Folate-targeted selenium nanoparticles deliver therapeutic siRNA to improve hepatocellular carcinoma therapy. RSC Adv. 2018, 8, 25932–25940. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Wu, X.; Zhou, B.; Chen, X.; Chen, T.; Yang, F. Targeting selenium nanoparticles combined with baicalin to treat HBV-infected liver cancer. RSC Adv. 2017, 7, 8178–8185. [Google Scholar] [CrossRef] [Green Version]
- Al-Nadaf, A.H.; Dahabiyeh, L.A.; Bardaweel, S.; Mahmoud, N.N.; Jawarneh, S. Functionalized mesoporous silica nanoparticles by lactose and hydrophilic polymer as a hepatocellular carcinoma drug delivery system. J. Drug Deliv. Sci. Technol. 2020, 56, 101504. [Google Scholar] [CrossRef]
- Yang, H.; Liu, H.-s.; Hou, W.; Gao, J.-x.; Duan, Y.; Wei, D.; Gong, X.-q.; Wang, H.-j.; Wu, X.-l.; Chang, J. An NIR-responsive mesoporous silica nanosystem for synergetic photothermal-immunoenhancement therapy of hepatocellular carcinoma. J. Mater. Chem. B 2020, 8, 251–259. [Google Scholar] [CrossRef]
- Niu, Y.; Tang, E.; Zhang, Q. Cytotoxic effect of silica nanoparticles against hepatocellular carcinoma cells through necroptosis induction. Toxicol. Res. 2019, 8, 1042–1049. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Zhu, D.; Li, J.; Chen, X.; Xie, W.; Jiang, X.; Wu, L.; Wang, G.; Xiao, Y.; Liu, Z. Coating biomimetic nanoparticles with chimeric antigen receptor T cell-membrane provides high specificity for hepatocellular carcinoma photothermal therapy treatment. Theranostics 2020, 10, 1281. [Google Scholar] [CrossRef]
- Wang, Z.; Chang, Z.; Lu, M.; Shao, D.; Yue, J.; Yang, D.; Zheng, X.; Li, M.; He, K.; Zhang, M. Shape-controlled magnetic mesoporous silica nanoparticles for magnetically-mediated suicide gene therapy of hepatocellular carcinoma. Biomaterials 2018, 154, 147–157. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, P.; He, Z.; He, H.; Rong, W.; Li, J.; Zhou, D.; Huang, Y. Mesoporous silica nanoparticles with lactose-mediated targeting effect to deliver platinum (IV) prodrug for liver cancer therapy. J. Mater. Chem. B 2017, 5, 7591–7597. [Google Scholar] [CrossRef]
- Xue, H.; Yu, Z.; Liu, Y.; Yuan, W.; Yang, T.; You, J.; He, X.; Lee, R.J.; Li, L.; Xu, C. Delivery of miR-375 and doxorubicin hydrochloride by lipid-coated hollow mesoporous silica nanoparticles to overcome multiple drug resistance in hepatocellular carcinoma. Int. J. Nanomed. 2017, 12, 5271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Y.; Li, J.; Chen, H.; Bai, Y.; Zhang, L. Glycyrrhetinic acid-functionalized mesoporous silica nanoparticles as hepatocellular carcinoma-targeted drug carrier. Int. J. Nanomed. 2017, 12, 4361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.-k.; Zhou, Y.-y.; Guo, S.-j.; Wang, Y.-y.; Nie, C.-j.; Wang, H.-l.; Wang, J.-l.; Zhao, Y.; Li, X.-y.; Chen, X.-j. Cetuximab conjugated and doxorubicin loaded silica nanoparticles for tumor-targeting and tumor microenvironment responsive binary drug delivery of liver cancer therapy. Mater. Sci. Eng. C 2017, 76, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, X.; Liu, Y.; Fan, L.; Lin, L.; Xu, Y.; Chen, S.; Shao, J. pH-Sensitive mesoporous silica nanoparticles anticancer prodrugs for sustained release of ursolic acid and the enhanced anti-cancer efficacy for hepatocellular carcinoma cancer. Eur. J. Pharm. Sci. 2017, 96, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.-T.; Liu, C.-H.; Yu, J.; Wu, K.C. Liver cancer cells: Targeting and prolonged-release drug carriers consisting of mesoporous silica nanoparticles and alginate microspheres. Int. J. Nanomed. 2014, 9, 2767. [Google Scholar]
- Karimzadeh, K.; Bakhshi, N.; Ramzanpoor, M. Biogenic silver nanoparticles using Oxalis corniculata characterization and their clinical implications. J. Drug Deliv. Sci. Technol. 2019, 54, 101263. [Google Scholar] [CrossRef]
- Ahmadian, E.; Dizaj, S.M.; Rahimpour, E.; Hasanzadeh, A.; Eftekhari, A.; Halajzadeh, J.; Ahmadian, H. Effect of silver nanoparticles in the induction of apoptosis on human hepatocellular carcinoma (HepG2) cell line. Mater. Sci. Eng. C 2018, 93, 465–471. [Google Scholar] [CrossRef]
- Saratale, R.G.; Shin, H.S.; Kumar, G.; Benelli, G.; Kim, D.-S.; Saratale, G.D. Exploiting antidiabetic activity of silver nanoparticles synthesized using Punica granatum leaves and anticancer potential against human liver cancer cells (HepG2). Artif. Cells Nanomed. Biotechnol. 2018, 46, 211–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaganathan, A.; Murugan, K.; Panneerselvam, C.; Madhiyazhagan, P.; Dinesh, D.; Vadivalagan, C.; Chandramohan, B.; Suresh, U.; Rajaganesh, R.; Subramaniam, J. Earthworm-mediated synthesis of silver nanoparticles: A potent tool against hepatocellular carcinoma, Plasmodium falciparum parasites and malaria mosquitoes. Parasitol. Int. 2016, 65, 276–284. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Guo, Y.; Lu, J.; Veeraraghavan, V.P.; Mohan, S.K.; Wang, C.; Yu, X. Synthesis of Zinc oxide nanoparticles from Marsdenia tenacissima inhibits the cell proliferation and induces apoptosis in laryngeal cancer cells (Hep-2). J. Photochem. Photobiol. B Biol. 2019, 201, 111624. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, C.; Wang, Y.; Koivisto, O.; Zhou, J.; Shu, Y.; Zhang, H. Nanotechnology-based delivery of CRISPR/Cas9 for cancer treatment. Adv. Drug Deliv. Rev. 2021, 176, 113891. [Google Scholar] [CrossRef]
- Skoulidis, F.; Heymach, J.V. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat. Rev. Cancer 2019, 19, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Jiménez, F.; Muiños, F.; Sentís, I.; Deu-Pons, J.; Reyes-Salazar, I.; Arnedo-Pac, C.; Mularoni, L.; Pich, O.; Bonet, J.; Kranas, H. A compendium of mutational cancer driver genes. Nat. Rev. Cancer 2020, 20, 555–572. [Google Scholar] [CrossRef]
- Azangou-Khyavy, M.; Ghasemi, M.; Khanali, J.; Boroomand-Saboor, M.; Jamalkhah, M.; Soleimani, M.; Kiani, J. CRISPR/Cas: From tumor gene editing to T cell-based immunotherapy of cancer. Front. Immunol. 2020, 11, 2062. [Google Scholar] [CrossRef]
- Montaño-Samaniego, M.; Bravo-Estupiñan, D.M.; Méndez-Guerrero, O.; Alarcón-Hernández, E.; Ibáñez-Hernández, M. Strategies for Targeting Gene Therapy in Cancer Cells With Tumor-Specific Promoters. Front. Oncol. 2020, 10, 2671. [Google Scholar] [CrossRef] [PubMed]
- Reghupaty, S.C.; Sarkar, D. Current status of gene therapy in hepatocellular carcinoma. Cancers 2019, 11, 1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabernero, J.; Shapiro, G.I.; LoRusso, P.M.; Cervantes, A.; Schwartz, G.K.; Weiss, G.J.; Paz-Ares, L.; Cho, D.C.; Infante, J.R.; Alsina, M. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013, 3, 406–417. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Huang, H.-L.; Li, J.; Qian, F.-C.; Li, L.-Q.; Niu, P.-P.; Dai, L.-C. Development of hybrid-type modified chitosan derivative nanoparticles for the intracellular delivery of midkine-siRNA in hepatocellular carcinoma cells. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 82–89. [Google Scholar] [CrossRef]
- Wang, D.; Chang, R.; Wang, G.; Hu, B.; Qiang, Y.; Chen, Z. Polo-like kinase 1-targeting Chitosan Nanoparticles suppress the progression of hepatocellular carcinoma. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Cancer Agents) 2017, 17, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Bogorad, R.L.; Yin, H.; Zeigerer, A.; Nonaka, H.; Ruda, V.M.; Zerial, M.; Anderson, D.G.; Koteliansky, V. Nanoparticle-formulated siRNA targeting integrins inhibits hepatocellular carcinoma progression in mice. Nat. Commun. 2014, 5, 3869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, S.-h.; Yu, B.; Wang, X.; Lu, Y.; Schmidt, C.R.; Lee, R.J.; Lee, L.J.; Jacob, S.T.; Ghoshal, K. Cationic lipid nanoparticles for therapeutic delivery of siRNA and miRNA to murine liver tumor. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1169–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Xu, X.-L.; Zhang, J.-Z.; Mao, X.-H.; Xie, M.-W.; Cheng, Z.-L.; Lu, L.-J.; Duan, X.-H.; Zhang, L.-M.; Shen, J. Magnetic cationic amylose nanoparticles used to deliver survivin-small interfering RNA for gene therapy of hepatocellular carcinoma in vitro. Nanomaterials 2017, 7, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Kievit, F.M.; Sham, J.G.; Jeon, M.; Stephen, Z.R.; Bakthavatsalam, A.; Park, J.O.; Zhang, M. Iron-oxide-based nanovector for tumor targeted siRNA delivery in an orthotopic hepatocellular carcinoma xenograft mouse model. Small 2016, 12, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, D.; Srivastava, J.; Ebeid, K.; Gredler, R.; Akiel, M.; Jariwala, N.; Robertson, C.L.; Shen, X.-N.; Siddiq, A.; Fisher, P.B. Combination of nanoparticle-delivered siRNA for astrocyte elevated gene-1 (AEG-1) and all-trans retinoic acid (ATRA): An effective therapeutic strategy for hepatocellular carcinoma (HCC). Bioconjugate Chem. 2015, 26, 1651–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, G.; Zhao, R.; Xu, A.; Shen, Z.; Chen, X.; Shao, J. Co-delivery of sorafenib and siVEGF based on mesoporous silica nanoparticles for ASGPR mediated targeted HCC therapy. Eur. J. Pharm. Sci. 2018, 111, 492–502. [Google Scholar] [CrossRef]
