External Evaluation of Population Pharmacokinetic Models and Bayes-Based Dosing of Infliximab
Abstract
:1. Introduction
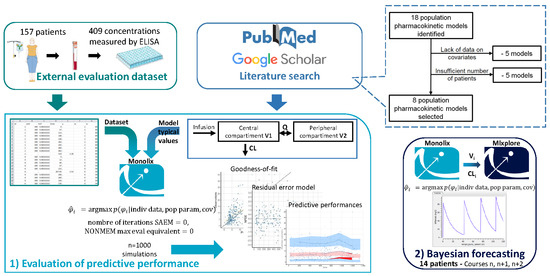
2. Materials and Methods
2.1. Literature Search
- -
- (Infliximab [MeSH Terms]) AND (“1900” [Date-Publication]: “2020/02” [Date-Publication])
- -
- (Infliximab/pharmacokinetics [MeSH Terms]) AND (“1900” [Date-Publication]: “2020/02” [Date-Publication])
- -
- (Infliximab [MeSH Terms]) AND (Pharmacology [MeSH Terms]) OR (Pharmacology, Clinical [MeSH Terms]) AND (“1900” [Date-Publication]: “2020/02” [Date-Publication])
- -
- (Infliximab) AND (“1900” [Date-Publication]: “2020/02” [Date-Publication]) AND (population) AND (pharmacokinetic)
2.2. External Evaluation Dataset
2.3. Evaluation of Predictive Performance
2.4. Bayesian Forecasting
3. Results
3.1. Literature Search
3.2. External Evaluation Dataset
3.3. Evaluation of Predictive Performance
3.3.1. Goodness-of-Fit
3.3.2. Residual Error
3.4. Bayesian Forecasting
4. Discussion
- The population on which the model was built: Although an effort was made to ensure that each model was evaluated on a population that closely resembled the population on which it had been developed, not all patient characteristics were considered. Indeed, only pathology and the detection or not of ADA were used to select patients. Differences in other parameters, such as ethnicity, weight, albumin levels, disease severity, duration of infliximab treatment, or co-medication, may persist and partly explain the models’ non-applicability to our evaluation population. For example, model E was developed on a population with a median Harvey–Bradshaw index (score assessing the severity of Crohn’s disease) much higher than that of our population, showing a greater disease severity, which may influence the PK of infliximab [11,14].
- The model construction study design: A mastered experimental design is necessary for a good estimation of the parameters. In PK studies, the crucial points to optimize are the number of subjects and samples as well as the sampling times, in order to minimize errors in the estimates [47]. Models A, B, C, E, and F have been developed using only residual concentrations, which may not be sufficient to estimate the distribution parameters correctly. However, the residual standard errors (RSE) of the population parameters were acceptable for all models except for the peripheral volume of model E (RSE(V2) = 32%). The RSE represents the estimate’s precision and must be under 30% for fixed parameters and under 50% for parameters of inter-individual variability [48].
- The applied covariates: Many covariates influence the PK of infliximab, and those taken into account varied greatly from one model to another. Models A and B were the ones that considered the largest number of covariates and showed the best results in the external evaluation. However, how covariates are taken into account could be improved in some models. Indeed, if we take weight as an example, our results showed that the concentrations of patients with extreme weights were poorly predicted (p < 0.05). Moreover, the consideration of ADA by models E and F was also problematic (p < 0.0001). As shown by the internal evaluation results during model E’s development, the uncertainty of the relative effect of the antibody covariate on clearance was considerable (CI: 0.1–1.6). These