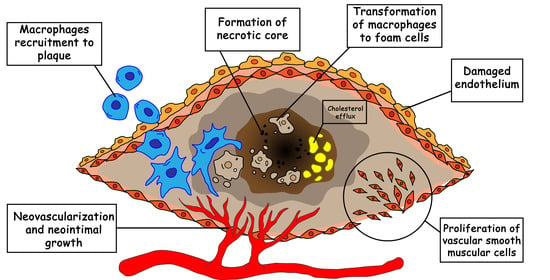
Nanoparticle-Based Approaches towards the Treatment of Atherosclerosis
Abstract
:1. Introduction
2. Nanocarriers-Based Drug Targeting Principles
2.1. ανβ3 Integrin
2.2. Stabilin-2
2.3. CD44
2.4. IL-1 Receptor Antagonist (IL-1ra)
2.5. Vascular Cell Adhesion Molecule-1 (VCAM-1)
2.6. LDL Targeting
3. Methods for the Synthesis of Particles
3.1. Organic Particles
3.1.1. Liposomes
3.1.2. Polymer-Based Particles
3.1.3. Solid Lipid Particles
3.2. Inorganic Nanoparticles
3.2.1. Metal Particles
3.2.2. Metal-Oxide Particles
4. Approaches towards the Treatment of Atherosclerosis
| Model | NPs Composition | NPs Size | Drug Load | Targeted Modality | Results | Ref. |
|---|---|---|---|---|---|---|
| Human monocyte-derived macrophages | Amphiphilic polysaccharide, mucic acid | 100–400 nm | - | Self-bind to scavenger receptors MSR1 and CD36 | Inhibition of oxLDL uptake by macrophages. | [57] |
| Immortalized murine aortic endothelial cells | Au nanospheres | 5, 10, 20, 50 nm | Anti-miR-712 | VCAM-1 targeting peptide | Shown internalization of NPs by cells. The best size for accumulation | [79] |
| RAW264.7 (transformed into inflammatory and foam cells) and HUVECs cells | Oxidation-sensitive chitosan oligosaccharide nanoparticles coated/not coated in macrophages membrane | ∼204 to ∼227 nm | Atorvastatin | Phagocytosis | No signs of cytotoxicity. Better viability of diseased macrophages after treatment with NPs. Reduced NO production and apoptosis. Coating NPs in macrophages membranes effectively reduces uptake by macrophages. | [106] |
| RAW264.7 cells | Cyclodextrin NPs coated with phospholipids | ~100 nm | Simvastatin | Phagocytosis | Dissolution of cholesterol crystals inside cells. Reduced cholesterol levels in the media. Reduced secretion of MCP-1 and TNF-α. Inhibition of cell proliferation. | [45] |
| HUVECs and L929 cells | Apoptotic body biomimetic liposomes | 90 to 140 nm | Pioglitazone | ανβ3 integrin targeting cRGDfK peptide | Reduced expression of IL-1β, IL-6, and TNF-α, shifting of macrophages phenotype from M1 to M2. | [20] |
| Model | NPs Composition | NPs Size | Drug Load | Targeted Modality | Results | Ref. |
|---|---|---|---|---|---|---|
| Ldlr−/− mice | PLGA-PEG | <100 nm | Ac2-26 peptide | Collagen IV | Increase in collagen layer, a decrease in the necrotic core size, reduced oxidative stress. | [107] |
| ApoE−/− mice | Amphiphilic polysaccharide, mucic acid | 100–400 nm | - | Self-bind to scavenger receptors MSR1 and CD36 | Reduced inflammation through lowered lipid content, neointimal hyperplasia, and inflammatory signaling. Reduced necrotic core size. | [57] |
| ApoE−/− mice | Cationic lipoparticles | 144 ± 55 nm | Anti-miR-712 | VCAM1 targeting peptide | Significantly reduced lesion development. | [54] |
| C57BL/6 mice | Au nanospheres | 5, 10, 20, 50 nm | Anti-miR-712 | VCAM-1 targeting peptide | Au nanoparticles with a size of 5 nm have the best accumulation rate in the left carotid artery. | [79] |
| Ldlr−/− mice | PLGA-b-PEG | 156.6 ± 10.3 nm | Synthetic LXR agonist GW3965 | Phosphatidylserine (optional) | Suppressed TNFα and MCP-1 levels. Reduced total cholesterol level in blood. Reduction in macrophage content in plaques. 50% reduced inflammation and lesion area. | [46] |
| ApoE−/− mice | NPs from cyclic polysaccharide β-cyclodextrin | 128 ± 1 nm | Tempol and phenylboronic acid pinacol ester | Phagocytosis by macrophages | Decrease in macrophage content and amount of cholesterol crystals. Decrease in the necrotic core volume and lowered ROS accumulation. Suppressed MMP 9 expression. | [44] |
| ApoE−/− mice | High-density lipoprotein nanoparticle | <220 nm | Simvastatin | Phagocytosis by macrophages | 43% reduced plaque size. Reduced macrophages proliferation and numbers by 65%. Suppressed plaque inflammation by silencing pro-inflammatory genes. | [108] |
| ApoE−/− mice | Single-walled carbon nanotubes | 5–6 nm in diameter, >60 nm in length | Src homology 2 domain-containing phosphatase-1 inhibitor | Phagocytosis by Ly-6Chi monocytes | Promoted efferocytosis resulting in the reduced necrotic core, lesion area, and debris amount. | [109] |
| ApoE−/− mice | Oxidation-sensitive chitosan oligosaccharide nanoparticles coated in macrophages membrane | ∼204 to ∼227 nm | Atorvastatin | Phagocytosis by macrophages | Reduced plaque area compared to the free drug (8% vs. 15%). Reduced the number of monocytes and MMP 9 levels. Thicker fibrous cap and increased proliferation of VSMCs, leading to overall plaque stability. General reduction in inflammation. Macrophages membranes were found to remove inflammatory cytokines or chemokines. Inhibited neovessel endothelial proliferation. | [106] |
| ApoE−/− mice | Cyclodextrin NPs coated with phospholipids | ~100 nm | Simvastatin | Phagocytosis by macrophages | Prolonged circulation time in blood and accumulation within plaque compared to free statin. Reduced proliferation of macrophages and plaque cholesterol levels. Plaque growth inhibition in the early stages of formation. Regression of existing plaques but no impact on blood cholesterol levels effect was observed. | [45] |
| Ldlr−/− mice | PLGA core with a lipid-PEG shell | 116.2 ± 2.5 nm | siRNA against Camk2g gene | S2P peptide, targeting macrophage stabilin-2 receptor | Significant reduction (2–3 times) in CaMKIIγ level proving the silencing of the corresponding gene by siRNA. Efferocytosis promotion. About 20% reduction in necrotic core volume. Twice increased fibrous cap thickness. Overall reduced lesion area. | [47] |
| ApoE−/− mice | α-Cyclodextrin based pH-sensitive NPs | 147.5 ± 2.1 nm | miR-33 | Cyclic pentapeptide (cRGDfK) targeting ανβ3 integrin | Promotion of cholesterol efflux from macrophages. Reduced necrotic core. Increased VSMCs content. | [48] |
| Fat-feed New Zealand white rabbits | Janus particles with a silica core and covered in platelet membrane shell | 300–400 nm | Paclitaxel | Direct delivery, anti-VCAM-1 antibody | Elimination of inflammatory macrophages, long-term anti-proliferation effect | [95] |
| ApoE−/− mice | Apoptotic body biomimetic liposomes | 90–140 nm | Pioglitazone | ανβ3 integrin targeting cRGDfK peptide | Significantly decreased expression of IL-1β and TNF-α due to reduced numbers of M1 macrophages in plaque. Increased collagen amount in fibrous cap, slightly reduced plaque area. | [20] |
4.1. Angiogenesis Prevention
4.2. PDGF Receptor Inhibitor
4.3. Protection of VSMCs
4.4. Inhibition of Pro-Inflammatory Factors
4.5. Protection of Macrophages
4.6. Reduction in Macrophage Proliferation
4.7. Efferocytosis Mediation
4.8. Preventing Low Shear Stress Consequences
4.9. Combined Approaches
5. Magnetic Nanocarriers for Atherosclerosis Therapy
6. Utilization of Theranostic Agents
6.1. Photodynamic Therapy (PDT)
- deeper light penetration into the tissues;
- greater selectivity due to chemical moieties allowing the drug to be activated only in macrophages;
- utilization of drug delivery systems as additional targeting modality;
- higher sensitivity and 1O2 production due to improved spectral characteristics;
- lower toxicity;
- the reduced amount of off-target effects of the drug due to drug encapsulation.