- Younis, M.A.; Khalil, I.A.; Abd Elwakil, M.M.; Harashima, H. A multifunctional lipid-based nanodevice for the highly specific codelivery of sorafenib and midkine siRNA to hepatic cancer cells. Mol. Pharm. 2019, 16, 4031–4044. [Google Scholar] [CrossRef]
- Yang, T.; Chen, Y.; Zhao, P.; Xue, H.; You, J.; Li, B.; Liu, Y.; He, C.; Zhang, X.; Fan, L. Enhancing the therapeutic effect via elimination of hepatocellular carcinoma stem cells using Bmi1 siRNA delivered by cationic cisplatin nanocapsules. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2009–2021. [Google Scholar] [CrossRef]
- Yang, J.D.; Nakamura, I.; Roberts, L.R. The tumor microenvironment in hepatocellular carcinoma: Current status and therapeutic targets. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 35–43. [Google Scholar]
- Friedman, S.L.; Roll, F.J.; Boyles, J.; Bissell, D.M. Hepatic lipocytes: The principal collagen-producing cells of normal rat liver. Proc. Natl. Acad. Sci. USA 1985, 82, 8681–8685. [Google Scholar] [CrossRef] [Green Version]
- Pietras, K.; Östman, A. Hallmarks of cancer: Interactions with the tumor stroma. Exp. Cell Res. 2010, 316, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Benetti, A.; Berenzi, A.; Gambarotti, M.; Garrafa, E.; Gelati, M.; Dessy, E.; Portolani, N.; Piardi, T.; Giulini, S.M.; Caruso, A. Transforming growth factor-β1 and CD105 promote the migration of hepatocellular carcinoma–derived endothelium. Cancer Res. 2008, 68, 8626–8634. [Google Scholar] [CrossRef] [Green Version]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef] [Green Version]
- Whiteside, T. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruoslahti, E. Antiangiogenics meet nanotechnology. Cancer Cell 2002, 2, 97–98. [Google Scholar] [CrossRef] [Green Version]
- Siemann, D.W. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor-vascular disrupting agents. Cancer Treat. Rev. 2011, 37, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Byrne, J.D.; Betancourt, T.; Brannon-Peppas, L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Bosch, J. Vascular deterioration in cirrhosis: The big picture. J. Clin. Gastroenterol. 2007, 41, S247–S253. [Google Scholar] [CrossRef]
- Trédan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug resistance and the solid tumor microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, J.W.; Bae, Y.H. Odyssey of a cancer nanoparticle: From injection site to site of action. Nano Today 2012, 7, 606–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, F.A.; Kettunen, M.I.; Day, S.E.; Hu, D.-E.; Ardenkjaer-Larsen, J.H.; Jensen, P.R.; Karlsson, M.; Golman, K.; Lerche, M.H.; Brindle, K.M. Magnetic resonance imaging of pH in vivo using hyperpolarized 13 C-labelled bicarbonate. Nature 2008, 453, 940–943. [Google Scholar] [CrossRef]
- Urano, Y.; Asanuma, D.; Hama, Y.; Koyama, Y.; Barrett, T.; Kamiya, M.; Nagano, T.; Watanabe, T.; Hasegawa, A.; Choyke, P.L. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat. Med. 2009, 15, 104–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hong Nguyen, B.-J.L. Protein corona: A new approach for nanomedicine design. Int. J. Nanomed. 2017, 12, 3137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, R.; Chen, C. The crown and the scepter: Roles of the protein corona in nanomedicine. Adv. Mater. 2019, 31, 1805740. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef] [Green Version]
- Ke, P.C.; Lin, S.; Parak, W.J.; Davis, T.P.; Caruso, F. A decade of the protein corona. Am. Chem. Soc. Nano 2017, 11, 11773–11776. [Google Scholar] [CrossRef] [PubMed]
- Rampado, R.; Crotti, S.; Caliceti, P.; Pucciarelli, S.; Agostini, M. Recent advances in understanding the protein corona of nanoparticles and in the formulation of “Stealthy” Nanomaterials. Front. Bioeng. Biotechnol. 2020, 8, 166. [Google Scholar] [CrossRef]
- Xu, F.; Reiser, M.; Yu, X.; Gummuluru, S.; Wetzler, L.; Reinhard, B.M. Lipid-mediated targeting with membrane-wrapped nanoparticles in the presence of corona formation. Am. Chem. Soc. Nano 2016, 10, 1189–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magro, M.; Baratella, D.; Bonaiuto, E.; de Almeida, R.J.; Chemello, G.; Pasquaroli, S.; Mancini, L.; Olivotto, I.; Zoppellaro, G.; Ugolotti, J. Stealth iron oxide nanoparticles for organotropic drug targeting. Biomacromolecules 2019, 20, 1375–1384. [Google Scholar] [CrossRef]
- García-Álvarez, R.; Hadjidemetriou, M.; Sánchez-Iglesias, A.; Liz-Marzán, L.M.; Kostarelos, K. In vivo formation of protein corona on gold nanoparticles. The effect of their size and shape. Nanoscale 2018, 10, 1256–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, K.; Rahimi, M.; Yazdani, M.; Kim, S.T.; Moyano, D.F.; Hou, S.; Das, R.; Mout, R.; Rezaee, F.; Mahmoudi, M. Regulation of macrophage recognition through the interplay of nanoparticle surface functionality and protein corona. Am. Chem. Soc. Nano 2016, 10, 4421–4430. [Google Scholar] [CrossRef] [Green Version]
- Almalik, A.; Benabdelkamel, H.; Masood, A.; Alanazi, I.O.; Alradwan, I.; Majrashi, M.A.; Alfadda, A.A.; Alghamdi, W.M.; Alrabiah, H.; Tirelli, N. Hyaluronic acid coated chitosan nanoparticles reduced the immunogenicity of the formed protein corona. Sci. Rep. 2017, 7, 10542. [Google Scholar] [CrossRef] [PubMed]
- Partikel, K.; Korte, R.; Mulac, D.; Humpf, H.-U.; Langer, K. Serum type and concentration both affect the protein-corona composition of PLGA nanoparticles. Beilstein J. Nanotechnol. 2019, 10, 1002–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monopoli, M.P.; Åberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef]
- Vroman, L.; Lukosevicius, A. Ellipsometer recordings of changes in optical thickness of adsorbed films associated with surface activation of blood clotting. Nature 1964, 204, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Vroman, L.; Adams, A.; Fischer, G.; Munoz, P. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood 1980, 55, 156–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjidemetriou, M.; Kostarelos, K. Evolution of the nanoparticle corona. Nat. Nanotechnol. 2017, 12, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Maiolo, D.; Del Pino, P.; Metrangolo, P.; Parak, W.J.; Baldelli Bombelli, F. Nanomedicine delivery: Does protein corona route to the target or off road? Nanomedicine 2015, 10, 3231–3247. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Kim, H.S.; Palanikumar, L.; Go, E.M.; Jana, B.; Park, S.A.; Kim, H.Y.; Kim, K.; Seo, J.K.; Kwak, S.K. Cloaking nanoparticles with protein corona shield for targeted drug delivery. Nat. Commun. 2018, 9, 4548. [Google Scholar] [CrossRef] [Green Version]
- Mizuhara, T.; Saha, K.; Moyano, D.F.; Kim, C.S.; Yan, B.; Kim, Y.K.; Rotello, V.M. Acylsulfonamide-functionalized zwitterionic gold nanoparticles for enhanced cellular uptake at tumor pH. Angew. Chem. 2015, 127, 6667–6670. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.; Okwieka, P.; Schöttler, S.; Winzen, S.; Langhanki, J.; Mohr, K.; Opatz, T.; Mailänder, V.; Landfester, K.; Wurm, F.R. Carbohydrate-based nanocarriers exhibiting specific cell targeting with minimum influence from the protein corona. Angew. Chem. Int. Ed. 2015, 54, 7436–7440. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Walkey, C.; Chan, W.C. Polyethylene glycol backfilling mitigates the negative impact of the protein corona on nanoparticle cell targeting. Angew. Chem. Int. Ed. 2014, 53, 5093–5096. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Kelly, P.M.; Mahon, E.; Stockmann, H.; Rudd, P.M.; Caruso, F.; Dawson, K.A.; Yan, Y.; Monopoli, M.P. The “sweet” side of the protein corona: Effects of glycosylation on nanoparticle–cell interactions. Am. Chem. Soc. Nano 2015, 9, 2157–2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbo, C.; Molinaro, R.; Tabatabaei, M.; Farokhzad, O.C.; Mahmoudi, M. Personalized protein corona on nanoparticles and its clinical implications. Biomater. Sci. 2017, 5, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Cai, R.; Wang, J.; Daniyal, M.; Baimanov, D.; Liu, Y.; Yin, D.; Liu, Y.; Miao, Q.; Zhao, Y. Precision nanomedicine development based on specific opsonization of human cancer patient-personalized protein coronas. Nano Lett. 2019, 19, 4692–4701. [Google Scholar] [CrossRef]