results can be explained by the fact that none of the studies predicted the power required to estimate the covariates’ effects [49]. Indeed, the number of patients presenting these covariates in the construction populations was low (Supplementary Materials Tables S3, S5 and S6), and the imprecision on the parameters associated with the covariates was sometimes large (models B and C: RSE (βMTX) = 49% and 50%, respectively). Moreover, the small number of data or the presence of uninformative data predisposes to the phenomenon of shrinkage, thus explaining imprecise and potentially biased predictions [20]. This is particularly the case for model F, where shrinkage exceeded 40% for the estimation of all population parameters.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klotz, U.; Teml, A.; Schwab, M. Clinical Pharmacokinetics and Use of Infliximab. Clin. Pharmacokinet. 2007, 46, 645–660. [Google Scholar] [CrossRef]
- Wong, U.; Cross, R.K. Primary and Secondary Nonresponse to Infliximab: Mechanisms and Countermeasures. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.J.; Ward, M.G.; Rentsch, C.; Friedman, A.B.; Taylor, K.M.; Sparrow, M.P.; Gibson, P.R. Review Article: Determination of the Therapeutic Range for Therapeutic Drug Monitoring of Adalimumab and Infliximab in Patients with Inflammatory Bowel Disease. Aliment. Pharm. 2020, 51, 612–628. [Google Scholar] [CrossRef] [PubMed]
- St.Clair, E.W.; Wagner, C.L.; Fasanmade, A.A.; Wang, B.; Schaible, T.; Kavanaugh, A.; Keystone, E.C. The Relationship of Serum Infliximab Concentrations to Clinical Improvement in Rheumatoid Arthritis: Results from ATTRACT, a Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheum. 2002, 46, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Mulleman, D.; Chu Miow Lin, D.; Ducourau, E.; Emond, P.; Ternant, D.; Magdelaine-Beuzelin, C.; Valat, J.-P.; Paintaud, G.; Goupille, P. Trough Infliximab Concentrations Predict Efficacy and Sustained Control of Disease Activity in Rheumatoid Arthritis. Drug Monit. 2010, 32, 232–236. [Google Scholar] [CrossRef]
- Wolbink, G.J. Relationship between Serum through Infliximab Levels, Pretreatment C Reactive Protein Levels, and Clinical Response to Infliximab Treatment in Patients with Rheumatoid Arthritis. Ann. Rheum. Dis. 2005, 64, 704–707. [Google Scholar] [CrossRef]
- Chu Miow Lin, D.; Mulleman, D.; Azzopardi, N.; Griffoul-Espitalier, I.; Valat, J.-P.; Paintaud, G.; Goupille, P. Trough Infliximab Concentration May Predict Long-Term Maintenance of Infliximab in Ankylosing Spondylitis. Scand. J. Rheumatol. 2010, 39, 97–98. [Google Scholar] [CrossRef]
- Adedokun, O.J.; Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Xu, Z.; Marano, C.W.; Johanns, J.; Zhou, H.; Davis, H.M.; Cornillie, F.; et al. Association Between Serum Concentration of Infliximab and Efficacy in Adult Patients with Ulcerative Colitis. Gastroenterology 2014, 147, 1296–1307.e5. [Google Scholar] [CrossRef] [Green Version]
- Maser, E.A.; Villela, R.; Silverberg, M.S.; Greenberg, G.R. Association of Trough Serum Infliximab to Clinical Outcome after Scheduled Maintenance Treatment for Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2006, 4, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Nestle, F.O.; Papp, K.; Ortonne, J.-P.; Evans, R.; Guzzo, C.; Li, S.; Dooley, L.T.; Griffiths, C.E.M. EXPRESS study investigators Infliximab Induction and Maintenance Therapy for Moderate-to-Severe Psoriasis: A Phase III, Multicentre, Double-Blind Trial. Lancet 2005, 366, 1367–1374. [Google Scholar] [CrossRef]