6.2. Photothermal Therapy (PTT)
6.3. Other Theranostic Approaches
7. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Raggi, P.; Genest, J.; Giles, J.T.; Rayner, K.J.; Dwivedi, G.; Beanlands, R.S.; Gupta, M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansson, G.K. Mechanisms of disease: Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, P.F. Endothelial mechanisms of flow-mediated athero-protection and susceptibility. Circ. Res. 2007, 101, 10–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.; Tempel, D.; van Haperen, R.; van der Baan, A.; Grosveld, F.; Daemen, M.J.A.P.; Krams, R.; de Crom, R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 2006, 113, 2744–2753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.Y.; Li, C.J.; Hou, M.F.; Chu, P.Y. New insights into the role of inflammation in the pathogenesis of atherosclerosis. Int. J. Mol. Sci. 2017, 18, 2034. [Google Scholar] [CrossRef]
- Nabel, E.G.; Braunwald, E. A tale of coronary artery disease and myocardial infarction. N. Engl. J. Med. 2012, 366, 54–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namiki, M.; Kawashima, S.; Yamashita, T.; Ozaki, M.; Hirase, T.; Ishida, T.; Inoue, N.; Hirata, K.I.; Matsukawa, A.; Morishita, R.; et al. Local overexpression of monocyte chemoattractant protein-1 at vessel wall induces infiltration of macrophages and formation of atherosclerotic lesion: Synergism with hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Galkina, E.; Ley, K. Immune and inflammatory mechanisms of atherosclerosis. Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef] [Green Version]
- Lamharzi, N.; Renard, C.B.; Kramer, F.; Pennathur, S.; Heinecke, J.W.; Chait, A.; Bornfeldt, K.E. Hyperlipidemia in concert with hyperglycemia stimulates the proliferation of macrophages in atherosclerotic lesions: Potential role of glucose-oxidized LDL. Diabetes 2004, 53, 3217–3225. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Ortiz, A.; Badimon, J.J.; Falk, E.; Fuster, V.; Meyer, B.; Mailhac, A.; Weng, D.; Shah, P.K.; Badimon, L. Characterization of the relative thrombogenicity of atherosclerotic plaque components: Implications for consequences of plaque rupture. J. Am. Coll. Cardiol. 1994, 23, 1562–1569. [Google Scholar] [CrossRef] [Green Version]
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of vulnerable/unstable plaque. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1282–1292. [Google Scholar] [CrossRef] [Green Version]
- Donis, N.; D’emal, C.; Hulin, A.; Gustin, M.; Dulgheru, R.E.; Lancellotti, P.; Oury, C. Effect of cholesterol- and palm oil-enriched diets on rabbit aorta and aortic valve calcification. Arch. Cardiovasc. Dis. Suppl. 2020, 12, 251. [Google Scholar] [CrossRef]
- Gupta, K.K.; Ali, S.; Sanghera, R.S. Pharmacological Options in Atherosclerosis: A Review of the Existing Evidence. Cardiol. Ther. 2019, 8, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Martinet, W.; Schrijvers, D.M.; de Meyer, G.R.Y. Pharmacological modulation of cell death in atherosclerosis: A promising approach towards plaque stabilization? Br. J. Pharmacol. 2011, 164, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ross, J.S.; Stagliano, N.E.; Donovan, M.J.; Breitbart, R.E.; Ginsburg, G.S. Atherosclerosis and cancer common molecular pathways of disease development and progression. Ann. N. Y. Acad. Sci. 2001, 947, 271–293. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting early atherosclerosis: A focus on oxidative stress and inflammation. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef]
- Calin, M.; Butoi, E.; Manea, S.-A.; Simionescu, M.; Manea, A. Lessons from experimental-induced atherosclerosis: Valuable for the precision medicine of tomorrow. In Arterial Revascularization of the Head and Neck; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Calin, M.; Manduteanu, I. Emerging Nanocarriers-based Approaches to Diagnose and Red uce Vascular Inflammation in Atherosclerosis. Curr. Med. Chem. 2017, 24, 550–567. [Google Scholar] [CrossRef]
- Monti, M.; Iommelli, F.; de Rosa, V.; Carriero, M.V.; Miceli, R.; Camerlingo, R.; di Minno, G.; del Vecchio, S. Integrin-dependent cell adhesion to neutrophil extracellular traps through engagement of fibronectin in neutrophil-like cells. PLoS ONE 2017, 12, e0171362. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Dai, L.L.; Wang, Q.Q.; Xue, L.J.; Su, Z.; Zhang, C. An apoptotic body-biomimic liposome in situ upregulates anti-inflammatory macrophages for stabilization of atherosclerotic plaques. J. Control. Release 2019, 316, 236–249. [Google Scholar] [CrossRef]
- Pandey, M.S.; Baggenstoss, B.A.; Washburn, J.; Harris, E.N.; Weigel, P.H. The hyaluronan receptor for endocytosis (HARE) activates NF-κB-mediated gene expression in response to 40-400-kDa, but not smaller or larger, hyaluronans. J. Biol. Chem. 2013, 288, 14068–14079. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.Y.; Kim, J.H.; Oh, G.T.; Lee, B.H.; Kwon, I.C.; Kim, I.S. Molecular targeting of atherosclerotic plaques by a stabilin-2-specific peptide ligand. J. Control. Release 2011, 155, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Kim, J.H.; Choi, K.Y.; Yoon, H.Y.; Kim, K.; Kwon, I.C.; Choi, K.; Lee, B.H.; Park, J.H.; Kim, I.S. Hyaluronic acid nanoparticles for active targeting atherosclerosis. Biomaterials 2015, 53, 341–348. [Google Scholar] [CrossRef]
- Cuff, C.A.; Kothapalli, D.; Azonobi, I.; Chun, S.; Zhang, Y.; Belkin, R.; Yeh, C.; Secreto, A.; Assoian, R.K.; Rader, D.J.; et al. The adhesion receptor CD44 promotes atherosclerosis by mediating inflammatory cell recruitment and vascular cell activation. J. Clin. Investig. 2001, 108, 1031–1040. [Google Scholar] [CrossRef]
- Lazaar, A.L.; Albelda, S.M.; Pilewski, J.M.; Brennan, B.; Puré, E.; Panettieri, R.A. T Lymphocytes adhere to airway smooth muscle cells via integrins and CD44 and induce smooth muscle cell DNA synthesis. J. Exp. Med. 1994, 180, 807–816. [Google Scholar] [CrossRef] [Green Version]
- Hodge-Dufour, J.; Noble, P.W.; Horton, M.R.; Bao, C.; Wysoka, M.; Burdick, M.D.; Strieter, R.M.; Trinchieri, G.; Puré, E. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J. Immunol. 1997, 159, 2492–2500. [Google Scholar]
- Aruffo, A.; Stamenkovic, I.; Melnick, M.; Underhill, C.B.; Seed, B. CD44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61, 1303–1313. [Google Scholar] [CrossRef]
- Vendrov, A.E.; Madamanchi, N.R.; Niu, X.L.; Molnar, K.C.; Runge, M.; Szyndralewiez, C.; Page, P.; Runge, M.S. NADPH oxidases regulate CD44 and hyaluronic acid expression in thrombin-treated vascular smooth muscle cells and in atherosclerosis. J. Biol. Chem. 2010, 285, 26545–26557. [Google Scholar] [CrossRef] [Green Version]