- Barui, A.K.; Oh, J.Y.; Jana, B.; Kim, C.; Ryu, J.H. Cancer-targeted nanomedicine: Overcoming the barrier of the protein corona. Adv. Ther. 2020, 3, 1900124. [Google Scholar] [CrossRef]
- Parmar, K.; Patel, J.K. Surface Modification of Nanoparticles to Oppose Uptake by the Mononuclear Phagocyte System. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2019; pp. 221–236. [Google Scholar]
- Allen, T. The use of glycolipids and hydrophilic polymers in avoiding rapid uptake of liposomes by the mononuclear phagocyte system. Adv. Drug Deliv. Rev. 1994, 13, 285–309. [Google Scholar] [CrossRef]
- Crispe, I.N. Liver antigen-presenting cells. J. Hepatol. 2011, 54, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O.; Dunbar, P.R.; Bartlett, A.; Phillips, A. The immunophenotype of antigen presenting cells of the mononuclear phagocyte system in normal human liver—A systematic review. J. Hepatol. 2015, 62, 458–468. [Google Scholar] [CrossRef] [Green Version]
- Jenne, C.N.; Kubes, P. Immune surveillance by the liver. Nat. Immunol. 2013, 14, 996–1006. [Google Scholar] [CrossRef]
- Geissmann, F.; Gordon, S.; Hume, D.A.; Mowat, A.M.; Randolph, G.J. Unravelling mononuclear phagocyte heterogeneity. Nat. Rev. Immunol. 2010, 10, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Wake, K. Karl Wilhelm Kupffer and his contributions to modern hepatology. Comp. Hepatol. 2004, 3, S2. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Wang, J.; Ling, D. Surface engineering of nanoparticles for targeted delivery to hepatocellular carcinoma. Small 2018, 14, 1702037. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C. Nanoparticle–liver interactions: Cellular uptake and hepatobiliary elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.J.; Poon, W.; Zhang, Y.-N.; Dai, Q.; Besla, R.; Ding, D.; Ouyang, B.; Li, A.; Chen, J.; Zheng, G. Effect of removing Kupffer cells on nanoparticle tumor delivery. Proc. Natl. Acad. Sci. USA 2017, 114, E10871–E10880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadauskas, E.; Wallin, H.; Stoltenberg, M.; Vogel, U.; Doering, P.; Larsen, A.; Danscher, G. Kupffer cells are central in the removal of nanoparticles from the organism. Part. Fibre Toxicol. 2007, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Owens, D.E., III; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Karakoti, A.S.; Das, S.; Thevuthasan, S.; Seal, S. PEGylated inorganic nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 1980–1994. [Google Scholar] [CrossRef] [PubMed]
- García, K.P.; Zarschler, K.; Barbaro, L.; Barreto, J.A.; O’Malley, W.; Spiccia, L.; Stephan, H.; Graham, B. Zwitterionic-coated “stealth” nanoparticles for biomedical applications: Recent advances in countering biomolecular corona formation and uptake by the mononuclear phagocyte system. Small 2014, 10, 2516–2529. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Abrams, M.; Sepp-Lorenzino, L. Expression of asialoglycoprotein receptor 1 in human hepatocellular carcinoma. J. Histochem. Cytochem. 2013, 61, 901–909. [Google Scholar] [CrossRef] [Green Version]
- Varshosaz, J.; Hassanzadeh, F.; Sadeghi, H.; Khadem, M. Galactosylated nanostructured lipid carriers for delivery of 5-FU to hepatocellular carcinoma. J. Liposome Res. 2012, 22, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Iwai, M.; Kawakami, S.; Yamashita, F.; Hashida, M. Novel PEG-matrix metalloproteinase-2 cleavable peptide-lipid containing galactosylated liposomes for hepatocellular carcinoma-selective targeting. J. Control. Release 2006, 111, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Leung, T.W.; Brown, G.; Moyses, C.; Chan, A.T.; Yeo, W.; Wong, H.; Chak, K.; Johnson, P. A phase I safety and pharmacokinetic study of OGT 719 in patients with liver cancer. Acta Oncol. 2004, 43, 245–251. [Google Scholar] [CrossRef] [Green Version]
- D’souza, A.A.; Devarajan, P.V. Asialoglycoprotein receptor mediated hepatocyte targeting—Strategies and applications. J. Control. Release 2015, 203, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Wall, D.A.; Hubbard, A.L. Galactose-specific recognition system of mammalian liver: Receptor distribution on the hepatocyte cell surface. J. Cell Biol. 1981, 90, 687–696. [Google Scholar] [CrossRef]
- Matsuura, S.; Nakada, H.; Sawamura, T.; Tashiro, Y. Distribution of an asialoglycoprotein receptor on rat hepatocyte cell surface. J. Cell Biol. 1982, 95, 864–875. [Google Scholar] [CrossRef] [Green Version]
- Spiess, M. The asialoglycoprotein receptor: A model for endocytic transport receptors. Biochemistry 1990, 29, 10009–10018. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Hammer, R.E.; Herz, J. Asialoglycoprotein receptor deficiency in mice lacking the minor receptor subunit. J. Biol. Chem. 1994, 269, 27803–27806. [Google Scholar] [CrossRef]
- Rozema, D.B.; Lewis, D.L.; Wakefield, D.H.; Wong, S.C.; Klein, J.J.; Roesch, P.L.; Bertin, S.L.; Reppen, T.W.; Chu, Q.; Blokhin, A.V. Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc. Natl. Acad. Sci. USA 2007, 104, 12982–12987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trere, D.; Fiume, L.; De Giorgi, L.B.; Di Stefano, G.; Migaldi, M.; Derenzini, M. The asialoglycoprotein receptor in human hepatocellular carcinomas: Its expression on proliferating cells. Br. J. Cancer 1999, 81, 404–408. [Google Scholar] [CrossRef] [Green Version]
- Julyan, P.J.; Seymour, L.W.; Ferry, D.R.; Daryani, S.; Boivin, C.M.; Doran, J.; David, M.; Anderson, D.; Christodoulou, C.; Young, A.M. Preliminary clinical study of the distribution of HPMA copolymers bearing doxorubicin and galactosamine. J. Control. Release 1999, 57, 281–290. [Google Scholar] [CrossRef]
- Seymour, L.W.; Ferry, D.R.; Anderson, D.; Hesslewood, S.; Julyan, P.J.; Poyner, R.; Doran, J.; Young, A.M.; Burtles, S.; Kerr, D.J. Hepatic drug targeting: Phase I evaluation of polymer-bound doxorubicin. J. Clin. Oncol. 2002, 20, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Ise, H.; Nikaido, T.; Negishi, N.; Sugihara, N.; Suzuki, F.; Akaike, T.; Ikeda, U. Effective hepatocyte transplantation using rat hepatocytes with low asialoglycoprotein receptor expression. Am. J. Pathol. 2004, 165, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Xing, M.M.; Zhong, W. Shell cross-linked and hepatocyte-targeting nanoparticles containing doxorubicin via acid-cleavable linkage. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, T.; Maeda, A.; Kagatani, S.; Konno, Y.; Sonobe, T.; Fukui, M.; Hashimoto, H.; Hara, K.; Fujita, K. Specific interaction between galactose branched-cyclodextrins and hepatocytes in vitro. Int. J. Pharm. 1998, 167, 147–154. [Google Scholar] [CrossRef]
- Zou, Y.; Song, Y.; Yang, W.; Meng, F.; Liu, H.; Zhong, Z. Galactose-installed photo-crosslinked pH-sensitive degradable micelles for active targeting chemotherapy of hepatocellular carcinoma in mice. J. Control. Release 2014, 193, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wei, W.; Tanaka, H.; Kohama, K.; Ma, G.; Dobashi, T.; Maki, Y.; Wang, H.; Bi, J.; Dai, S. A galactosamine-mediated drug delivery carrier for targeted liver cancer therapy. Pharmacol. Res. 2011, 64, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Xu, Y.; Zou, Q.; Tu, L.; Tang, C.; Xu, T.; Deng, L.; Wu, C. Hepatocellular carcinoma targeting effect of PEGylated liposomes modified with lactoferrin. Eur. J. Pharm. Sci. 2012, 46, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Filmus, J.; Capurro, M. Glypican-3: A marker and a therapeutic target in hepatocellular carcinoma. FEBS J. 2013, 280, 2471–2476. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Ho, M. Glypican-3 antibodies: A new therapeutic target for liver cancer. FEBS Lett. 2014, 588, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filmus, J.; Selleck, S.B. Glypicans: Proteoglycans with a surprise. J. Clin. Investig. 2001, 108, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Kim, H. Glypican-3: A new target for cancer immunotherapy. Eur. J. Cancer 2011, 47, 333–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiguro, T.; Sugimoto, M.; Kinoshita, Y.; Miyazaki, Y.; Nakano, K.; Tsunoda, H.; Sugo, I.; Ohizumi, I.; Aburatani, H.; Hamakubo, T. Anti–glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res. 2008, 68, 9832–9838. [Google Scholar] [CrossRef] [Green Version]