- Van den Bemt, B.J.F.; den Broeder, A.A.; Wolbink, G.-J.; van den Maas, A.; Hekster, Y.A.; van Riel, P.L.C.M.; Benraad, H.B.; van den Hoogen, F.H.J. The Combined Use of Disease Activity and Infliximab Serum Trough Concentrations for Early Prediction of (Non-)Response to Infliximab in Rheumatoid Arthritis: Predictive Value of Infliximab Serum Levels. Br. J. Clin. Pharmacol. 2013, 76, 939–945. [Google Scholar] [CrossRef] [Green Version]
- Papamichael, K.; Chachu, K.A.; Vajravelu, R.K.; Vaughn, B.P.; Ni, J.; Osterman, M.T.; Cheifetz, A.S. Improved Long-Term Outcomes of Patients with Inflammatory Bowel Disease Receiving Proactive Compared with Reactive Monitoring of Serum Concentrations of Infliximab. Clin. Gastroenterol. Hepatol. 2017, 15, 1580–1588.e3. [Google Scholar] [CrossRef]
- Brandse, J.F.; Mould, D.; Smeekes, O.; Ashruf, Y.; Kuin, S.; Strik, A.; van den Brink, G.R.; DʼHaens, G.R. A Real-Life Population Pharmacokinetic Study Reveals Factors Associated with Clearance and Immunogenicity of Infliximab in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 650–660. [Google Scholar] [CrossRef] [Green Version]
- Passot, C.; Mulleman, D.; Bejan-Angoulvant, T.; Aubourg, A.; Willot, S.; Lecomte, T.; Picon, L.; Goupille, P.; Paintaud, G.; Ternant, D. The Underlying Inflammatory Chronic Disease Influences Infliximab Pharmacokinetics. mAbs 2016, 8, 1407–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dotan, I.; Ron, Y.; Yanai, H.; Becker, S.; Fishman, S.; Yahav, L.; Ben Yehoyada, M.; Mould, D.R. Patient Factors That Increase Infliximab Clearance and Shorten Half-Life in Inflammatory Bowel Disease: A Population Pharmacokinetic Study. Inflamm. Bowel Dis. 2014, 20, 2247–2259. [Google Scholar] [CrossRef]
- Wojciechowski, J.; Upton, R.N.; Mould, D.R.; Wiese, M.D.; Foster, D.J.R. Infliximab Maintenance Dosing in Inflammatory Bowel Disease: An Example for In Silico Assessment of Adaptive Dosing Strategies. AAPS J. 2017, 19, 1136–1147. [Google Scholar] [CrossRef]
- Mould, D.R.; Upton, R.N.; Wojciechowski, J.; Phan, B.L.; Tse, S.; Dubinsky, M.C. Dashboards for Therapeutic Monoclonal Antibodies: Learning and Confirming. AAPS J. 2018, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Strik, A.S.; Löwenberg, M.; Mould, D.R.; Berends, S.E.; Ponsioen, C.I.; van den Brande, J.M.H.; Jansen, J.M.; Hoekman, D.R.; Brandse, J.F.; Duijvestein, M.; et al. Efficacy of Dashboard Driven Dosing of Infliximab in Inflammatory Bowel Disease Patients; a Randomized Controlled Trial. Scand. J. Gastroenterol. 2021, 56, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Santacana Juncosa, E.; Rodríguez-Alonso, L.; Padullés Zamora, A.; Guardiola, J.; Rodríguez-Moranta, F.; Serra Nilsson, K.; Bas Minguet, J.; Morandeira Rego, F.; Colom Codina, H.; Padullés Zamora, N. Bayes-based Dosing of Infliximab in Inflammatory Bowel Diseases: Short-term Efficacy. Br. J. Clin. Pharm. 2021, 87, 494–505. [Google Scholar] [CrossRef]
- Nguyen, T.H.T.; Mouksassi, M.; Holford, N.; Al-Huniti, N.; Freedman, I.; Hooker, A.C.; John, J.; Karlsson, M.O.; Mould, D.R.; Pérez Ruixo, J.J.; et al. Model Evaluation of Continuous Data Pharmacometric Models: Metrics and Graphics. Cpt: Pharmacomet. Syst. Pharmacol. 2017, 6, 87–109. [Google Scholar] [CrossRef]