- Dewberry, R.; Holden, H.; Crossman, D.; Francis, S. Interleukin-1 receptor antagonist expression in human endothelial cells and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2394–2400. [Google Scholar] [CrossRef] [Green Version]
- Gabay, C. Cytokine inhibitors in the treatment of rheumatoid arthritis. Expert Opin. Biol. Ther. 2002, 2, 135–149. [Google Scholar] [CrossRef]
- Hoffman, H.M.; Patel, D.D. Genomic-Based Therapy: Targeting Interleukin-1 for Autoinflammatory Diseases. Arthritis Rheum. 2004, 50, 345–349. [Google Scholar] [CrossRef]
- Elhage, R.; Maret, A.; Pieraggi, M.T.; Thiers, J.C.; Arnal, J.F.; Bayard, F. Differential effects of interleukin-1 receptor antagonist and tumor necrosis factor binding protein on fatty-streak formation in apolipoprotein E-deficient mice. Circulation 1998, 97, 242–244. [Google Scholar] [CrossRef] [Green Version]
- Alexander, M.R.; Moehle, C.W.; Johnson, J.L.; Yang, Z.; Lee, J.K.; Jackson, C.L.; Owens, G.K. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J. Clin. Investig. 2012, 122, 70–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aricescu, A.R.; Jones, E.Y. Immunoglobulin superfamily cell adhesion molecules: Zippers and signals. Curr. Opin. Cell Biol. 2007, 19, 543–550. [Google Scholar] [CrossRef]
- Galkina, E.; Ley, K. Vascular adhesion molecules in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2292–2301. [Google Scholar] [CrossRef]
- Fotis, L.; Agrogiannis, G.; Vlachos, I.S.; Pantopoulou, A.; Margoni, A.; Kostaki, M.; Verikokos, C.; Tzivras, D.; Mikhailidis, D.P.; Perrea, D. Intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 at the early stages of atherosclerosis in a rat model. In Vivo 2012, 26, 243–250. [Google Scholar]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Peetz, D.; Hafner, G.; Tiret, L.; Meyer, J. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation 2001, 104, 1336–1342. [Google Scholar] [CrossRef] [Green Version]
- Sobot, D.; Mura, S.; Rouquette, M.; Vukosavljevic, B.; Cayre, F.; Buchy, E.; Pieters, G.; Garcia-Argote, S.; Windbergs, M.; Desmaële, D.; et al. Circulating Lipoproteins: A Trojan Horse Guiding Squalenoylated Drugs to LDL-Accumulating Cancer Cells. Mol. Ther. 2017, 25, 1596–1605. [Google Scholar] [CrossRef] [Green Version]
- Sobot, D.; Mura, S.; Yesylevskyy, S.O.; Dalbin, L.; Cayre, F.; Bort, G.; Mougin, J.; Desmaële, D.; Lepetre-Mouelhi, S.; Pieters, G.; et al. Conjugation of squalene to gemcitabine as unique approach exploiting endogenous lipoproteins for drug delivery. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Brusini, R.; Dormont, F.; Cailleau, C.; Nicolas, V.; Peramo, A.; Varna, M.; Couvreur, P. Squalene-based nanoparticles for the targeting of atherosclerotic lesions. Int. J. Pharm. 2020, 581, 119282. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Chaudhuri, R.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Jaffar, S.; Nam, K.T.; Khademhosseini, A.; Xing, J.; Langer, R.S.; Belcher, A.M. Layer-by-layer surface modification and patterned electrostatic deposition of quantum dots. Nano Lett. 2004, 4, 1421–1425. [Google Scholar] [CrossRef]
- Nobs, L.; Buchegger, F.; Gurny, R.; Allémann, E. Surface modification of poly(lactic acid) nanoparticles by covalent attachment of thiol groups by means of three methods. Int. J. Pharm. 2003, 250, 327–337. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Zhao, W.; Dou, Y.; An, H.; Tao, H.; Xu, X.; Jia, Y.; Lu, S.; Zhang, J.; et al. Targeted Therapy of Atherosclerosis by a Broad-Spectrum Reactive Oxygen Species Scavenging Nanoparticle with Intrinsic Anti-inflammatory Activity. ACS Nano 2018, 12, 8943–8960. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Kumar, S.; Kang, D.W.; Jo, H.; Park, J.H. Affinity-Driven Design of Cargo-Switching Nanoparticles to Leverage a Cholesterol-Rich Microenvironment for Atherosclerosis Therapy. ACS Nano 2020, 14, 6519–6531. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Even-Or, O.; Xu, X.; van Rosmalen, M.; Lim, L.; Gadde, S.; Farokhzad, O.C.; Fisher, E.A. Nanoparticles containing a liver X receptor agonist inhibit inflammation and atherosclerosis. Adv. Healthc. Mater. 2015, 4, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Tao, W.; Yurdagul, A.; Kong, N.; Li, W.; Wang, X.; Doran, A.C.; Feng, C.; Wang, J.; Islam, M.A.; Farokhzad, O.C.; et al. SiRNA nanoparticles targeting CaMKIIγ in lesional macrophages improve atherosclerotic plaque stability in mice. Sci. Transl. Med. 2020, 12, eaay1063. [Google Scholar] [CrossRef]
- Li, C.; Dou, Y.; Chen, Y.; Qi, Y.; Li, L.; Han, S.; Jin, T.; Guo, J.; Chen, J.; Zhang, J. Site-Specific MicroRNA-33 Antagonism by pH-Responsive Nanotherapies for Treatment of Atherosclerosis via Regulating Cholesterol Efflux and Adaptive Immunity. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [Green Version]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef]
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2019, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Li, L.; Xing, J.; Cai, J.; Chen, J.; Liu, P.; Gu, N.; Ji, M. Layer-by-layer construction of lipid bilayer on mesoporous silica nanoparticle to improve its water suspensibility and hemocompatibility. J. Sol-Gel Sci. Technol. 2017, 82, 490–499. [Google Scholar] [CrossRef]
- Huang, X.; Xu, M.Q.; Zhang, W.; Ma, S.; Guo, W.; Wang, Y.; Zhang, Y.; Gou, T.; Chen, Y.; Liang, X.J.; et al. ICAM-1-Targeted Liposomes Loaded with Liver X Receptor Agonists Suppress PDGF-Induced Proliferation of Vascular Smooth Muscle Cells. Nanoscale Res. Lett. 2017, 12, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kheirolomoom, A.; Kim, C.W.; Seo, J.W.; Kumar, S.; Son, D.J.; Gagnon, M.K.J.; Ingham, E.S.; Ferrara, K.W.; Jo, H. Multifunctional Nanoparticles Facilitate Molecular Targeting and miRNA Delivery to Inhibit Atherosclerosis in ApoE-/- Mice. ACS Nano 2015, 9, 8885–8897. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.L.T.; Maranhão, R.C.; Tavares, E.R.; Carvalho, P.O.; Higuchi, M.L.; Mattos, F.R.; Pitta, F.G.; Hatab, S.A.; Kalil-Filho, R.; Serrano, C.V. Regression of Atherosclerotic Plaques of Cholesterol-Fed Rabbits by Combined Chemotherapy With Paclitaxel and Methotrexate Carried in Lipid Core Nanoparticles. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Pustulka, K.M.; Wohl, A.R.; Lee, H.S.; Michel, A.R.; Han, J.; Hoye, T.R.; McCormick, A.V.; Panyam, J.; Macosko, C.W. Flash nanoprecipitation: Particle structure and stability. Mol. Pharm. 2013, 10, 4367–4377. [Google Scholar] [CrossRef] [Green Version]
