CYP3A Excipient-Based Microemulsion Prolongs the Effect of Magnolol on Ischemia Stroke Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Construction of Pseudo-Ternary Phase Diagrams
2.3. Characterization of Microemulsion
2.3.1. Measurement of Droplet Size and Zeta Potential of Microemulsion
2.3.2. Viscosity of Microemulsion
2.3.3. Determination of the Maximum Solubility of Magnolol in ME
2.3.4. Stability Studies
2.3.5. Serum Stability Study
2.3.6. Transmission Electron Microscopy (TEM)
2.4. Animals
2.5. Blood and Brain Sample Collection for Pharmacokinetic Study
2.6. High-Performance Liquid Chromatography System and Method Validation
2.7. Calculation of Pharmacokinetic Parameters
2.8. Surgical Procedures for Inducing a Reversible Ischemic Stroke
2.8.1. Behavioral Tests
EBST
Grip Strength Assessment
2.9. Magnolol Administration for Pharmacodynamic Study
Evaluation of the Size of the Ischemic Injury Using 2,3,5-triphenyl tetrazolium Chloride Staining
2.10. Statistical Analysis
3. Results
3.1. Physicochemical Properties of Selected Microemulsions
3.2. Pharmacokinetic Parameters of Magnolol Loaded ME
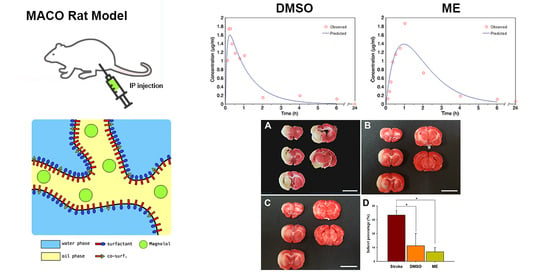
3.3. Brain Distribution and Effects of Magnolol on an Induced Ischemic Stroke
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alonso-Castro, A.J.; Zapata-Bustos, R.; Dominguez, F.; Garcia-Carranca, A.; Salazar-Olivo, L.A. Magnolia dealbata Zucc and its active principles honokiol and magnolol stimulate glucose uptake in murine and human adipocytes using the insulin-signaling pathway. Phytomedicine 2011, 18, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, F.; Chavez, M.; Garduno-Ramirez, M.L.; Chavez-Avila, V.M.; Mata, M.; Cruz-Sosa, F. Honokiol and magnolol production by in vitro micropropagated plants of Magnolia dealbata, an endangered endemic Mexican species. Nat. Prod. Commun. 2010, 5, 235–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuribara, H.; Kishi, E.; Kimura, M.; Weintraub, S.T.; Maruyama, Y. Comparative assessment of the anxiolytic-like activities of honokiol and derivatives. Pharmacol. Biochem. Behav. 2000, 67, 597–601. [Google Scholar] [CrossRef]
- Lin, Y.R.; Chen, H.H.; Ko, C.H.; Chan, M.H. Neuroprotective activity of honokiol and magnolol in cerebellar granule cell damage. Eur. J. Pharmacol. 2006, 537, 64–69. [Google Scholar] [CrossRef]
- Lin, Y.R.; Chen, H.H.; Lin, Y.C.; Ko, C.H.; Chan, M.H. Antinociceptive actions of honokiol and magnolol on glutamatergic and inflammatory pain. J. Biomed. Sci. 2009, 16, 94. [Google Scholar] [CrossRef] [Green Version]
- Qiang, L.Q.; Wang, C.P.; Wang, F.M.; Pan, Y.; Yi, L.T.; Zhang, X.; Kong, L.D. Combined administration of the mixture of honokiol and magnolol and ginger oil evokes antidepressant-like synergism in rats. Arch. Pharm. Res. 2009, 32, 1281–1292. [Google Scholar] [CrossRef]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef]
- Ko, C.H.; Chen, H.H.; Lin, Y.R.; Chan, M.H. Inhibition of smooth muscle contraction by magnolol and honokiol in porcine trachea. Planta Med. 2003, 69, 532–536. [Google Scholar] [CrossRef]
- Chen, L.C.; Liu, Y.C.; Liang, Y.C.; Ho, Y.S.; Lee, W.S. Magnolol inhibits human glioblastoma cell proliferation through upregulation of p21/Cip1. J. Agric. Food Chem. 2009, 57, 7331–7337. [Google Scholar] [CrossRef]
- Hoi, C.P.; Ho, Y.P.; Baum, L.; Chow, A.H. Neuroprotective effect of honokiol and magnolol, compounds from Magnolia officinalis, on beta-amyloid-induced toxicity in PC12 cells. Phytother. Res. 2010, 24, 1538–1542. [Google Scholar] [CrossRef]
- Muroyama, A.; Fujita, A.; Lv, C.; Kobayashi, S.; Fukuyama, Y.; Mitsumoto, Y. Magnolol Protects against MPTP/MPP(+)-Induced Toxicity via Inhibition of Oxidative Stress in In Vivo and In Vitro Models of Parkinson’s Disease. Parkinsons Dis. 2012, 2012, 985157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.H.; Kuo, H.C.; Lee, K.F.; Tsai, T.H. Magnolol protects neurons against ischemia injury via the downregulation of p38/MAPK, CHOP and nitrotyrosine. Toxicol. Appl. Pharmacol. 2014, 279, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Kuo, H.C.; Lee, K.F.; Tsai, T.H. Global proteomic analysis of brain tissues in transient ischemia brain damage in rats. Int. J. Mol. Sci. 2015, 16, 11873–11891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Liu, C.; Liu, S.; Liu, Z.; Li, S.; Wang, Y. In vitro metabolism of magnolol and honokiol in rat liver microsomes and their interactions with seven cytochrome P substrates. Rapid Commun. Mass Spectrom 2019, 33, 229–238. [Google Scholar] [CrossRef]