- Djerada, Z.; Feliu, C.; Cazaubon, Y.; Smati, F.; Gomis, P.; Guerrot, D.; Charbit, B.; Fernandes, O.; Malinovsky, J.-M. Population Pharmacokinetic-Pharmacodynamic Modeling of Ropivacaine in Spinal Anesthesia. Clin. Pharm. 2018, 57, 1135–1147. [Google Scholar] [CrossRef]
- Yano, Y.; Beal, S.L.; Sheiner, L.B. Evaluating Pharmacokinetic/Pharmacodynamic Models Using the Posterior Predictive Check. J. Pharm. Pharm. 2001, 28, 171–192. [Google Scholar] [CrossRef]
- Comets, E.; Brendel, K.; Mentré, F. Model Evaluation in Nonlinear Mixed Effect Models, with Applications to Pharmacokinetics. J. De La SociÉTÉ Française De Stat. 2010, 151, 106–128. [Google Scholar]
- Cazaubon, Y.; Mauprivez, C.; Feliu, C.; Binet, L.; Oget, O.; Gozalo, C.; Djerada, Z. Population Pharmacokinetics of Articaine with 1:200,000 Epinephrine during Third Molar Surgery and Simulation of High-Dose Regimens. Eur. J. Pharm. Sci. 2018, 114, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Feliu, C.; Millart, H.; Guillemin, H.; Vautier, D.; Binet, L.; Fouley, A.; Djerada, Z. Validation of a Fast UPLC–MS/MS Method for Quantitative Analysis of Opioids, Cocaine, Amphetamines (and Their Derivatives) in Human Whole Blood. Bioanalysis 2015, 7, 2685–2700. [Google Scholar] [CrossRef] [PubMed]
- Corchia, A.; Wynckel, A.; Journet, J.; Moussi Frances, J.; Skandrani, N.; Lautrette, A.; Zafrani, L.; Lewandowski, E.; Reboul, P.; Vrigneaud, L.; et al. Metformin-Related Lactic Acidosis with Acute Kidney Injury: Results of a French Observational Multicenter Study. Clin. Toxicol. 2020, 58, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Cazaubon, Y.; Talineau, Y.; Feliu, C.; Konecki, C.; Russello, J.; Mathieu, O.; Djerada, Z. Population Pharmacokinetics Modelling and Simulation of Mitotane in Patients with Adrenocortical Carcinoma: An Individualized Dose Regimen to Target All Patients at Three Months? Pharmaceutics 2019, 11, 566. [Google Scholar] [CrossRef] [Green Version]
- Allard, Q.; Djerada, Z.; Pouplard, C.; Repessé, Y.; Desprez, D.; Galinat, H.; Frotscher, B.; Berger, C.; Harroche, A.; Ryman, A.; et al. Real Life Population Pharmacokinetics Modelling of Eight Factors VIII in Patients with Severe Haemophilia A: Is It Always Relevant to Switch to an Extended Half-Life? Pharmaceutics 2020, 12, 380. [Google Scholar] [CrossRef]
- Brendel, K.; Dartois, C.; Comets, E.; Lemenuel-Diot, A.; Laveille, C.; Tranchand, B.; Girard, P.; Laffont, C.M.; Mentré, F. Are Population Pharmacokinetic and/or Pharmacodynamic Models Adequately Evaluated? A Survey of the Literature from 2002 to 2004. Clin. Pharm. 2007, 46, 221–234. [Google Scholar] [CrossRef]
- Santacana, E.; Rodríguez-Alonso, L.; Padullés, A.; Guardiola, J.; Rodríguez-Moranta, F.; Serra, K.; Bas, J.; Morandeira Biology, F.; Colom, H.; Padullés, N. External Evaluation of Population Pharmacokinetic Models of Infliximab in Patients with Inflammatory Bowel Disease. Drug Monit. 2018, 40, 120–129. [Google Scholar] [CrossRef]
- Monolix, version 2019R2; Lixoft: Antony, France. Available online: http://lixoft.com/ (accessed on 8 May 2020).
- Brendel, K.; Comets, E.; Laffont, C.; Laveille, C.; Mentré, F. Metrics for External Model Evaluation with an Application to the Population Pharmacokinetics of Gliclazide. Pharm Res. 2006, 23, 2036–2049. [Google Scholar] [CrossRef] [Green Version]