- Lewis, D.R.; Petersen, L.K.; York, A.W.; Zablocki, K.R.; Joseph, L.B.; Kholodovych, V.; Prud’Homme, R.K.; Uhrich, K.E.; Moghe, P.V. Sugar-based amphiphilic nanoparticles arrest atherosclerosis in vivo. Proc. Natl. Acad. Sci. USA 2015, 112, 2693–2698. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Singh, S.K.; Arya, S.K.; Kundu, S.C.; Kapoor, S. Protein Nanoparticles: Promising Platforms for Drug Delivery Applications. ACS Biomater. Sci. Eng. 2018, 4, 3939–3961. [Google Scholar] [CrossRef]
- De Frates, K.; Markiewicz, T.; Gallo, P.; Rack, A.; Weyhmiller, A.; Jarmusik, B.; Hu, X. Protein polymer-based nanoparticles: Fabrication and medical applications. Int. J. Mol. Sci. 2018, 19, 1717. [Google Scholar] [CrossRef] [Green Version]
- Prilepskii, A.; Schekina, A.; Vinogradov, V. Magnetically controlled protein nanocontainers as a drug depot for the hemostatic agent. Nanotechnol. Sci. Appl. 2019, 12, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef]
- Langer, K.; Balthasar, S.; Vogel, V.; Dinauer, N.; von Briesen, H.; Schubert, D. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int. J. Pharm. 2003, 257, 169–180. [Google Scholar] [CrossRef]
- Khlebtsov, B.; Prilepskii, A.; Lomova, M.; Khlebtsov, N. Au-nanocluster-loaded human serum albumin nanoparticles with enhanced cellular uptake for fluorescent imaging. J. Innov. Opt. Health Sci. 2016, 9, 1650004. [Google Scholar] [CrossRef] [Green Version]
- Triplett, M.D.; Rathman, J.F. Optimization of β-carotene loaded solid lipid nanoparticles preparation using a high shear homogenization technique. J. Nanopart. Res. 2009, 11, 601–614. [Google Scholar] [CrossRef]
- Jenning, V.; Lippacher, A.; Gohla, S.H. Medium scale production of solid lipid nanoparticles (SLN) by high pressure homogenization. J. Microencapsul. 2002, 19, 1–10. [Google Scholar] [CrossRef]
- Trotta, M.; Debernardi, F.; Caputo, O. Preparation of solid lipid nanoparticles by a solvent emulsification-diffusion technique. Int. J. Pharm. 2003, 257, 153–160. [Google Scholar] [CrossRef]
- Siekmann, B.; Westesen, K. Investigations on solid lipid nanoparticles prepared by precipitation in o/w emulsions. Eur. J. Pharm. Biopharm. 1996, 42, 104–109. [Google Scholar]
- Kuchur, O.A.; Tsymbal, S.A.; Shestovskaya, M.V.; Serov, N.S.; Dukhinova, M.S.; Shtil, A.A. Metal-derived nanoparticles in tumor theranostics: Potential and limitations. J. Inorg. Biochem. 2020, 209, 111117. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications Toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Bibikova, O.; Popov, A.; Skovorodkin, I.; Prilepskyi, A.; Pylaev, T.; Bykov, A.; Staroverov, S.; Bogatyrev, V.; Tuchin, V.; Kinnunen, M.; et al. Plasmon-resonant gold nanoparticles with variable morphology as optical labels and drug carriers for cytological research. In Proceedings of the European Conference on Biomedical Optics, Munich, Gemrany, 12–16 May 2013; Volume 8801. [Google Scholar]
- Khan, A.; Rashid, A.; Younas, R.; Chong, R. A chemical reduction approach to the synthesis of copper nanoparticles. Int. Nano Lett. 2016, 6, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Daruich de Souza, C.; Ribeiro Nogueira, B.; Rostelato, M.E.C.M. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Hu, S.; Hsieh, Y. Lo Silver nanoparticle synthesis using lignin as reducing and capping agents: A kinetic and mechanistic study. Int. J. Biol. Macromol. 2016, 82, 856–862. [Google Scholar] [CrossRef] [Green Version]
- Egorova, E.M.; Kubatiev, A.A.; Schvets, V.I.; Egorova, E.M.; Kubatiev, A.A.; Schvets, V.I. Antimicrobial Activity of Nanoparticles Stabilized with Synthetic Surfactant. In Biological Effects of Metal Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Hirsch, T.; Zharnikov, M.; Shaporenko, A.; Stahl, J.; Weiss, D.; Wolfbeis, O.S.; Mirsky, V.M. Size-controlled electrochemical synthesis of metal nanoparticles on monomolecular templates. Angew. Chem. Int. Ed. 2005, 44, 6775–6778. [Google Scholar] [CrossRef]
- Kim, F.; Song, J.H.; Yang, P. Photochemical synthesis of gold nanorods. J. Am. Chem. Soc. 2002, 124, 14316–14317. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.J.; Scurrell, M.S. Polymer stabilized silver nanoparticles: A photochemical synthesis route. J. Mater. Sci. 2004, 39, 4459–4463. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Sun, T.; Simmons, R.; Huo, D.; Pang, B.; Zhao, X.; Kim, C.W.; Jo, H.; Xia, Y. Targeted delivery of anti-miR-712 by VCAM1-binding Au nanospheres for atherosclerosis therapy. ChemNanoMat 2016, 2, 400–406. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “Green” Synthesis and Stabilization of Metal Nanoparticles. J. Am. Chem. Soc. 2003, 46, 13940–13941. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Chumakov, D.S.; Sokolov, A.O.; Bogatyrev, V.A.; Sokolov, O.I.; Selivanov, N.Y.; Dykman, L.A. Green Synthesis of Gold Nanoparticles using Arabidopsis thaliana and Dunaliella salina Cell Cultures. Nanotechnol. Russ. 2018, 13, 539–545. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. 2010, 156, 1–13. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Fabrication of Metal Nanoparticles from Fungi and Metal Salts: Scope and Application. Nanoscale Res. Lett. 2016, 11, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divakaran, D.; Lakkakula, J.R.; Thakur, M.; Kumawat, M.K.; Srivastava, R. Dragon fruit extract capped gold nanoparticles: Synthesis and their differential cytotoxicity effect on breast cancer cells. Mater. Lett. 2019, 236, 498–502. [Google Scholar] [CrossRef]
- Chumakov, D.; Pylaev, T.; Avdeeva, E.; Dykman, L.; Khlebtsov, N.; Bogatyrev, V. Anticancer properties of gold nanoparticles biosynthesized by reducing of chloroaurate ions with Dunaliella salina aqueous extract. In Proceedings of the Saratov Fall Meeting 2019: VII International Symposium on Optics and Biophotonics, 2019, Saratov, Russia, 23–27 September 2019; Volume 11457, p. 1145715. [Google Scholar]
- Fakhardo, A.F.; Anastasova, E.I.; Gabdullina, S.R.; Solovyeva, A.S.; Saparova, V.B.; Chrishtop, V.V.; Koshevaya, E.D.; Krivoshapkina, E.F.; Krivoshapkin, P.V.; Kiselev, G.O.; et al. Toxicity Patterns of Clinically Relevant Metal Oxide Nanoparticles. ACS Appl. Bio Mater. 2019, 2, 4427–4435. [Google Scholar] [CrossRef]
- Dukhinova, M.S.; Prilepskii, A.Y.; Vinogradov, V.V.; Shtil, A.A. Metal oxide nanoparticles in therapeutic regulation of macrophage functions. Nanomaterials 2019, 9, 1631. [Google Scholar] [CrossRef] [Green Version]