- Nakano, K.; Ishiguro, T.; Konishi, H.; Tanaka, M.; Sugimoto, M.; Sugo, I.; Igawa, T.; Tsunoda, H.; Kinoshitam, Y.; Habu, K. Generation of a humanized anti-glypican 3 antibody by CDR grafting and stability optimization. Anti-Cancer Drugs 2010, 21, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Orita, T.; Nezu, J.; Yoshino, T.; Ohizumi, I.; Sugimoto, M.; Furugaki, K.; Kinoshita, Y.; Ishiguro, T.; Hamakubo, T. Anti-glypican 3 antibodies cause ADCC against human hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2009, 378, 279–284. [Google Scholar] [CrossRef]
- Zhu, A.X.; Gold, P.J.; El-Khoueiry, A.B.; Abrams, T.A.; Morikawa, H.; Ohishi, N.; Ohtomo, T.; Philip, P.A. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2013, 19, 920–928. [Google Scholar] [CrossRef] [Green Version]
- Takai, H.; Kato, A.; Kinoshita, Y.; Ishiguro, T.; Takai, Y.; Ohtani, Y.; Sugimoto, M.; Suzuki, M. Histopathological analyses of the antitumor activity of anti-glypican-3 antibody (GC33) in human liver cancer xenograft models: The essential role of macrophages. Cancer Biol. Ther. 2009, 8, 930–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phung, Y.; Gao, W.; Man, Y.-G.; Nagata, S.; Ho, M. High-affinity monoclonal antibodies to cell surface tumor antigen glypican-3 generated through a combination of peptide immunization and flow cytometry screening. mAbs 2012, 4, 592–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, M.; Gao, W.; Wang, R.; Chen, W.; Man, Y.-G.; Figg, W.D.; Wang, X.W.; Dimitrov, D.S.; Ho, M. Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 2013, 110, E1083–E1091. [Google Scholar] [CrossRef] [Green Version]
- Park, J.O.; Stephen, Z.; Sun, C.; Veiseh, O.; Kievit, F.M.; Fang, C.; Leung, M.; Mok, H.; Zhang, M. Glypican-3 targeting of liver cancer cells using multifunctional nanoparticles. Mol. Imaging 2011, 10, 00048. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, Y.; Zhang, D.; Wu, M.; Wu, L.; Huang, A.; Yang, H.; Liu, X.; Liu, J. Glypican-3 antibody functionalized Prussian blue nanoparticles for targeted MR imaging and photothermal therapy of hepatocellular carcinoma. J. Mater. Chem. B 2014, 2, 3686–3696. [Google Scholar] [CrossRef]
- Kolhatkar, R.; Lote, A.; Khambhati, H. Active tumor targeting of nanomaterials using folic acid, transferrin and integrin receptors. Curr. Drug Discov. Technol. 2011, 8, 197–206. [Google Scholar] [CrossRef]
- Crielaard, B.J.; Lammers, T.; Rivella, S. Targeting iron metabolism in drug discovery and delivery. Nat. Rev. Drug Discov. 2017, 16, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmström, P.; Gåfvels, M.; Eriksson, L.C.; Dzikaite, V.; Hultcrantz, R.; Eggertsen, G.; Stål, P. Expression of iron regulatory genes in a rat model of hepatocellular carcinoma. Liver Int. 2006, 26, 976–985. [Google Scholar] [CrossRef]
- Sakurai, K.; Sohda, T.; Ueda, S.-i.; Tanaka, T.; Hirano, G.; Yokoyama, K.; Morihara, D.; Aanan, A.; Takeyama, Y.; Irie, M. Immunohistochemical demonstration of transferrin receptor 1 and 2 in human hepatocellular carcinoma tissue. Hepato-Gastroenterology 2014, 61, 426–430. [Google Scholar] [PubMed]
- Tang, J.; Wang, Q.; Yu, Q.; Qiu, Y.; Mei, L.; Wan, D.; Wang, X.; Li, M.; He, Q. A stabilized retro-inverso peptide ligand of transferrin receptor for enhanced liposome-based hepatocellular carcinoma-targeted drug delivery. Acta Biomater. 2019, 83, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.R.; Delgado, T.; Rodriguez, J.A.; Helguera, G.; Penichet, M.L. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin. Immunol. 2006, 121, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Prutki, M.; Poljak-Blazi, M.; Jakopovic, M.; Tomas, D.; Stipancic, I.; Zarkovic, N. Altered iron metabolism, transferrin receptor 1 and ferritin in patients with colon cancer. Cancer Lett. 2006, 238, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, W.A.; Brandon, M.R.; Hunt, S.V.; Williams, A.F.; Gatter, K.C.; Mason, D.Y. Transferrin receptor on endothelium of brain capillaries. Nature 1984, 312, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ke, W.; Liu, Y.; Jiang, C.; Pei, Y. The use of lactoferrin as a ligand for targeting the polyamidoamine-based gene delivery system to the brain. Biomaterials 2008, 29, 238–246. [Google Scholar] [CrossRef]
- Gomme, P.T.; McCann, K.B.; Bertolini, J. Transferrin: Structure, function and potential therapeutic actions. Drug Discov. Today 2005, 10, 267–273. [Google Scholar] [CrossRef]
- Huang, R.-Q.; Qu, Y.-H.; Ke, W.-L.; Zhu, J.-H.; Pei, Y.-Y.; Jiang, C. Efficient gene delivery targeted to the brain using a transferrin-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. Fed. Am. Soc. Exp. Biol. J. 2007, 21, 1117–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Chen, J.-T.; Yan, X.-P. Fabrication of transferrin functionalized gold nanoclusters/graphene oxide nanocomposite for turn-on near-infrared fluorescent bioimaging of cancer cells and small animals. Anal. Chem. 2013, 85, 2529–2535. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin-and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Malarvizhi, G.L.; Retnakumari, A.P.; Nair, S.; Koyakutty, M. Transferrin targeted core-shell nanomedicine for combinatorial delivery of doxorubicin and sorafenib against hepatocellular carcinoma. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1649–1659. [Google Scholar] [CrossRef]
- Parab, H.J.; Huang, J.-H.; Lai, T.-C.; Jan, Y.-H.; Liu, R.-S.; Wang, J.-L.; Hsiao, M.; Chen, C.-H.; Hwu, Y.-K.; Tsai, D.P. Biocompatible transferrin-conjugated sodium hexametaphosphate-stabilized gold nanoparticles: Synthesis, characterization, cytotoxicity and cellular uptake. Nanotechnology 2011, 22, 395706. [Google Scholar] [CrossRef] [Green Version]
- Golla, K.; Cherukuvada, B.; Ahmed, F.; Kondapi, A.K. Efficacy, safety and anticancer activity of protein nanoparticle-based delivery of doxorubicin through intravenous administration in rats. PLoS ONE 2012, 7, e51960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Huang, R.; Li, J.; Liu, S.; Huang, S.; Jiang, C. Plasmid pORF-hTRAIL and doxorubicin co-delivery targeting to tumor using peptide-conjugated polyamidoamine dendrimer. Biomaterials 2011, 32, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.-Z.; Jin, H.-W.; Huang, H.-Q. Transferrin–cisplatin specifically deliver cisplatin to HepG2 cells in vitro and enhance cisplatin cytotoxicity. J. Proteom. 2012, 77, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Teng, G.-J.; Zhang, Y.; Niu, H.-Z.; Zhu, G.-Y.; An, Y.-L.; Yu, H.; Li, G.-Z.; Qiu, D.-H.; Wu, C.-G. Enhancement of p53 gene transfer efficiency in hepatic tumor mediated by transferrin receptor through trans-arterial delivery. Cancer Biol. Ther. 2008, 7, 218–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, F.; Li, J.; Liu, D.; Wang, C.; Sui, Z. Dual ligands modified double targeted nano-system for liver targeted gene delivery. Pharm. Biol. 2013, 51, 643–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.-L.; Cai, J.-Y.; Qin, L.; Wang, H. Atomic force microscopy-based cell nanostructure for ligand-conjugated quantum dot endocytosis. Acta Biochim. Biophys. Sin. 2006, 38, 646–652. [Google Scholar] [CrossRef] [Green Version]
- Seol, J.; Heo, D.; Kim, H.; Yoon, J.; Choi, B.; Lee, H.; Kim, N.; Kim, C. Selective gene expression in hepatic tumor with trans-arterial delivery of DNA/liposome/transferrin complex. In Vivo 2000, 14, 513–517. [Google Scholar]