- Ternant, D.; Passot, C.; Aubourg, A.; Goupille, P.; Desvignes, C.; Picon, L.; Lecomte, T.; Mulleman, D.; Paintaud, G. Model-Based Therapeutic Drug Monitoring of Infliximab Using a Single Serum Trough Concentration. Clin. Pharmacokinet. 2017, 57, 1173–1184. [Google Scholar] [CrossRef]
- Aubourg, A.; Picon, L.; Lecomte, T.; Bejan-Angoulvant, T.; Paintaud, G.; Ternant, D. A Robust Estimation of Infliximab Pharmacokinetic Parameters in Crohn’s Disease. Eur. J. Clin. Pharmacol. 2015, 71, 1541–1542. [Google Scholar] [CrossRef]
- Buurman, D.J.; Maurer, J.M.; Keizer, R.J.; Kosterink, J.G.W.; Dijkstra, G. Population Pharmacokinetics of Infliximab in Patients with Inflammatory Bowel Disease: Potential Implications for Dosing in Clinical Practice. Aliment. Pharmacol. Ther. 2015, 42, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Fasanmade, A.A.; Adedokun, O.J.; Blank, M.; Zhou, H.; Davis, H.M. Pharmacokinetic Properties of Infliximab in Children and Adults with Crohn’s Disease: A Retrospective Analysis of Data from 2 Phase III Clinical Trials. Clin. Ther. 2011, 33, 946–964. [Google Scholar] [CrossRef]
- Ternant, D.; Berkane, Z.; Picon, L.; Gouilleux-Gruart, V.; Colombel, J.-F.; Allez, M.; Louis, E.; Paintaud, G. Assessment of the Influence of Inflammation and FCGR3A Genotype on Infliximab Pharmacokinetics and Time to Relapse in Patients with Crohn’s Disease. Clin. Pharmacokinet. 2014, 54, 551–562. [Google Scholar] [CrossRef]
- Dreesen, E.; Faelens, R.; Van Assche, G.; Ferrante, M.; Vermeire, S.; Gils, A.; Bouillon, T. Optimising Infliximab Induction Dosing for Patients with Ulcerative Colitis. Br. J. Clin. Pharm. 2019, 85, 782–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Seitz, K.; Fasanmade, A.; Ford, J.; Williamson, P.; Xu, W.; Davis, H.M.; Zhou, H. Population Pharmacokinetics of Infliximab in Patients with Ankylosing Spondylitis. J. Clin. Pharmacol. 2008, 48, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Ternant, D.; Mulleman, D.; Lauféron, F.; Vignault, C.; Ducourau, E.; Wendling, D.; Goupille, P.; Paintaud, G. Influence of Methotrexate on Infliximab Pharmacokinetics and Pharmacodynamics in Ankylosing Spondylitis: Infliximab in Spondylitis. Br. J. Clin. Pharmacol. 2012, 73, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasanmade, A.A.; Adedokun, O.J.; Ford, J.; Hernandez, D.; Johanns, J.; Hu, C.; Davis, H.M.; Zhou, H. Population Pharmacokinetic Analysis of Infliximab in Patients with Ulcerative Colitis. Eur. J. Clin. Pharmacol. 2009, 65, 1211–1228. [Google Scholar] [CrossRef] [Green Version]
- Ternant, D.; Ducourau, E.; Perdriger, A.; Corondan, A.; Le Goff, B.; Devauchelle-Pensec, V.; Solau-Gervais, E.; Watier, H.; Goupille, P.; Paintaud, G.; et al. Relationship between Inflammation and Infliximab Pharmacokinetics in Rheumatoid Arthritis: Infliximab Pharmacokinetics in Rheumatoid Arthritis. Br. J. Clin. Pharmacol. 2014, 78, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Palaparthy, R.; Rehman, M.I.; von Richter, O.; Yin, D. Population Pharmacokinetics of PF-06438179/GP1111 (an Infliximab Biosimilar) and Reference Infliximab in Patients with Moderately to Severely Active Rheumatoid Arthritis. Expert Opin. Biol. Ther. 2019, 19, 1065–1074. [Google Scholar] [CrossRef]