- Prilepskii, A.Y.; Fakhardo, A.F.; Drozdov, A.S.; Vinogradov, V.V.; Dudanov, I.P.; Shtil, A.A.; Bel’Tyukov, P.P.; Shibeko, A.M.; Koltsova, E.M.; Nechipurenko, D.Y.; et al. Urokinase-Conjugated Magnetite Nanoparticles as a Promising Drug Delivery System for Targeted Thrombolysis: Synthesis and Preclinical Evaluation. ACS Appl. Mater. Interfaces 2018, 10, 36764–36775. [Google Scholar] [CrossRef]
- Chapurina, Y.E.; Drozdov, A.S.; Popov, I.; Vinogradov, V.V.; Dudanov, I.P.; Vinogradov, V.V. Streptokinase@ alumina nanoparticles as a promising thrombolytic colloid with prolonged action. J. Mater. Chem. B 2016, 4, 5921–5928. [Google Scholar] [CrossRef]
- Roca, A.G.; Gutiérrez, L.; Gavilán, H.; Fortes Brollo, M.E.; Veintemillas-Verdaguer, S.; del Morales, M.P. Design strategies for shape-controlled magnetic iron oxide nanoparticles. Adv. Drug Deliv. Rev. 2019, 138, 68–104. [Google Scholar] [CrossRef]
- Hossaini Nasr, S.; Tonson, A.; El-Dakdouki, M.H.; Zhu, D.C.; Agnew, D.; Wiseman, R.; Qian, C.; Huang, X. Effects of Nanoprobe Morphology on Cellular Binding and Inflammatory Responses: Hyaluronan-Conjugated Magnetic Nanoworms for Magnetic Resonance Imaging of Atherosclerotic Plaques. ACS Appl. Mater. Interfaces 2018, 10, 11495–11507. [Google Scholar] [CrossRef]
- Drozdov, A.S.; Ivanovski, V.; Avnir, D.; Vinogradov, V.V. A universal magnetic ferrofluid: Nanomagnetite stable hydrosol with no added dispersants and at neutral pH. J. Colloid Interface Sci. 2016, 468, 307–312. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, J.; Ding, Q.; Lin, D.; Dong, R.; Yang, L.; Liu, J. Sea-urchin-like Fe3O4@C@Ag particles: An efficient SERS substrate for detection of organic pollutants. Nanoscale 2013, 5, 5887–5895. [Google Scholar] [CrossRef]
- Huang, Y.; Li, T.; Gao, W.; Wang, Q.; Li, X.; Mao, C.; Zhou, M.; Wan, M.; Shen, J. Platelet-derived nanomotor coated balloon for atherosclerosis combination therapy. J. Mater. Chem. B 2020, 8, 5765–5775. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Cha, J.; Lee, J.K. Synthesis of various magnetite nanoparticles through simple phase transformation and their shape-dependent magnetic properties. RSC Adv. 2013, 3, 8365–8371. [Google Scholar] [CrossRef]
- Kasparis, G.; Erdocio, A.S.; Tuffnell, J.M.; Thanh, N.T.K. Synthesis of size-tuneable β-FeOOH nanoellipsoids and a study of their morphological and compositional changes by reduction. CrystEngComm 2019, 21, 1293–1301. [Google Scholar] [CrossRef] [Green Version]
- Jazirehpour, M.; Ebrahimi, S.A.S. Synthesis of magnetite nanostructures with complex morphologies and effect of these morphologies on magnetic and electromagnetic properties. Ceram. Int. 2016, 42, 16512–16520. [Google Scholar] [CrossRef]
- Sun, H.; Chen, B.; Jiao, X.; Jiang, Z.; Qin, Z.; Chen, D. Solvothermal synthesis of tunable electroactive magnetite nanorods by controlling the side reaction. J. Phys. Chem. C 2012, 116, 5476–5481. [Google Scholar] [CrossRef]
- Massart, R. Preparation of Aqueous Magnetic Liquids in Alkaline and Acidic Media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Y.C.; Feldmann, C. Polyol synthesis of nanoparticles: Status and options regarding metals, oxides, chalcogenides, and non-metal elements. Green Chem. 2015, 17, 4107–44132. [Google Scholar] [CrossRef]
- Kang, G.S.; Gillespie, P.A.; Gunnison, A.; Moreira, A.L.; Tchou-Wong, K.M.; Chen, L.C. Long-term inhalation exposure to nickel nanoparticles exacerbated atherosclerosis in a susceptible mouse model. Environ. Health Perspect. 2011, 119, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Ma, R.; Liu, X.; Chen, T.; Li, Y.; Yu, Y.; Duan, J.; Zhou, X.; Li, Y.; Sun, Z. Silica nanoparticles promote oxLDL-induced macrophage lipid accumulation and apoptosis via endoplasmic reticulum stress signaling. Sci. Total Environ. 2018, 631, 570–579. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J. Endothelial cells dysfunction induced by silica nanoparticles through oxidative stress via JNK/P53 and NF-κB pathways. Biomaterials 2010, 31, 8198–8209. [Google Scholar] [CrossRef]
- Duan, J.; Du, J.; Jin, R.; Zhu, W.; Liu, L.; Yang, L.; Li, M.; Gong, Q.; Song, B.; Anderson, J.M.; et al. Iron oxide nanoparticles promote vascular endothelial cells survival from oxidative stress by enhancement of autophagy. Regen. Biomater. 2019, 6, 221–229. [Google Scholar] [CrossRef]
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.T.; Yue, L.; Wan, J.B.; Lee, S.M.Y.; Wang, R. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Fredman, G.; Kamaly, N.; Spolitu, S.; Milton, J.; Ghorpade, D.; Chiasson, R.; Kuriakose, G.; Perretti, M.; Farokzhad, O.; Tabas, I. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci. Transl. Med. 2015, 7, 275ra20. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Lobatto, M.E.; Hassing, L.; van der Staay, S.; van Rijs, S.M.; Calcagno, C.; Braza, M.S.; Baxter, S.; Fay, F.; Sanchez-Gaytan, B.L.; et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci. Adv. 2015, 1, e1400223. [Google Scholar] [CrossRef] [Green Version]
- Flores, A.M.; Hosseini-Nassab, N.; Jarr, K.U.; Ye, J.; Zhu, X.; Wirka, R.; Koh, A.L.; Tsantilas, P.; Wang, Y.; Nanda, V.; et al. Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nat. Nanotechnol. 2020, 15, 154–161. [Google Scholar] [CrossRef]
- Ingber, D.; Fujita, T.; Kishimoto, S.; Sudo, K.; Kanamaru, T.; Brem, H.; Folkman, J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 1990, 348, 555–557. [Google Scholar] [CrossRef]
- Winter, P.M.; Neubauer, A.M.; Caruthers, S.D.; Harris, T.D.; Robertson, J.D.; Williams, T.A.; Schmieder, A.H.; Hu, G.; Allen, J.S.; Lacy, E.K.; et al. Endothelial ανβ3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2103–2109. [Google Scholar] [CrossRef] [Green Version]
- Maranhão, R.C.; Tavares, E.R.; Padoveze, A.F.; Valduga, C.J.; Rodrigues, D.G.; Pereira, M.D. Paclitaxel associated with cholesterol-rich nanoemulsions promotes atherosclerosis regression in the rabbit. Atherosclerosis 2008, 197, 959–966. [Google Scholar] [CrossRef]
- Bulgarelli, A.; Martins Dias, A.A.; Caramelli, B.; Maranhão, R.C. Treatment with methotrexate inhibits atherogenesis in cholesterol-fed rabbits. J. Cardiovasc. Pharmacol. 2012, 59, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Meneghini, B.C.; Tavares, E.R.; Guido, M.C.; Tavoni, T.M.; Stefani, H.A.; Kalil-Filho, R.; Maranhão, R.C. Lipid core nanoparticles as vehicle for docetaxel reduces atherosclerotic lesion, inflammation, cell death and proliferation in an atherosclerosis rabbit model. Vascul. Pharmacol. 2019, 115, 46–54. [Google Scholar] [CrossRef]