- Liu, M.-C.; Liu, L.; Wang, X.-R.; Shuai, W.-P.; Hu, Y.; Han, M.; Gao, J.-Q. Folate receptor-targeted liposomes loaded with a diacid metabolite of norcantharidin enhance antitumor potency for H22 hepatocellular carcinoma both in vitro and in vivo. Int. J. Nanomed. 2016, 11, 1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabizon, A.; Horowitz, A.T.; Goren, D.; Tzemach, D.; Shmeeda, H.; Zalipsky, S. In vivo fate of folate-targeted polyethylene-glycol liposomes in tumor-bearing mice. Clin. Cancer Res. 2003, 9, 6551–6559. [Google Scholar] [PubMed]
- Huang, Y.; Yang, T.; Zhang, W.; Lu, Y.; Ye, P.; Yang, G.; Li, B.; Qi, S.; Liu, Y.; He, X. A novel hydrolysis-resistant lipophilic folate derivative enables stable delivery of targeted liposomes in vivo. Int. J. Nanomed. 2014, 9, 4581. [Google Scholar]
- Gao, J.-Q.; Lv, Q.; Li, L.-M.; Tang, X.-J.; Li, F.-Z.; Hu, Y.-L.; Han, M. Glioma targeting and blood–brain barrier penetration by dual-targeting doxorubincin liposomes. Biomaterials 2013, 34, 5628–5639. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.B.; Lee, R.J. Tumor-selective targeted delivery of genes and antisense oligodeoxyribonucleotides via the folate receptor. Adv. Drug Deliv. Rev. 2004, 56, 1193–1204. [Google Scholar] [CrossRef]
- Koirala, N.; Das, D.; Fayazzadeh, E.; Sen, S.; McClain, A.; Puskas, J.E.; Drazba, J.A.; McLennan, G. Folic acid conjugated polymeric drug delivery vehicle for targeted cancer detection in hepatocellular carcinoma. J. Biomed. Mater. Res. Part A 2019, 107, 2522–2535. [Google Scholar] [CrossRef] [PubMed]
- Zwicke, G.L.; Ali Mansoori, G.; Jeffery, C.J. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012, 3, 18496. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.; Turk, M.J.; Westrick, E.; Lewis, J.D.; Low, P.S.; Leamon, C.P. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005, 338, 284–293. [Google Scholar] [CrossRef]
- Kelemen, L.E. The role of folate receptor α in cancer development, progression and treatment: Cause, consequence or innocent bystander? Int. J. Cancer 2006, 119, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-W.; Mason, J.B. Folate and carcinogenesis: An integrated scheme. J. Nutr. 2000, 130, 129–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blount, B.C.; Mack, M.M.; Wehr, C.M.; MacGregor, J.T.; Hiatt, R.A.; Wang, G.; Wickramasinghe, S.N.; Everson, R.B.; Ames, B.N. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. Proc. Natl. Acad. Sci. USA 1997, 94, 3290–3295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghalehkhondabi, V.; Soleymani, M.; Fazlali, A. Folate-targeted nanomicelles containing silibinin as an active drug delivery system for liver cancer therapy. J. Drug Deliv. Sci. Technol. 2021, 61, 102157. [Google Scholar] [CrossRef]
- Bwatanglang, I.B.; Mohammad, F.; Yusof, N.A.; Abdullah, J.; Alitheen, N.B.; Hussein, M.Z.; Abu, N.; Mohammed, N.E.; Nordin, N.; Zamberi, N.R. In vivo tumor targeting and anti-tumor effects of 5-fluororacil loaded, folic acid targeted quantum dot system. J. Colloid Interface Sci. 2016, 480, 146–158. [Google Scholar] [CrossRef]
- Abdelmoneem, M.A.; Mahmoud, M.; Zaky, A.; Helmy, M.W.; Sallam, M.; Fang, J.-Y.; Elkhodairy, K.A.; Elzoghby, A.O. Dual-targeted casein micelles as green nanomedicine for synergistic phytotherapy of hepatocellular carcinoma. J. Control. Release 2018, 287, 78–93. [Google Scholar] [CrossRef]
- Maghsoudinia, F.; Tavakoli, M.B.; Samani, R.K.; Motaghi, H.; Hejazi, S.H.; Mehrgardi, M.A. Bevacizumab and folic acid dual-targeted gadolinium-carbon dots for fluorescence/magnetic resonance imaging of hepatocellular carcinoma. J. Drug Deliv. Sci. Technol. 2021, 61, 102288. [Google Scholar] [CrossRef]
- Gao, W.; Jia, X.; Wu, J.; Song, Y.; Yin, J.; Zhang, M.; Qiu, N.; Li, X.; Wu, P.; Qi, X. Preparation and evaluation of folate-decorated human serum albumin nanoparticles for the targeted delivery of sorafenib to enhance antihepatocarcinoma efficacy. J. Drug Deliv. Sci. Technol. 2019, 54, 101349. [Google Scholar] [CrossRef]
- Shi, X.; He, D.; Tang, G.; Tang, Q.; Xiong, R.; Ouyang, H.; Yu, C.-y. Fabrication and characterization of a folic acid-bound 5-fluorouracil loaded quantum dot system for hepatocellular carcinoma targeted therapy. RSC Adv. 2018, 8, 19868–19878. [Google Scholar] [CrossRef] [Green Version]
- Bi, J.; Lu, Y.; Dong, Y.; Gao, P. Synthesis of folic acid-modified DOX@ ZIF-8 nanoparticles for targeted therapy of liver Cancer. J. Nanomater. 2018, 2018, 1357812. [Google Scholar] [CrossRef]
- Lu, T.; Nong, Z.; Wei, L.; Wei, M.; Li, G.; Wu, N.; Liu, C.; Tang, B.; Qin, Q.; Li, X. Preparation and anti-cancer activity of transferrin/folic acid double-targeted graphene oxide drug delivery system. J. Biomater. Appl. 2020, 35, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Zagami, R.; Rapozzi, V.; Piperno, A.; Scala, A.; Triolo, C.; Trapani, M.; Xodo, L.E.; Monsù Scolaro, L.; Mazzaglia, A. Folate-decorated amphiphilic Cyclodextrins as cell-targeted Nanophototherapeutics. Biomacromolecules 2019, 20, 2530–2544. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Duan, S.; Wang, J.; Bao, S.; Qiu, X.; Li, C.; Liu, Y.; Yan, L.; Zhang, Z.; Hu, Y. Folic-acid-mediated functionalized gold nanocages for targeted delivery of anti-miR-181b in combination of gene therapy and photothermal therapy against hepatocellular carcinoma. Adv. Funct. Mater. 2016, 26, 2532–2544. [Google Scholar] [CrossRef]
- Shao, D.; Li, J.; Pan, Y.; Zhang, X.; Zheng, X.; Wang, Z.; Zhang, M.; Zhang, H.; Chen, L. Noninvasive theranostic imaging of HSV-TK/GCV suicide gene therapy in liver cancer by folate-targeted quantum dot-based liposomes. Biomater. Sci. 2015, 3, 833–841. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W.; Shang, Y.; Cao, P.; Cui, J.; Li, Z.; Yin, X.; Li, Y. Folic acid-nanoscale gadolinium-porphyrin metal-organic frameworks: Fluorescence and magnetic resonance dual-modality imaging and photodynamic therapy in hepatocellular carcinoma. Int. J. Nanomed. 2019, 14, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, D.; Xia, H.; Park, W.; Hackett, M.J.; Song, C.; Na, K.; Hui, K.M.; Hyeon, T. pH-sensitive nanoformulated triptolide as a targeted therapeutic strategy for hepatocellular carcinoma. Am. Chem. Soc. Nano 2014, 8, 8027–8039. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-J.; Azhar, S.; Kraemer, F.B. SR-B1: A unique multifunctional receptor for cholesterol influx and efflux. Annu. Rev. Physiol. 2018, 80, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-J.; Azhar, S.; Kraemer, F.B. Lipid droplets and steroidogenic cells. Exp. Cell Res. 2016, 340, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Hu, X.-H.; Li, Q.; Xiong, Y.; Hu, G.-J.; Xu, J.-J.; Zhao, X.-N.; Wei, X.-X.; Chang, C.C.; Liu, Y.-K. A specific cholesterol metabolic pathway is established in a subset of HCCs for tumor growth. J. Mol. Cell Biol. 2013, 5, 404–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacko, A.G.; Nair, M.; Paranjape, S.; Johnson, S.; McConathy, W.J. High density lipoprotein complexes as delivery vehicles for anticancer drugs. Anticancer Res. 2002, 22, 2045–2050. [Google Scholar] [PubMed]
- Trigatti, B.L. SR-B1 and PDZK1: Partners in HDL regulation. Curr. Opin. Lipidol. 2017, 28, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Mooberry, L.K.; Sabnis, N.A.; Panchoo, M.; Nagarajan, B.; Lacko, A.G. Targeting the SR-B1 receptor as a gateway for cancer therapy and imaging. Front. Pharmacol. 2016, 7, 466. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, S.; Ricciuti, B.; Pirro, M.; Butler, A.E.; Atkin, S.L.; Banach, M.; Sahebkar, A. High-density lipoprotein components and functionality in cancer: State-of-the-art. Trends Endocrinol. Metab. 2019, 30, 12–24. [Google Scholar] [CrossRef]
- Plebanek, M.P.; Bhaumik, D.; Thaxton, C.S. HDL and the golden key to cancer immunity? Oncoscience 2018, 5, 164. [Google Scholar] [CrossRef]
- Henrich, S.E.; Thaxton, C.S. An update on synthetic high-density lipoprotein-like nanoparticles for cancer therapy. Expert Rev. Anticancer Ther. 2019, 19, 515–528. [Google Scholar] [CrossRef]
- Luthi, A.J.; Lyssenko, N.N.; Quach, D.; McMahon, K.M.; Millar, J.S.; Vickers, K.C.; Rader, D.J.; Phillips, M.C.; Mirkin, C.A.; Thaxton, C.S. Robust passive and active efflux of cellular cholesterol to a designer functional mimic of high density lipoprotein. J. Lipid Res. 2015, 56, 972–985. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Jin, H.; Chen, J.; Ding, L.; Ng, K.K.; Lin, Q.; Lovell, J.F.; Zhang, Z.; Zheng, G. Efficient cytosolic delivery of siRNA using HDL-mimicking nanoparticles. Small 2011, 7, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, C.; Zhai, Y.; Cai, Y.; Lee, R.J.; Xing, J.; Wang, H.; Zhu, H.H.; Teng, L.; Li, Y. High-density lipoprotein modulates tumor-associated macrophage for chemoimmunotherapy of hepatocellular carcinoma. Nano Today 2021, 37, 101064. [Google Scholar] [CrossRef]