- Kevans, D.; Murthy, S.; Mould, D.R.; Silverberg, M.S. Accelerated Clearance of Infliximab Is Associated with Treatment Failure in Patients with Corticosteroid-Refractory Acute Ulcerative Colitis. J. Crohn’s Colitis 2018, 12, 662–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ternant, D.; Aubourg, A.; Magdelaine-Beuzelin, C.; Degenne, D.; Watier, H.; Picon, L.; Paintaud, G. Infliximab Pharmacokinetics in Inflammatory Bowel Disease Patients. Drug Monit. 2008, 30, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Kaguelidou, F.; Biran, V.; Zhang, D.; Allegaert, K.; Capparelli, E.V.; Holford, N.; Kimura, T.; Lo, Y.-L.; Peris, J.-E.; et al. External Evaluation of Population Pharmacokinetic Models of Vancomycin in Neonates: The Transferability of Published Models to Different Clinical Settings. Br. J. Clin. Pharm. 2013, 75, 1068–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aarons, L.; Ogungbenro, K. Optimal Design of Pharmacokinetic Studies. Basic Clin. Pharmacol. Toxicol. 2010, 106, 250–255. [Google Scholar] [CrossRef]
- Mould, D.R.; Upton, R.N. Basic Concepts in Population Modeling, Simulation, and Model-Based Drug Development—Part 2: Introduction to Pharmacokinetic Modeling Methods. Cpt: Pharm. Syst. Pharm. 2013, 2, e38. [Google Scholar] [CrossRef]
- Bonate, P.L. Pharmacokinetic-Pharmacodynamic Modeling and Simulation; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-9485-1. [Google Scholar]
- Nanda, K.S.; Cheifetz, A.S.; Moss, A.C. Impact of Antibodies to Infliximab on Clinical Outcomes and Serum Infliximab Levels in Patients with Inflammatory Bowel Disease (IBD): A Meta-Analysis. Am. J. Gastroenterol. 2013, 108, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Abrantes, J.A.; Jönsson, S.; Karlsson, M.O.; Nielsen, E.I. Handling Interoccasion Variability in Model-based Dose Individualization Using Therapeutic Drug Monitoring Data. Br. J. Clin. Pharm. 2019, 85, 1326–1336. [Google Scholar] [CrossRef]
- Holford, N.H.G. Concentration Controlled Therapy. Int. Congr. Ser. 2001, 1220, 135–144. [Google Scholar] [CrossRef]






| Model | A | B | C | D |
|---|---|---|---|---|
| Reference | Passot et al., 2016 | Ternant et al., 2017 PK | Ternant et al., 2017 TLD | Aubourg et al., 2015 |
| Patients | 218 patients with Crohn’s disease, UC, AS, PsA or RA, adults and children, no ADA detected | 143 patients with Crohn’s disease, UC, AS, PsA or RA, adults and children, no ADA detected | 143 patients with Crohn’s disease, UC, AS, PsA or RA, adults and children, no ADA detected | 133 patients with Crohn’s disease, 6 first months of treatment, no ADA detected |
| Infliximab measurement | 870 trough concentrations measured by ELISA (in-house) | Trough concentrations measured by ELISA (in-house) | Trough concentrations measured by ELISA (in-house) | Peak and trough concentrations measured by ELISA (in-house) |
| Tested covariates | Weight, sex, age (2 categories: pediatrics (<15) and adults (≥15)), disease (Crohn, UC, AS, PsA, RA), MTX treatment | Weight, sex, age (2 categories: pediatrics (<15) and adults (≥15)), disease (IBD, AS, PsA, RA), MTX treatment | Weight, sex, age (2 categories: pediatrics (<15) and adults (≥15)), disease (IBD, AS, PsA, RA), MTX treatment | Weight and sex |
| Clearance(s) (L/day) | CL = 0.23 × × e0.181 × Sex × e0.384 × Crohn × e0.472 × UC × e0.392 × RA × e−0.336 × MTX | CL = 0.24 × × e0.36 × IBD × e0.44 × RA × e−0.37 × MTX | CL = 0.20 × × e0.43 × IBD × e0.50 × RA × e−0.47 × MTX | CL = 0.336 × e0.305 × Sex Q = 1.992 |