- Fiorelli, A.I.; Lourenço-Filho, D.D.; Tavares, E.R.; Carvalho, P.O.; Marques, A.F.; Gutierrez, P.S.; Maranhão, R.C.; Stolf, N.A.G. Methotrexate associated to lipid core nanoparticles improves cardiac allograft vasculopathy and the inflammatory profile in a rabbit heart graft model. Braz. J. Med. Biol. Res. 2017, 50, e6225. [Google Scholar] [CrossRef] [Green Version]
- Shiozaki, A.A.; Senra, T.; Morikawa, A.T.; Deus, D.F.; Paladino-Filho, A.T.; Pinto, I.M.F.; Maranhão, R.C. Treatment of patients with aortic atherosclerotic disease with paclitaxel-associated lipid nanoparticles. Clinics 2016, 71, 435–439. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [Green Version]
- Mondy, J.S.; Lindner, V.; Miyashiro, J.K.; Berk, B.C.; Dean, R.H.; Geary, R.L. Platelet-derived growth factor ligand and receptor expression in response to altered blood flow in vivo. Circ. Res. 1997, 81, 320–327. [Google Scholar] [CrossRef]
- Negoro, N.; Kanayama, Y.; Haraguchi, M.; Umetani, N.; Nishimura, M.; Konishi, Y.; Iwai, J.; Okamura, M.; Inoue, T.; Takeda, T. Blood pressure regulates platelet-derived growth factor A-chain gene expression in vascular smooth muscle cells in vivo. An autocrine mechanism promoting hypertensive vascular hypertrophy. J. Clin. Investig. 1995, 95, 1140–1150. [Google Scholar] [CrossRef]
- Ross, R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 1993, 362, 801–809. [Google Scholar] [CrossRef]
- He, C.; Medley, S.C.; Hu, T.; Hinsdale, M.E.; Lupu, F.; Virmani, R.; Olson, L.E. PDGFRβ signalling regulates local inflammation and synergizes with hypercholesterolaemia to promote atherosclerosis. Nat. Commun. 2015, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, W.D.; Wang, K.Q.; Li, T.; Song, S.H.; Yuan, B. Expression of platelet-derived growth factor in the vascular walls of patients with lower extremity arterial occlusive disease. Exp. Ther. Med. 2015, 9, 1223–1228. [Google Scholar] [CrossRef]
- Esfandyari-Manesh, M.; Abdi, M.; Talasaz, A.H.; Ebrahimi, S.M.; Atyabi, F.; Dinarvand, R. S2P peptide-conjugated PLGA-Maleimide-PEG nanoparticles containing Imatinib for targeting drug delivery to atherosclerotic plaques. DARU J. Pharm. Sci. 2020, 28, 131–138. [Google Scholar] [CrossRef]
- Cohen, M.H.; Dagher, R.; Griebel, D.J.; Ibrahim, A.; Martin, A.; Scher, N.S.; Sokol, G.H.; Williams, G.A.; Pazdur, R.U.S. Food and Drug Administration Drug Approval Summaries: Imatinib Mesylate, Mesna Tablets, and Zoledronic Acid. Oncologist 2002, 7, 393–400. [Google Scholar] [CrossRef]
- Pouwer, M.G.; Pieterman, E.J.; Verschuren, L.; Caspers, M.P.M.; Kluft, C.; Garcia, R.A.; Aman, J.; Jukema, J.W.; Princen, H.M.G. The BCR-ABL1 Inhibitors Imatinib and Ponatinib Decrease Plasma Cholesterol and Atherosclerosis, and Nilotinib and Ponatinib Activate Coagulation in a Translational Mouse Model. Front. Cardiovasc. Med. 2018, 5, 55. [Google Scholar] [CrossRef] [Green Version]
- Gacic, J.; Vorkapic, E.; Olsen, R.S.; Söderberg, D.; Gustafsson, T.; Geffers, R.; Skoglund, K.; Matussek, A.; Wågsäter, D. Imatinib reduces cholesterol uptake and matrix metalloproteinase activity in human THP-1 macrophages. Pharmacol. Rep. 2016, 68, 1–6. [Google Scholar] [CrossRef]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Grootaert, M.O.J.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.R.; de Meyer, G.R.Y. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef]
- Reddy, M.K.; Vasir, J.K.; Sahoo, S.K.; Jain, T.K.; Yallapu, M.M.; Labhasetwar, V. Inhibition of apoptosis through localized delivery of rapamycin-loaded nanoparticles prevented neointimal hyperplasia and reendothelialized injured artery. Circ. Cardiovasc. Interv. 2008, 1, 209–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grootaert, M.O.J.; Schrijvers, D.M.; Hermans, M.; van Hoof, V.O.; de Meyer, G.R.Y.; Martinet, W. Caspase-3 Deletion Promotes Necrosis in Atherosclerotic Plaques of ApoE Knockout Mice. Oxid. Med. Cell. Longev. 2016, 2016, 3087469. [Google Scholar] [CrossRef]
- Grootaert, M.O.J.; Schrijvers, D.M.; van Spaendonk, H.; Breynaert, A.; Hermans, N.; van Hoof, V.O.; Takahashi, N.; Vandenabeele, P.; Kim, S.H.; de Meyer, G.R.Y.; et al. NecroX-7 reduces necrotic core formation in atherosclerotic plaques of Apoe knockout mice. Atherosclerosis 2016, 252, 166–174. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, D.; Maguire, E.M.; He, S.; Chen, J.; An, W.; Yang, M.; Afzal, T.A.; Luong, L.A.; Zhang, L.; et al. Cbx3 inhibits vascular smooth muscle cell proliferation, migration, and neointima formation. Cardiovasc. Res. 2018, 114, 443–455. [Google Scholar] [CrossRef]
- Bedel, A.; Nègre-Salvayre, A.; Heeneman, S.; Grazide, M.H.; Thiers, J.C.; Salvayre, R.; Maupas-Schwalm, F. E-Cadherin/β-Catenin/T-cell factor pathway is involved in smooth muscle cell proliferation elicited by oxidized low-density lipoprotein. Circ. Res. 2008, 103, 694–701. [Google Scholar] [CrossRef] [Green Version]
- Raines, E.W.; Garton, K.J.; Ferri, N. Beyond the Endothelium: NF-κB Regulation of Smooth Muscle Function. Circ. Res. 2004, 94, 706–708. [Google Scholar] [CrossRef] [Green Version]
- Ouimet, M.; Ediriweera, H.; Afonso, M.S.; Ramkhelawon, B.; Singaravelu, R.; Liao, X.; Bandler, R.C.; Rahman, K.; Fisher, E.A.; Rayner, K.J.; et al. MicroRNA-33 Regulates Macrophage Autophagy in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1058–1067. [Google Scholar] [CrossRef] [Green Version]
- Robbins, C.S.; Hilgendorf, I.; Weber, G.F.; Theurl, I.; Iwamoto, Y.; Figueiredo, J.L.; Gorbatov, R.; Sukhova, G.K.; Gerhardt, L.M.S.; Smyth, D.; et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 2013, 19, 1166. [Google Scholar] [CrossRef]
- Senokuchi, T.; Matsumura, T.; Sakai, M.; Yano, M.; Taguchi, T.; Matsuo, T.; Sonoda, K.; Kukidome, D.; Imoto, K.; Nishikawa, T.; et al. Statins suppress oxidized low density lipoprotein-induced macrophage proliferation by inactivation of the small G protein-p38 MAPK pathway. J. Biol. Chem. 2005, 280, 6627–6633. [Google Scholar] [CrossRef] [Green Version]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; De Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef]
- Van Vré, E.A.; Ait-Oufella, H.; Tedgui, A.; Mallat, Z. Apoptotic cell death and efferocytosis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 887–893. [Google Scholar] [CrossRef] [Green Version]
- Thorp, E.; Tabas, I. Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J. Leukoc. Biol. 2009, 86, 1089–1095. [Google Scholar] [CrossRef] [Green Version]
- Yurdagul, A.; Doran, A.C.; Cai, B.; Fredman, G.; Tabas, I.A. Mechanisms and Consequences of Defective Efferocytosis in Atherosclerosis. Front. Cardiovasc. Med. 2018, 4, 86. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of toxicity studies of carbon nanotubes. J. Occup. Health 2017, 59, 394–407. [Google Scholar] [CrossRef] [Green Version]