- Cruz, W.; Huang, H.; Barber, B.; Pasini, E.; Ding, L.; Zheng, G.; Chen, J.; Bhat, M. Lipoprotein-Like Nanoparticle Carrying Small Interfering RNA Against Spalt-Like Transcription Factor 4 Effectively Targets Hepatocellular Carcinoma Cells and Decreases Tumor Burden. Hepatol. Commun. 2020, 4, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, W.; Wang, B.; Zhu, H.; Zhang, B.; Feng, M. Delivery of hydrophilic drug doxorubicin hydrochloride-targeted liver using apoAI as carrier. J. Drug Target. 2013, 21, 367–374. [Google Scholar] [CrossRef]
- Jiang, Y.-Q.; Wang, H.-R.; Li, H.-P.; Hao, H.-J.; Zheng, Y.-L.; Gu, J. Targeting of hepatoma cell and suppression of tumor growth by a novel 12mer peptide fused to superantigen TSST-1. Mol. Med. 2006, 12, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Savier, E.; Simon-Gracia, L.; Charlotte, F.; Tuffery, P.; Teesalu, T.; Scatton, O.; Rebollo, A. Bi-Functional Peptides as a New Therapeutic Tool for Hepatocellular Carcinoma. Pharmaceutics 2021, 13, 1631. [Google Scholar] [CrossRef]
- Nie, X.; Liu, Y.; Li, M.; Yu, X.; Yuan, W.; Huang, S.; Ren, D.; Wang, Y.; Wang, Y. SP94 Peptide-Functionalized PEG-PLGA nanoparticle loading with cryptotanshinone for targeting therapy of hepatocellular carcinoma. AAPS PharmSciTech 2020, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Yan, L.; Zhang, J.; Zhou, M.; Shi, G.; Tian, X.; Fan, K.; Hao, C.; Yan, X. Biomineralization synthesis of the cobalt nanozyme in SP94-ferritin nanocages for prognostic diagnosis of hepatocellular carcinoma. Am. Chem. Soc. Appl. Mater. Interfaces 2019, 11, 9747–9755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Cheng, L.; Yuan, J.; Zhong, Z. SP94 peptide mediating highly specific and efficacious delivery of polymersomal doxorubicin hydrochloride to hepatocellular carcinoma in vivo. Colloids Surf. B Biointerfaces 2021, 197, 111399. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Filipenko, P.; Huaman, J.; Lyudmer, M.; Hossain, M.; Santamaria, C.; Huang, K. Antitumor effects of Tv1 venom peptide in liver cancer. bioRxiv 2019, 518340. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Chen, X.; Xie, S.; Zhang, C.; Qiu, Z.; Zhu, F. Variant 1 of KIAA0101, overexpressed in hepatocellular carcinoma, prevents doxorubicin-induced apoptosis by inhibiting p53 activation. Hepatology 2012, 56, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Chen, X.; Wang, M.; Zhou, Y.; Zhou, P.; Li, W.; Zhu, F. Variant 2 of KIAA0101, antagonizing its oncogenic variant 1, might be a potential therapeutic strategy in hepatocellular carcinoma. Oncotarget 2017, 8, 43990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, X.; Wu, Y.; Shen, Y.-W.; Zhang, H.; Zhou, Y.-D.; Chen, H.-Z.; Nagle, D.G.; Zhang, W.-D. Cytotoxic and antitumor peptides as novel chemotherapeutics. Nat. Prod. Rep. 2021, 38, 7–17. [Google Scholar] [CrossRef]
- Liu, B.H.; Jobichen, C.; Chia, C.B.; Chan, T.H.M.; Tang, J.P.; Chung, T.X.; Li, J.; Poulsen, A.; Hung, A.W.; Koh-Stenta, X. Targeting cancer addiction for SALL4 by shifting its transcriptome with a pharmacologic peptide. Proc. Natl. Acad. Sci. USA 2018, 115, E7119–E7128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruskal, J.B.; Robson, S.C.; Franks, J.J.; Kirsch, R.E. Elevated fibrin-related and fibrinogen-related antigens in patients with liver disease. Hepatology 1992, 16, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Schmithals, C.; Köberle, V.; Korkusuz, H.; Pleli, T.; Kakoschky, B.; Augusto, E.A.; Ibrahim, A.A.; Arencibia, J.M.; Vafaizadeh, V.; Groner, B. Improving drug penetrability with iRGD leverages the therapeutic response to sorafenib and doxorubicin in hepatocellular carcinoma. Cancer Res. 2015, 75, 3147–3154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-T.; Ma, R.; Sun, S.-C.; Zhu, X.-F.; Xu, X.-L.; Mu, Q. Synthesis and biological evaluation of cyclopeptide GG-8-6 and its analogues as anti-hepatocellular carcinoma agents. Bioorg. Med. Chem. 2018, 26, 609–622. [Google Scholar] [CrossRef]
- Gong, F.; Wang, R.; Zhu, Z.; Duan, J.; Teng, X.; Cui, Z.-K. Drug-interactive mPEG-b-PLA-Phe (Boc) micelles enhance the tolerance and anti-tumor efficacy of docetaxel. Drug Deliv. 2020, 27, 238–247. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lin, C.; Chan, W.; Liu, K.; Lu, A.; Lin, G.; Hu, R.; Shi, H.; Zhang, H.; Yang, Z. Dual-functional liposomes with carbonic anhydrase IX antibody and BR2 peptide modification effectively improve intracellular delivery of cantharidin to treat orthotopic hepatocellular carcinoma mice. Molecules 2019, 24, 3332. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhu, J.; Liu, Y. A novel anti-adhesion peptide (β3) inhibits hepatocellular carcinoma activity in vitro and in vivo. Oncol. Lett. 2016, 12, 4744–4748. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-L.S.; Li, J.-H.; Yu, C.-Y.; Lin, C.-J.; Chiu, P.-H.; Chen, P.-W.; Lin, C.-C.; Chen, W.-J. Novel cationic antimicrobial peptide GW-H1 induced caspase-dependent apoptosis of hepatocellular carcinoma cell lines. Peptides 2012, 36, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Kardani, K.; Bolhassani, A. Antimicrobial/anticancer peptides: Bioactive molecules and therapeutic agents. Immunotherapy 2021, 13, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.D.; de Oliveira, M.D.; de Paula, S.O.; Baracat-Pereira, M.C.; Breukink, E.; Mantovani, H.C. Toxicity of bovicin HC5 against mammalian cell lines and the role of cholesterol in bacteriocin activity. Microbiology 2012, 158, 2851–2858. [Google Scholar] [CrossRef]
- Pittala, S.; Krelin, Y.; Shoshan-Barmatz, V. Targeting liver cancer and associated pathologies in mice with a mitochondrial VDAC1-based peptide. Neoplasia 2018, 20, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, Y.; Liu, Y.; Zhang, W.; Liu, Y.; Yang, X.; Cao, Y.; Wang, S. C7 peptide inhibits hepatocellular carcinoma metastasis by targeting the HGF/c-met signaling pathway. Cancer Biol. Ther. 2019, 20, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Tsang, F.H.; Lee, N.P.; Luk, J.M. The use of small peptides in the diagnosis and treatment of hepatocellular carcinoma. Protein Pept. Lett. 2009, 16, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Yoshikawa, T.; Ofuji, K.; Yoshimura, M.; Tsuchiya, N.; Takahashi, M.; Nobuoka, D.; Gotohda, N.; Takahashi, S.; Kato, Y. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology 2016, 5, e1129483. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, Z.; Tao, J.; Zhao, M.; Zhang, W.; Li, P.; Tang, L.; Gu, Y. An innovative peptide with high affinity to GPC3 for hepatocellular carcinoma diagnosis. Biomater. Sci. 2019, 7, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, N.; Yoshikawa, T.; Fujinami, N.; Saito, K.; Mizuno, S.; Sawada, Y.; Endo, I.; Nakatsura, T. Immunological efficacy of glypican-3 peptide vaccine in patients with advanced hepatocellular carcinoma. Oncoimmunology 2017, 6, e1346764. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Wu, Y.; Ma, J.; Yang, J.; Zhang, F. The antibacterial peptide from Bombyx mori cecropinXJ induced growth arrest and apoptosis in human hepatocellular carcinoma cells. Oncol. Lett. 2016, 12, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobuoka, D.; Yoshikawa, T.; Sawada, Y.; Fujiwara, T.; Nakatsura, T. Peptide vaccines for hepatocellular carcinoma. Hum. Vaccines Immunother. 2013, 9, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, F.; Yao, Z.; Jia, C.; Xiong, Z.; Liang, H.; Lin, N.; Deng, M. Inhibition of cyclin E1 sensitizes hepatocellular carcinoma cells to regorafenib by mcl-1 suppression. Cell Commun. Signal. 2019, 17, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Xue, Y.; Wang, G.; Gu, T.; Li, Y.; Zhu, Y.Y.; Chen, L. Multi-target siRNA: Therapeutic strategy for hepatocellular carcinoma. J. Cancer 2016, 7, 1317. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, T.; Liu, Y.; Zhang, N. Co-delivery of sorafenib and VEGF-siRNA via pH-sensitive liposomes for the synergistic treatment of hepatocellular carcinoma. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1374–1383. [Google Scholar] [CrossRef] [Green Version]