- Duan, J.; Xiao, J.; Chen, Y.; Han, F.-M. Inhibition of Magnolol and Honokiol on Cytochrome P450 Enzymes in Rat and Human Liver Microsomes. Chin. Herb. Med. 2015, 7. [Google Scholar] [CrossRef]
- Bravo Gonzalez, R.C.; Huwyler, J.; Boess, F.; Walter, I.; Bittner, B. In vitro investigation on the impact of the surface-active excipients Cremophor EL, Tween 80 and Solutol HS 15 on the metabolism of midazolam. Biopharm. Drug Dispos. 2004, 25, 37–49. [Google Scholar] [CrossRef]
- Mountfield, R.J.; Senepin, S.; Schleimer, M.; Walter, I.; Bittner, B. Potential inhibitory effects of formulation ingredients on intestinal cytochrome P450. Int. J. Pharm. 2000, 211, 89–92. [Google Scholar] [CrossRef]
- Rao, Z.; Si, L.; Guan, Y.; Pan, H.; Qiu, J.; Li, G. Inhibitive effect of cremophor RH40 or tween 80-based self-microemulsiflying drug delivery system on cytochrome P450 3A enzymes in murine hepatocytes. J. Huazhong Univ. Sci. Technol. Med. Sci. 2010, 30, 562–568. [Google Scholar] [CrossRef]
- Lin, S.P.; Tsai, S.Y.; Chao, P.D.L.; Chen, Y.C.; Hou, Y.C. Pharmacokinetics, bioavailability, and tissue distribution of magnolol following single and repeated dosing of magnolol to rats. Planta Med. 2011, 77, 1800–1805. [Google Scholar] [CrossRef] [Green Version]
- PubChem. PubChem Compound Summary for CID 72300, Magnolol; National Library of Medicine (USA), National Center for Biotechnology Information: Bethesda, MD, USA, 2004. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Magnolol (accessed on 5 August 2020).
- Brayton, C.F. Dimethyl sulfoxide (DMSO): A review. Cornell Vet. 1986, 76, 61–90. [Google Scholar]
- McKim, A.S.; Strub, R. Advances in the regulated pharmaceutical use of dimethyl sulfoxide USP, Ph.Eur. Pharm. Technol. 2016, 28, S32–S37. [Google Scholar]
- Patel, D.; Wairkar, S. Recent advances in cyclosporine drug delivery: Challenges and opportunities. Drug Deliv. Transl. Res. 2019, 9, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Zakir, F.; Mirza, M.A.; Anwer, M.K.; Ahmad, F.J.; Iqbal, Z. Development of Curcumin loaded chitosan polymer based nanoemulsion gel: In vitro, ex vivo evaluation and in vivo wound healing studies. Int. J. Biol. Macromol. 2017, 101, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cao, C.; Li, N.; Yuan, S. SYL3C aptamer-anchored microemulsion co-loading beta-elemene and PTX enhances the treatment of colorectal cancer. Drug Deliv. 2019, 26, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, D.A.; Aboelwafa, A.A.; Hamza, M.Y.; Mohamed, M.I. Microemulsion for topical delivery of fenoprofen calcium: In vitro and in vivo evaluation. J. Liposome Res. 2018, 28, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Chou, C.J.; Chen, C.F. Pharmacokinetics and brain distribution of magnolol in the rat after intravenous bolus injection. J. Pharm. Pharmacol. 1996, 48, 57–59. [Google Scholar] [CrossRef]
- Tsai, T.H.; Chou, C.J.; Chen, C.F. Disposition of magnolol after intravenous bolus and infusion in rabbits. Drug Metab. Dispos. 1994, 22, 518–521. [Google Scholar]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- Smith, M.L.; Bendek, G.; Dahlgren, N.; Rosen, I.; Wieloch, T.; Siesjo, B.K. Models for studying long-term recovery following forebrain ischemia in the rat. 2. A 2-vessel occlusion model. Acta Neurol. Scand. 1984, 69, 385–401. [Google Scholar] [CrossRef]
- Dittmar, M.; Spruss, T.; Schuierer, G.; Horn, M. External carotid artery territory ischemia impairs outcome in the endovascular filament model of middle cerebral artery occlusion in rats. Stroke 2003, 34, 2252–2257. [Google Scholar] [CrossRef]