- Francis, A.P.; Devasena, T. Toxicity of carbon nanotubes: A review. Toxicol. Ind. Health 2018, 34, 200–210. [Google Scholar] [CrossRef]
- Souilhol, C.; Serbanovic-Canic, J.; Fragiadaki, M.; Chico, T.J.; Ridger, V.; Roddie, H.; Evans, P.C. Endothelial responses to shear stress in atherosclerosis: A novel role for developmental genes. Nat. Rev. Cardiol. 2020, 17, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kim, C.W.; Simmons, R.D.; Jo, H. Role of flow-sensitive microRNAs in endothelial dysfunction and atherosclerosis mechanosensitive athero-miRs. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2206–2216. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.W.; Kumar, S.; Son, D.J.; Jang, I.H.; Griendling, K.K.; Jo, H. Prevention of abdominal aortic aneurysm by anti-MicroRNA-712 or anti-MicroRNA-205 in angiotensin II-infused mice. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1412–1421. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Law, J.; Luo, M.; Gong, Z.; Yu, J.; Tang, W.; Zhang, Z.; Mei, X.; Huang, Z.; You, L.; et al. Magnetic Measurement and Stimulation of Cellular and Intracellular Structures. ACS Nano 2020, 14, 3805–3821. [Google Scholar] [CrossRef]
- Kim, J.W.; Jeong, H.K.; Southard, K.M.; Jun, Y.W.; Cheon, J. Magnetic Nanotweezers for Interrogating Biological Processes in Space and Time. Acc. Chem. Res. 2018, 51, 839–849. [Google Scholar] [CrossRef]
- Nuzhina, J.V.; Shtil, A.A.; Prilepskii, A.Y.; Vinogradov, V.V. Preclinical Evaluation and Clinical Translation of Magnetite-Based Nanomedicines. J. Drug Deliv. Sci. Technol. 2019, 54, 101282. [Google Scholar] [CrossRef]
- Ta, H.T.; Li, Z.; Hagemeyer, C.E.; Cowin, G.; Zhang, S.; Palasubramaniam, J.; Alt, K.; Wang, X.; Peter, K.; Whittaker, A.K. Molecular imaging of activated platelets via antibody-targeted ultra-small iron oxide nanoparticles displaying unique dual MRI contrast. Biomaterials 2017, 134, 31–42. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Luchinat, C. Relaxation Times. In Nuclear and Electron Relaxion. The Magnetic Nucleus-Unpaired Electron Coupling in Solution; VCH Publisher: New York, NY, USA, 1993; pp. 23–39. [Google Scholar]
- Liu, J.; Guo, X.; Zhao, Z.; Li, B.; Qin, J.; Peng, Z.; He, G.; Brett, D.J.L.; Wang, R.; Lu, X. Fe3S4 nanoparticles for arterial inflammation therapy: Integration of magnetic hyperthermia and photothermal treatment. Appl. Mater. Today 2020, 18, 100457. [Google Scholar] [CrossRef]
- Balanov, V.A.; Kiseleva, A.P.; Krivoshapkina, E.F.; Kashtanov, E.; Gimaev, R.R.; Zverev, V.I.; Krivoshapkin, P.V. Synthesis of (Mn(1−x)Znx)Fe2O4 nanoparticles for magnetocaloric applications. J. Sol-Gel Sci. Technol. 2020, 95, 795–800. [Google Scholar] [CrossRef]
- Matuszak, J.; Lutz, B.; Sekita, A.; Zaloga, J.; Alexiou, C.; Lyer, S.; Cicha, I. Drug delivery to atherosclerotic plaques using superparamagnetic iron oxide nanoparticles. Int. J. Nanomed. 2018, 13, 8443. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Chen, H.; Chen, C.; Zhang, X.; Tian, X.; Zhang, Y.; Shi, Z.; Liu, Q. Polymer-lipid hybrid theranostic nanoparticles co-delivering ultrasmall superparamagnetic iron oxide and paclitaxel for targeted magnetic resonance imaging and therapy in atherosclerotic plaque. J. Biomed. Nanotechnol. 2016, 12, 1245–1257. [Google Scholar] [CrossRef]
- Wu, G.; Wei, W.; Zhang, J.; Nie, W.; Yuan, L.; Huang, Y.; Zuo, L.; Huang, L.; Xi, X.; Xie, H.Y. A self-driven bioinspired nanovehicle by leukocyte membrane-hitchhiking for early detection and treatment of atherosclerosis. Biomaterials 2020, 250, 119963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tian, X.Y.; Chan, C.K.W.; Bai, Q.; Cheng, C.K.; Chen, F.M.; Cheung, M.S.H.; Yin, B.; Yang, H.; Yung, W.Y.; et al. Promoting the Delivery of Nanoparticles to Atherosclerotic Plaques by DNA Coating. ACS Appl. Mater. Interfaces 2019, 11, 13888–13904. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ke, H.; Dai, Z.; Liu, Z. Nanoscale theranostics for physical stimulus-responsive cancer therapies. Biomaterials 2015, 73, 214–230. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining imaging and therapy. Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, J.; Heitz, V.; Sour, A.; Bolze, F.; Kessler, P.; Flamigni, L.; Ventura, B.; Bonnet, C.S.; Tõth, É. A Theranostic Agent Combining a Two-Photon-Absorbing Photosensitizer for Photodynamic Therapy and a Gadolinium(III) Complex for MRI Detection. Chem. A Eur. J. 2016, 22, 2775–2786. [Google Scholar] [CrossRef]
- Park, D.; Cho, Y.; Goh, S.H.; Choi, Y. Hyaluronic acid–polypyrrole nanoparticles as pH-responsive theranostics. Chem. Commun. 2014, 95, 15014–15017. [Google Scholar] [CrossRef]
- Qin, J.; Peng, Z.; Li, B.; Ye, K.; Zhang, Y.; Yuan, F.; Yang, X.; Huang, L.; Hu, J.; Lu, X. Gold nanorods as a theranostic platform for in vitro and in vivo imaging and photothermal therapy of inflammatory macrophages. Nanoscale 2015, 7, 13991–14001. [Google Scholar] [CrossRef]
- De Oliveira Gonçalves, K.; da Silva, M.N.; Sicchieri, L.B.; de Oliveira Silva, F.R.; de Matos, R.A.; Courrol, L.C. Aminolevulinic acid with gold nanoparticles: A novel theranostic agent for atherosclerosis. Analyst 2015, 140, 1974–1980. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, X.; Zhang, S.; Wei, G.; Su, Z. Biomedical and bioactive engineered nanomaterials for targeted tumor photothermal therapy: A review. Mater. Sci. Eng. C 2019, 104, 109891. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kolemen, S.; Yoon, J.; Akkaya, E.U. Activatable Photosensitizers: Agents for Selective Photodynamic Therapy. Adv. Funct. Mater. 2017, 5, 1604053. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591. [Google Scholar] [CrossRef] [Green Version]
- Henderson, B.W.; Busch, T.M.; Vaughan, L.A.; Frawley, N.P.; Babich, D.; Sosa, T.A.; Zollo, J.D.; Dee, A.S.; Cooper, M.T.; Bellnier, D.A.; et al. Photofrin photodynamic therapy can significantly deplete or preserve oxygenation in human basal cell carcinomas during treatment, depending on fluence rate. Cancer Res. 2000, 60, 525–529. [Google Scholar]
- Peng, C.; Li, Y.; Liang, H.; Cheng, J.; Li, Q.; Sun, X.; Li, Z.; Wang, F.; Guo, Y.; Tian, Z.; et al. Detection and photodynamic therapy of inflamed atherosclerotic plaques in the carotid artery of rabbits. J. Photochem. Photobiol. B Biol. 2011, 102, 26–31. [Google Scholar] [CrossRef]