- Younis, M.A.; Khalil, I.A.; Elewa, Y.H.; Kon, Y.; Harashima, H. Ultra-small lipid nanoparticles encapsulating sorafenib and midkine-siRNA selectively-eradicate sorafenib-resistant hepatocellular carcinoma in vivo. J. Control. Release 2021, 331, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, S.; Sun, H.; Pan, Z.; Zhou, W.; Wu, M. Inhibition of tumorigenesis and invasion of hepatocellular carcinoma by siRNA-mediated silencing of the livin gene. Mol. Med. Rep. 2010, 3, 903–907. [Google Scholar]
- Cho, S.B.; Lee, W.S.; Park, Y.L.; Kim, N.; Oh, H.H.; Kim, M.Y.; Oak, C.Y.; Chung, C.Y.; Park, H.C.; Kim, J.S. Livin is associated with the invasive and oncogenic phenotypes of human hepatocellular carcinoma cells. Hepatol. Res. 2015, 45, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Yuan, Y.; Xie, L.; Ran, P.; Xiang, X.; Huang, Q.; Qi, G.; Guo, X.; Xiao, C.; Zheng, S. miRNA-320a inhibits tumor proliferation and invasion by targeting c-Myc in human hepatocellular carcinoma. OncoTargets Ther. 2017, 10, 885. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Li, Y.; Tian, L.; Shi, H.; Wang, J.; Liang, Y.; Sun, B.; Wang, S.; Zhou, M.; Wu, L. Gankyrin drives metabolic reprogramming to promote tumorigenesis, metastasis and drug resistance through activating β-catenin/c-Myc signaling in human hepatocellular carcinoma. Cancer Lett. 2019, 443, 34–46. [Google Scholar] [CrossRef]
- Li, H.; Fu, X.; Chen, Y.; Hong, Y.; Tan, Y.; Cao, H.; Wu, M.; Wang, H. Use of adenovirus-delivered siRNA to target oncoprotein p28GANK in hepatocellular carcinoma. Gastroenterology 2005, 128, 2029–2041. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Chiang, T.; Liu, C.-H.; Chern, G.-G.; Lin, T.-T.; Gao, D.-Y.; Chen, Y. Delivery of siRNA using CXCR4-targeted nanoparticles modulates tumor microenvironment and achieves a potent antitumor response in liver cancer. Mol. Ther. 2015, 23, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, R.; Wilcox, D.; Sarthy, A.; Lin, X.; Huang, X.; Tian, L.; Dande, P.; Hubbard, R.D.; Hansen, T.M. Developing lipid nanoparticle-based siRNA therapeutics for hepatocellular carcinoma using an integrated approach. Mol. Cancer Ther. 2013, 12, 2308–2318. [Google Scholar] [CrossRef] [Green Version]
- Ashley, C.E.; Carnes, E.C.; Phillips, G.K.; Padilla, D.; Durfee, P.N.; Brown, P.A.; Hanna, T.N.; Liu, J.; Phillips, B.; Carter, M.B. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nat. Mater. 2011, 10, 389–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Tang, C.; Yin, C. Oral delivery of shRNA and siRNA via multifunctional polymeric nanoparticles for synergistic cancer therapy. Biomaterials 2014, 35, 4589–4600. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, W.; Feng, M.; Wang, Y.; Zhou, J.; Ding, X.; Zhou, X.; Liu, C.; Wang, R.; Zhang, Q. A biomimetic nanovector-mediated targeted cholesterol-conjugated siRNA delivery for tumor gene therapy. Biomaterials 2012, 33, 8893–8905. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, S.; Tong, L.; Li, J.; Chen, F.; Han, Y.; Zhao, M.; Xiong, W. Superparamagnetic iron oxide nanoparticles mediated 131 I-hVEGF siRNA inhibits hepatocellular carcinoma tumor growth in nude mice. BMC Cancer 2014, 14, 114. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Gong, F.; Pang, P.; Shen, M.; Zhu, K.; Cheng, D.; Liu, Z.; Shan, H. An RGD-modified MRI-visible polymeric vector for targeted siRNA delivery to hepatocellular carcinoma in nude mice. PLoS ONE 2013, 8, e66416. [Google Scholar]
- Varshosaz, J.; Farzan, M. Nanoparticles for targeted delivery of therapeutics and small interfering RNAs in hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 12022. [Google Scholar] [CrossRef]
- Liu, C.; Yu, J.; Yu, S.; Lavker, R.M.; Cai, L.; Liu, W.; Yang, K.; He, X.; Chen, S. MicroRNA-21 acts as an oncomir through multiple targets in human hepatocellular carcinoma. J. Hepatol. 2010, 53, 98–107. [Google Scholar] [CrossRef]
- Su, H.; Yang, J.-R.; Xu, T.; Huang, J.; Xu, L.; Yuan, Y.; Zhuang, S.-M. MicroRNA-101, down-regulated in hepatocellular carcinoma, promotes apoptosis and suppresses tumorigenicity. Cancer Res. 2009, 69, 1135–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Targeting Moiety | Nanocarrier | Cargo Carried by Nanocarrier | In Vitro and/or In Vivo Studies and Results |
|---|---|---|---|
| Pullulan (Pul), Arabinogalactan (AGn), and Pul-AGn [45] | Polyethylene sebacate (PES) nanoparticles | Doxorubicin | ASGPR-mediated uptake in HepG2 cells, biodistribution and hepatic disposition in vivo and antitumor activity and toxicity testing in vivo showed that Pul and Pul-AGn labeling increased liver uptake with hepatocyte: nonparenchymal cell ratio of 85:15 |
| Lactose [211] | Shell cross-linking nanoparticles | Doxorubicin | In vitro cytotoxicity and cellular uptake in HepG2 cells showed that lactose conjugated NPs were internalized through a lactose-mediated mechanism |
| Galactose [212] | Cyclodextrins | Not applicable | In vitro and in vivo adherence of hepatocytes to formulation proved that the enzymatically synthesized NPs were specific to hepatocytes |
| Galactose [213] | Cross-linked pH-sensitive micelles | Paclitaxel | In vitro hepatoma targeting in HepG2 cells and in vivo biodistribution and antitumor studies showed that galactose conjugated NPs underwent receptor-mediated endocytosis mechanism in vitro with enhanced drug accumulation at the tumor sites in vivo |
| Galactosamine [214] | Albumin nanoparticles | Doxorubicin | In vitro cytotoxicity and cellular uptake in HepG2 concluded that the NPs were selectively taken up by HepG2 cells due to the surface ASGPR as opposed to ASGPR-negative cells |
| Lactoferrin [215] | PEGylated liposomes | Not applicable | In vitro cellular uptake in HepG2 lines and in vivo imaging for targeting in HepG2 bearing mice showed that cell uptake was efficiently associated with ASGPR-positive HepG2 cells compared to negative control along with increased drug accumulation in tumors treated with conjugated NPs |
| Antibody | Species | Antibody Form | Mechanism of Action | Development Status |
|---|---|---|---|---|
| GC33 [217,220,221,222,223,224] | Humanized mouse | IgG | Antibody-dependent cellular cytotoxicity (ADCC) | Phase II |
| YP7 [217,225] | Humanized mouse | IgG | ADCC | Preclinical |
| HN3 [217,226] | Human | VH-hFc | Inhibition of YAP signaling, direct inhibition of HCC cell proliferation | Preclinical |
| MDX-1414 [217] | Human | IgG | Not available | Preclinical |
| Targeting Moiety | Nanocarrier | Cargo Carried by Nanocarrier | In Vitro and/or In Vivo Studies and Results |
|---|---|---|---|
| Peptides specific for GPC3 [97] | Lipid nanoparticles | Sorafenib | In vitro cytotoxicity, cellular uptake in Hep3B and SK-Hep1 cells, and in vivo targeting and antitumor studies in Hep3B xenografts showed that the effective aqueous solubility of sorafenib was improved with increased uptake in vivo |
| GPC3 mAb [227] | Iron oxide nanoparticles | N/A | In vitro cell uptake and MRI in HepG2 and HLF lines confirmed that only GPC3-expressing cells were specifically targeted using these NPs and may increase pre-treatment MR imaging capability for HCC visualization |
| GPC3 mAb [228] | Citrate-coated nanoparticles | Prussian blue | In vitro cellular uptake and targeted MR imaging in HepG2 cells confirmed mAb targeting cells via receptor-mediated endocytosis with excellent MR imaging contrast enhancement ability and biocompatibility |
| Targeting Moiety | Nanocarrier | Cargo Carried by Nanocarrier | In Vitro and/or In Vivo Studies and Results |
|---|---|---|---|
| Transferrin [242] | PVA and albumin nanoshells | Doxorubicin and sorafenib | In vitro TFR1 expression, cytotoxicity, apoptosis, cellular uptake in HepG2 cells and 3D HCC spheroids showed synergistic cellular uptake and cytotoxicity in 92% of cells with efficient cell penetration |
| Transferrin [243] | Sodium hexametaphosphate gold nanoparticles | N/A | In vitro cytotoxicity and cellular uptake in J5 cells showed its biocompatibility along with higher cellular intake compared to non-conjugated NPs |
| Apotransferrin Lactoferrin [244] | Lipid nanoparticles | Doxorubicin | In vivo anti-cancer effect in diethylnitrosamine (DENA)-induced HCC rats confirmed significant antitumor potential by reduced liver nodules along with extended bioavailability of the designed NPs |