| Distribution volume(s) (L) | V = 5.2 × e0.209 × Sex × e0.399 × Crohn × e0.417 × UC × e−0.396 × Ped | V = 5.3 × e0.14 × Sex × e0.25 × IBD | V = 5.3 × e0.19 × Sex × e0.27 × IBD | V1 = 2.6 × e0.208 × Sex V2 = 4.5 |
| Residual error model | Combined error σadd = 0.72 mg/L; σprop = 22.3% | Combined error σadd = 0.80 mg/L; σprop = 22% | Proportional error σprop = 29% | Combined error σadd = 2.3 mg/L; σprop = 21% |
| Software (algorithm) | Monolix (SAEM) | Monolix (SAEM) | Monolix (SAEM) | Monolix (SAEM) |
| Internal evaluation procedure | Goodness of fit plots, distribution of residuals plots | Learning (2/3) and validation (1/3) subsets, goodness of fit plots, distribution of residuals plots | Learning (2/3) and validation (1/3) subsets, goodness of fit plots, distribution of residuals plots | Not specified |
| Model | E | F | G | H |
| Reference | Buurman et al., 2015 | Dotan et al., 2014 | Fasanmade et al., 2011 | Wojciechowski et al., 2017 Xu et al., 2012 |
| Patients | 42 patients with Crohn’s disease or UC | 54 patients with Crohn’s disease or UC, adults only | 692 patients with Crohn’s disease, children and adults | 788 patients with Crohn’s disease, UC, RA or Kawasaki, children and adults |
| Infliximab measurement | 188 trough concentrations measured by ELISA (in-house); ADA concentrations measured using a radioimmunoassay | 169 trough concentrations measured and ADA concentrations measured by ELISA (Immunodiagnostik) | 5757 peak and trough concentrations measured by ELISA | Not specified |
| Tested covariates | Clinical scores, duration and extension of disease, treatment period, weight, smoking, Crohn’s disease, UC, CRP, albumin, leucocytes, concomitant immunosuppressive drugs, prior infliximab use | Weight, sex, ADA, albumin | Age, weight, sex, race, AST, ALT, alkaline phosphatase, bilirubin, CRP, ALB, total protein, platelet and white blood cell counts, concurrent use of immunomodulators, ADA | Age, weight, albumin, disease type, ADA |
| Clearance(s) (L/day) | CL = 0.199 × 1.345Sex × 1.722ADA × 1.40Period Q = 0.068 | CL = 0.381 × × × (1 + 1.59 × ADA) Q = 0.122 × | CL = 0.3523 × 1.292ADA × 0.863IMM Q = 0.1469 | CL = 0.294 × e0.257 × ADA Q = 0.079 × |
| Distribution volume(s) (L) | V1 = 4.94 × 0.964 × (HBI-6) V2 = 3.13 | V1 = 2.37 × V2 = 1.37 × | V1 = 3.406 × V2 = 1.274 × | V1 = 3.33 × V2 = 1.14 × |
| Residual error model | Combined error σadd = 0.98 mg/L; σprop = 21.7% | Not specified | Combined error σadd = 0.371 mg/L; σprop = 29.2% | Proportional error σprop = 17.5561% |
| Software (algorithm) | NONMEM (FOCE) | NONMEM | NONMEM | NONMEM |
| Internal evaluation procedure | Visual predictive checks, bootstrap analysis | Goodness of fit plots, visual predictive checks | Bootstrap analysis, condition number | Not specified |
| Characteristic | Median [Min–Max] or Number (%) |
|---|---|
| Female | 82 (52.2) |
| Age (years) | 39.5 (8.5–87.7) |
| Weight (kg) | 68 (24–150) |
| Number of infusions per patient | 23 (2–45) |
| Time between two infusions (days) | 44 (14–79) |
| Administered dose per infusion (mg) | 400 (180–1000) |
| Number of samples per patient | 2 (1–6) |
| Infliximab concentration (mg/L) | 8.68 (0–439) |
| Antibody against infliximab (patients) | 12 (7.6) |
| Disease | |
| 116 (73.9) |
| 22 (14.0) |
| 18 (11.5) |
| 3 (1.9) |
| 3 (1.9) |
| Other immunomodulators | |
| 49 (31.2) |
| 31 (19.7) |
| 6 (3.8) |
| 2 (1.3) |