- Han, X.B.; Li, H.X.; Jiang, Y.Q.; Wang, H.; Li, X.S.; Kou, J.Y.; Zheng, Y.H.; Liu, Z.N.; Li, H.; Li, J.; et al. Upconversion nanoparticle-mediated photodynamic therapy induces autophagy and cholesterol efflux of macrophage-derived foam cells via ROS generation. Cell Death Dis. 2017, 8, e2864. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Jaffer, F.A.; Weissleder, R. A macrophage-targeted theranostic nanoparticle for biomedical applications. Small 2006, 2, 983–987. [Google Scholar] [CrossRef]
- Deguchi, J.O.; Aikawa, M.; Tung, C.H.; Aikawa, E.; Kim, D.E.; Ntziachristos, V.; Weissleder, R.; Libby, P. Inflammation in atherosclerosis: Visualizing matrix metalloproteinase action in macrophages in vivo. Circulation 2006, 114, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos-Antonakakis, N.; Sarantopoulou, E.; Trohopoulos, P.N.; Stefi, A.L.; Kollia, Z.; Gavriil, V.E.; Bourkoula, A.; Petrou, P.S.; Kakabakos, S.; Semashko, V.V.; et al. Selective aggregation of PAMAM dendrimer nanocarriers and PAMAM/ZnPc nanodrugs on human atheromatous carotid tissues: A photodynamic therapy for atherosclerosis. Nanoscale Res. Lett. 2015, 10, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, Y.; Kim, I.H.; Kim, K.; Choi, Y. ROS-Responsive activatable photosensitizing agent for imaging and photodynamic therapy of activated macrophages. Theranostics 2014, 4, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wennink, J.W.H.; Liu, Y.; Mäkinen, P.I.; Setaro, F.; de la Escosura, A.; Bourajjaj, M.; Lappalainen, J.P.; Holappa, L.P.; van den Dikkenberg, J.B.; al Fartousi, M.; et al. Macrophage selective photodynamic therapy by meta-tetra(hydroxyphenyl)chlorin loaded polymeric micelles: A possible treatment for cardiovascular diseases. Eur. J. Pharm. Sci. 2017, 107, 112–125. [Google Scholar] [CrossRef]
- Lu, K.Y.; Lin, P.Y.; Chuang, E.Y.; Shih, C.M.; Cheng, T.M.; Lin, T.Y.; Sung, H.W.; Mi, F.L. H2O2-Depleting and O2-Generating Selenium Nanoparticles for Fluorescence Imaging and Photodynamic Treatment of Proinflammatory-Activated Macrophages. ACS Appl. Mater. Interfaces 2017, 9, 5158–5172. [Google Scholar] [CrossRef]
- Lu, R.; Zhu, J.; Yu, C.; Nie, Z.; Gao, Y. Cu3BiS3 Nanocrystals as Efficient Nanoplatforms for CT Imaging Guided Photothermal Therapy of Arterial Inflammation. Front. Bioeng. Biotechnol. 2020, 8, 981. [Google Scholar] [CrossRef]
- Gao, W.; Sun, Y.; Cai, M.; Zhao, Y.; Cao, W.; Liu, Z.; Cui, G.; Tang, B. Copper sulfide nanoparticles as a photothermal switch for TRPV1 signaling to attenuate atherosclerosis. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Harel-Adar, T.; Ben Mordechai, T.; Amsalem, Y.; Feinberg, M.S.; Leor, J.; Cohen, S. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc. Natl. Acad. Sci. USA 2011, 108, 1827–1832. [Google Scholar] [CrossRef] [Green Version]
- Winter, P.M.; Morawski, A.M.; Caruthers, S.D.; Fuhrhop, R.W.; Zhang, H.; Williams, T.A.; Allen, J.S.; Lacy, E.K.; Robertson, J.D.; Lanza, G.M.; et al. Molecular Imaging of Angiogenesis in Early-Stage Atherosclerosis With αvβ3-Integrin-Targeted Nanoparticles. Circulation 2003, 108, 2270–2274. [Google Scholar] [CrossRef] [Green Version]
- Marrache, S.; Dhar, S. Biodegradable synthetic high-density lipoprotein nanoparticles for atherosclerosis. Proc. Natl. Acad. Sci. USA 2013, 110, 9440–9450. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, J.; Cheedu, D.; Sebastian, R.; Mascata, R.; Sidawy, A.; Mishra, L.; Nguyen, B. Abstract 139: PARP-1 Silencing Upregulates FOSL1 Transcription, Enhances Angiogenesis and Accelerates Ischemic-Diabetic Wound Healing. Arterioscler. Thromb. Vasc. Biol. 2018, 38, A139. [Google Scholar] [CrossRef]
- Kojima, Y.; Downing, K.; Kundu, R.; Miller, C.; Dewey, F.; Lancero, H.; Raaz, U.; Perisic, L.; Hedin, U.; Schadt, E.; et al. Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. J. Clin. Investig. 2014, 124, 1083–1097. [Google Scholar] [CrossRef] [Green Version]
- Barrett, H.E.; van der Heiden, K.; Farrell, E.; Gijsen, F.J.H.; Akyildiz, A.C. Calcifications in atherosclerotic plaques and impact on plaque biomechanics. J. Biomech. 2019, 87, 1–12. [Google Scholar] [CrossRef]
- Rogers, M.A.; Chen, J.; Nallamshetty, S.; Pham, T.; Goto, S.; Muehlschlegel, J.D.; Libby, P.; Aikawa, M.; Aikawa, E.; Plutzky, J. Retinoids repress human cardiovascular cell calcification with evidence for distinct selective retinoid modulator effects. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 656–669. [Google Scholar] [CrossRef]
- Karamched, S.R.; Nosoudi, N.; Moreland, H.E.; Chowdhury, A.; Vyavahare, N.R. Site-specific chelation therapy with EDTA-loaded albumin nanoparticles reverses arterial calcification in a rat model of chronic kidney disease. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Zhang, X.; Gu, Z.; Zhao, Y. Recent Advances in Upconversion Nanoparticles-Based Multifunctional Nanocomposites for Combined Cancer Therapy. Adv. Mater. 2015, 47, 7692–7712. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tao, H.; Cheng, L.; Liu, Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials 2011, 32, 6145–6154. [Google Scholar] [CrossRef]
- Karunakaran, S.C.; Babu, P.S.S.; Madhuri, B.; Marydasan, B.; Paul, A.K.; Nair, A.S.; Rao, K.S.; Srinivasan, A.; Chandrashekar, T.K.; Rao, C.M.; et al. In vitro demonstration of apoptosis mediated photodynamic activity and NIR nucleus imaging through a novel porphyrin. ACS Chem. Biol. 2013, 8, 127–132. [Google Scholar] [CrossRef]
- Wang, D.; Su, H.; Kwok, R.T.K.; Hu, X.; Zou, H.; Luo, Q.; Lee, M.M.S.; Xu, W.; Lam, J.W.Y.; Tang, B.Z. Rational design of a water-soluble NIR AIEgen, and its application in ultrafast wash-free cellular imaging and photodynamic cancer cell ablation. Chem. Sci. 2018, 9, 3685–3693. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; MacK, J.; Yang, Y.; Shen, Z. Structural modification strategies for the rational design of red/NIR region BODIPYs. Chem. Soc. Rev. 2014, 13, 4778–4823. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prilepskii, A.Y.; Serov, N.S.; Kladko, D.V.; Vinogradov, V.V. Nanoparticle-Based Approaches towards the Treatment of Atherosclerosis. Pharmaceutics 2020, 12, 1056. https://doi.org/10.3390/pharmaceutics12111056
Prilepskii AY, Serov NS, Kladko DV, Vinogradov VV. Nanoparticle-Based Approaches towards the Treatment of Atherosclerosis. Pharmaceutics. 2020; 12(11):1056. https://doi.org/10.3390/pharmaceutics12111056
Chicago/Turabian StylePrilepskii, Artur Y., Nikita S. Serov, Daniil V. Kladko, and Vladimir V. Vinogradov. 2020. "Nanoparticle-Based Approaches towards the Treatment of Atherosclerosis" Pharmaceutics 12, no. 11: 1056. https://doi.org/10.3390/pharmaceutics12111056