| TfR-specific peptide (HAIYPRH) [245] | Polyamidoamine dendrimer | pORF-hTRAIL and doxorubicin | In vitro cellular uptake and gene expression studies in Bel-7402 cells and in vivo antitumor and apoptotic effect in Bel-7402-derived xenografts showed increased cellular uptake and induction of apoptosis. In vivo study showed efficient tumor accumulation of NPs |
| Apotransferrin [246] | Inorganic nanoparticles | Cisplatin | In vitro cytotoxicity, cellular uptake, and pathway analysis in HepG2 cells showed specific targeting of NPs supported by apoptosis of HepG2 cells induced by the conjugate |
| Transferrin [247] | Liposomes | DNA | In vitro cellular uptake and gene expression in Huh7 and SK-Hep1 lines followed by in vivo gene transfer efficiency using VX2 rabbit hepatocarcinoma model depicted increased dose-dependent targeting using transferrin |
| Transferrin [248] | Lipid nanoparticles | N/A | In vitro cytotoxicity in HepG2 and KC cells and in vivo transfection activity in HepG2 xenografts concluded high loading efficiency along with excellent cell targeting ability of Tf-conjugated NPs |
| Transferrin [249] | Quantum dots (nanocrystales) | N/A | In vitro receptor activation analysis in HepG2 cells demonstrated that the Tf-conjugated quantum dots (QDs) were internalized and tightly bound to the cell receptors conferring it a useful technique in biological labeling of cells |
| Transferrin [250] | Liposomes | DNA | In vivo gene delivery analysis in VX2 hepatocarcinoma rabbit model demonstrated NPs accumulated only in tumor cells using transarterial injection compared to intra-tumoral injection that transfected peritumoral cells along with hepatic tumor cells |
| LT7 (L(HAIYPRH)) [233] | Liposomes | N/A | In vitro binding affinity and cellular uptake in HepG2 cells followed by in vivo antitumor effect in HepG2-derived xenografts showed better cellular uptake and enhanced antitumor effect using NPs along with enhanced drug accumulation in HCC |
| Targeting Moiety | Nanocarrier | Cargo Carried by Nanocarrier | In Vitro and/or In Vivo Studies and Results |
|---|---|---|---|
| Folic acid [262] | Pluronic F127 nanomicelles | Silibinin | In vitro cytotoxicity in HepG2 cells indicated that the viability of cells treated with conjugated NPs was significantly less than unconjugated NPs |
| Folic acid [263] | Mn-ZnS quantum dots with chitosan biopolymer | 5-Fluorouracil | In vitro drug release and in vivo sub-chronic toxicity assay and anti-4T1 breast cancer study indicated a controlled release behavior in vitro and accumulation of NPs in the tumor of the tumor-bearing mice |
| Folic acid [264] | Casein micelles | Berberine and diosmin | In vivo cytotoxicity and uptake studies using HepG2 cells supported with in vivo antitumor efficacy using DENA-induced HCC mouse model demonstrated superior cytotoxicity and cellular uptake in vitro along with increased antitumor efficacy in vivo |
| Folic acid and/or bevacizumab (dual targeting) [265] | Carbon dots | Gadolinium (imaging nanoprobe) | In vitro cytotoxicity assay, fluorescent imaging, and cellular uptake using Hepa1-6 and L929 cells indicated low toxicity with improved sensitivity and specificity as an ideal fluorophore nanosystem |
| Folic acid [266] | Human serum albumin nanoparticles | Sorafenib | In vitro cell viability assay, cellular uptake, and apoptosis analysis using BEL-7402 cells along with in vivo antitumor efficacy and safety evaluation and tissue distribution study using BEL-7402 xenograft model and pharmacokinetic study confirmed enhanced cytotoxicity, increased safety, and notably enhanced sorafenib accumulation in tumor tissues in vivo |
| Folic acid [267] | Quantum dots | 5-fluorouracil | In vitro cellular uptake using HepG2 cells and in vivo antitumor efficacy, toxicity, and biodistribution study using SMMC-7721 xenograft model indicated reduced cytotoxicity compared to free drug in vitro and enhanced tumor suppression in vivo |
| Folic acid [268] | ZIF-8 nanoparticles | Doxorubicin | In vitro cytotoxicity using HepG2 cells showed higher anti-cancer efficiency as a targeted therapy |
| Folic acid and Transferrin [269] | Graphene oxide DDS | Doxorubicin | In vitro cytotoxicity using SMMC-7721 cell line indicated that the double target drug delivery system exhibited controlled drug release, no toxicity, and better inhibitory effect on HCC cells |
| Adamantanyl-folic acid [270] | Ternary nanoassembly | Pheophorbide | In vitro cellular uptake, photodynamic therapy, and apoptosis assay in MCF-7 and PC3 cells demonstrated increased cellular uptake and improved phototoxicity |
| PEG-Folic acid [271] | Gold nanocages | Anti-miR-181b | In vitro cellular uptake and cytotoxicity assay using SMMC-7721 cells accompanied with in vivo biodistribution and antitumor effect in SMMC-7721 xenograft model indicated efficient cargo delivery in vitro and suppressing tumor growth and significantly reducing tumor volumes in vivo |
| Folic acid [272] | Liposomes | HSV-TK suicide gene | In vitro cellular uptake and cell viability using SMMC-7721, HepG2, and HL-7702 cells and in vivo tumor and tissue distribution imaging using Bel-7402 xenograft model and antitumor efficacy using Bel-7402 xenograft model demonstrated highly tumor-specific imaging and excellent antitumor activity devoid of any systemic toxicity |
| Folic acid [106] | Selenium nanoparticles | HES5 siRNA | In vitro cellular uptake, cytotoxicity, and apoptosis assay using HepG2 and Lo2 cell lines along with in vivo biodistribution and antitumor efficacy using HepG2 xenograft model indicated higher cellular uptake, enhanced gene silencing efficiency, and increased cytotoxicity in vitro. NPs conjugated with FA showed increased antitumor efficacy and low toxicity compared to unconjugated NPs |
| Folic acid [256] | Drug delivery vehicle | Fluorescein | In vitro cytotoxicity and apoptosis assay using N1S1 and U937 cell lines supported with in vivo biodistribution using N1S1 cell-induced murine model. Results indicated increased specific uptake due to cell surface folate receptors |
| Folic acid [273] | Porphyrin nanoparticles | Gadolinium | In vitro biotoxicity and imaging capability using HepG2 cells and in vivo biotoxicity and imaging capability in embryonic and larval zebrafish demonstrated excellent MRI capability both in vitro and in vivo due to its strong affinity for folate receptor on HCC tumor cells |
| Folic acid [274] | pH-sensitive nanoparticles | Triptolide | In vitro cytotoxicity and cellular uptake using Bel-7404 cells and in vivo distribution and antitumor effect using Bel-7404 xenograft model indicated that the pH-sensitive NPs increased cellular uptake mitigating the significant toxicity of triptolide |
| Targeting Moiety | Nanocarrier | Cargo Carried by Nanocarrier | In Vitro and/or In Vivo Studies and Results |
|---|---|---|---|
| ApoA-1 [287] | Lipid nanoparticles | SALL4 siRNA | In vitro cellular uptake assay and silencing efficacy in KB, HT1080, Hep3B, SNU398, and Huh7 cell lines. Along with in vivo biodistribution and antitumor effect in KB, HT1080, and Hep3B xenograft model. Results showed inhibited tumor growth with a 3-fold increase in apoptosis |
| ApoA-1 [288] | Lipid nanoparticles | Doxorubicin | In vitro cytotoxicity and cellular uptake assay in HepG2 cells indicated increased cytotoxicity and cellular uptake in SR-B1-positive liver cells |
| ApoA-1 [286] | Lipid nanoparticles | Vadimezan and gemcitabine | In vivo imaging, biodistribution, and antitumor effect in Hepa1-6 xenograft model demonstrated specific targeting to HCC tumors |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakrania, A.; Zheng, G.; Bhat, M. Nanomedicine in Hepatocellular Carcinoma: A New Frontier in Targeted Cancer Treatment. Pharmaceutics 2022, 14, 41. https://doi.org/10.3390/pharmaceutics14010041
Bakrania A, Zheng G, Bhat M. Nanomedicine in Hepatocellular Carcinoma: A New Frontier in Targeted Cancer Treatment. Pharmaceutics. 2022; 14(1):41. https://doi.org/10.3390/pharmaceutics14010041
Chicago/Turabian StyleBakrania, Anita, Gang Zheng, and Mamatha Bhat. 2022. "Nanomedicine in Hepatocellular Carcinoma: A New Frontier in Targeted Cancer Treatment" Pharmaceutics 14, no. 1: 41. https://doi.org/10.3390/pharmaceutics14010041