| Model | Observations vs. PPRED | Observations vs. IPRED | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R² (%) | ME (mg/L) [CI] | p | MPE (%) | RMSE (%) | R² (%) | ME (mg/L) [CI] | p | MPE (%) | RMSE (%) | |
| A | 34.6 | 0.04 [−0.81; 0.90] | 0.9180 | −58.4 | 279.0 | 90.3 | −0.33 [−0.63; −0.02] | 0.0343 | −10.7 | 88.6 |
| B | 34.7 | −0.59 [−1.48; 0.29] | 0.1877 | −41.9 | 236.0 | 90.5 | −0.35 [−0.64; −0.05] | 0.0212 | −9.5 | 84.8 |
| C | 32.0 | 2.45 [1.40; 3.50] | <0.0001 | −94.5 | 326.9 | 90.6 | −0.29 [−0.60; 0.03] | 0.0734 | −9.0 | 89.9 |
| D | 27.7 | −1.04 [−3.55; 1.46] | 0.3940 | −20.5 | 97.0 | 91.8 | −0.74 [−1.45; −0.02] | 0.0454 | −5.1 | 43.4 |
| E | 33.7 | −0.92 [−2.14; 0.31] | 0.1413 | −20.4 | 169.5 | 85.6 | −0.45 [−1.02; 0.13] | 0.1253 | −16.6 | 115.0 |
| F | 41.3 | −7.19 [−8.17; −6.21] | <0.0001 | 53.2 | 96.6 | 91.5 | −1.67 [−2.12; −1.22] | <0.0001 | 8.6 | 43.7 |
| G | 42.5 | −2.69 [−4.10; −1.28] | 0.0002 | 3.5 | 145.0 | 92.3 | −0.53 [−1.05; −0.01] | 0.0442 | −0.4 | 42.8 |
| H | 45.4 | 7.38 [5.88; 8.87] | <0.0001 | −151.8 | 428.0 | 87.0 | 1.02 [0.47; 1.57] | 0.0003 | −39.6 | 299.8 |
| Model | Mean | p | Variance | p | Normality (p) | Symmetry (p) |
|---|---|---|---|---|---|---|
| A | −0.062 | 0.0972 | 1.279 | 0.0100 | 0.0783 | 0.0917 |
| B | 0.158 | 0.0001 | 1.331 | 0.0026 | 0.0003 | 0.0044 |
| C | −0.140 | <0.0001 | 2.135 | <0.0001 | <0.0001 | 0.0023 |
| D | 0.062 | 0.7841 | 2.963 | 0.0067 | 0.7220 | 0.5446 |
| E | 0.441 | <0.0001 | 0.576 | <0.0001 | 0.0197 | 0.9934 |
| F | 1.595 | <0.0001 | 1.092 | 0.4333 | <0.0001 | 0.1131 |
| G | 0.447 | <0.0001 | 1.402 | 0.0062 | 0.0431 | 0.5513 |
| H | −1.199 | <0.0001 | 0.404 | <0.0001 | 0.0003 | 0.0514 |
| Model | Infusion n | Infusion n + 1 | Infusion n + 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ME (mg/L) | MPE (%) | RMSE (%) | ME (mg/L) | MPE (%) | RMSE (%) | ME (mg/L) | MPE (%) | RMSE (%) | |
| A | −0.38 | 2.64 | 12.29 | −1.35 | 14.98 | 23.38 | −4.31 | 37.42 | 39.44 |
| B | −0.50 | 4.71 | 14.07 | −1.28 | 16.38 | 24.88 | −4.52 | 40.60 | 41.96 |
| C | −0.44 | 5.29 | 7.29 | −1.33 | 16.23 | 25.10 | −4.47 | 37.84 | 39.82 |
| E | −0.93 | 3.54 | 48.75 | −2.02 | 22.58 | 32.36 | −4.78 | 47.62 | 50.73 |
| F | −2.08 | 28.58 | 34.46 | −3.59 | 37.23 | 42.67 | −6.58 | 63.62 | 64.33 |
| G | −1.02 | 13.37 | 14.22 | −0.12 | 16.34 | 32.22 | −4.45 | 40.43 | 43.56 |
| H | 0.57 | −12.91 | 28.66 | 0.50 | 0.96 | 22.65 | −2.83 | 26.58 | 32.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konecki, C.; Feliu, C.; Cazaubon, Y.; Giusti, D.; Tonye-Libyh, M.; Brixi, H.; Cadiot, G.; Biron, A.; Djerada, Z. External Evaluation of Population Pharmacokinetic Models and Bayes-Based Dosing of Infliximab. Pharmaceutics 2021, 13, 1191. https://doi.org/10.3390/pharmaceutics13081191
Konecki C, Feliu C, Cazaubon Y, Giusti D, Tonye-Libyh M, Brixi H, Cadiot G, Biron A, Djerada Z. External Evaluation of Population Pharmacokinetic Models and Bayes-Based Dosing of Infliximab. Pharmaceutics. 2021; 13(8):1191. https://doi.org/10.3390/pharmaceutics13081191
Chicago/Turabian StyleKonecki, Celine, Catherine Feliu, Yoann Cazaubon, Delphine Giusti, Marcelle Tonye-Libyh, Hedia Brixi, Guillaume Cadiot, Amélie Biron, and Zoubir Djerada. 2021. "External Evaluation of Population Pharmacokinetic Models and Bayes-Based Dosing of Infliximab" Pharmaceutics 13, no. 8: 1191. https://doi.org/10.3390/pharmaceutics13081191