- Schmid-Elsaesser, R.; Zausinger, S.; Hungerhuber, E.; Baethmann, A.; Reulen, H.J. A critical reevaluation of the intraluminal thread model of focal cerebral ischemia: Evidence of inadvertent premature reperfusion and subarachnoid hemorrhage in rats by laser-Doppler flowmetry. Stroke 1998, 29, 2162–2170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safakheil, M.; Safakheil, H. The Effect of Exosomes Derived from Bone Marrow Stem Cells in Combination with Rosuvastatin on Functional Recovery and Neuroprotection in Rats After Ischemic Stroke. J. Mol. Neurosci. 2020, 70, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Ingberg, E.; Gudjonsdottir, J.; Theodorsson, E.; Theodorsson, A.; Strom, J.O. Elevated body swing test after focal cerebral ischemia in rodents: Methodological considerations. BMC Neurosci. 2015, 16, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.L.; Guo, Y.; Zhang, Y.S.; Zhao, Y.; Zhang, L. Effects of Integrin beta1 on behavior and neurovascular regeneration in rats with cerebral ischemia-reperfusion injury. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3487–3494. [Google Scholar] [CrossRef]
- Erdo, F.; Berzsenyi, P.; Nemet, L.; Andrasi, F. Talampanel improves the functional deficit after transient focal cerebral ischemia in rats. A 30-day follow up study. Brain Res. Bull. 2006, 68, 269–276. [Google Scholar] [CrossRef]
- Swanson, R.A.; Sharp, F.R. Infarct measurement methodology. J. Cereb. Blood Flow Metab. 1994, 14, 697–698. [Google Scholar] [CrossRef]
- Huang, S.Y.; Tai, S.H.; Chang, C.C.; Tu, Y.F.; Chang, C.H.; Lee, E.J. Magnolol protects against ischemic-reperfusion brain damage following oxygen-glucose deprivation and transient focal cerebral ischemia. Int. J. Mol. Med. 2018, 41, 2252–2262. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, X.; Zhu, Y.; Wang, K.; Wang, Y. Effect of magnolol on cerebral injury and blood brain barrier dysfunction induced by ischemia-reperfusion in vivo and in vitro. Metab. Brain Dis. 2017, 32, 1109–1118. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Zhu, Y.; Liu, Y.; Wang, Y. Magnolol derivative 002C-3 protects brain against ischemia-reperfusion injury via inhibiting apoptosis and autophagy. Neurosci. Lett. 2015, 588, 178–183. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Q.W.; Xu, M.; Guo, J.J.; Shen, S.W.; Wang, Y.Q.; Sun, F.Y. New striatal neurons form projections to substantia nigra in adult rat brain after stroke. Neurobiol. Dis. 2012, 45, 601–609. [Google Scholar] [CrossRef]
- Hosp, J.A.; Greiner, K.L.; Martinez Arellano, L.; Roth, F.; Loffler, F.; Reis, J.; Fritsch, B. Progressive secondary exo-focal dopaminergic neurodegeneration occurs in not directly connected midbrain nuclei after pure motor-cortical stroke. Exp. Neurol. 2020, 327, 113211. [Google Scholar] [CrossRef] [PubMed]
- Durukan, A.; Tatlisumak, T. Ischemic stroke in mice and rats. Methods Mol. Biol. 2009, 573, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Xu, X.; Xu, L.; Yang, L.; Xu, X.; Zhu, J.; Wu, L.; Jiang, Y.; Liu, X. Optimization of behavioural tests for the prediction of outcomes in mouse models of focal middle cerebral artery occlusion. Brain Res. 2017, 1665, 88–94. [Google Scholar] [CrossRef] [PubMed]
- AboulFotouh, K.; Allam, A.A.; El-Badry, M.; El-Sayed, A.M. Development and in vitro/in vivo performance of self-nanoemulsifying drug delivery systems loaded with candesartan cilexetil. Eur. J. Pharm. Sci. 2017, 109, 503–513. [Google Scholar] [CrossRef]
- Li, X.; Tsibouklis, J.; Weng, T.; Zhang, B.; Yin, G.; Feng, G.; Cui, Y.; Savina, I.N.; Mikhalovska, L.I.; Sandeman, S.R.; et al. Nano carriers for drug transport across the blood-brain barrier. J. Drug Target. 2017, 25, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Subongkot, T.; Ngawhirunpat, T. Development of a novel microemulsion for oral absorption enhancement of all-trans retinoic acid. Int. J. Nanomed. 2017, 12, 5585–5599. [Google Scholar] [CrossRef] [Green Version]
- Kogan, A.; Shalev, D.E.; Raviv, U.; Aserin, A.; Garti, N. Formation and characterization of ordered bicontinuous microemulsions. J. Phys. Chem. B 2009, 113, 10669–10678. [Google Scholar] [CrossRef]







| Properties (Unit) | Formulation A | Formulation B |
|---|---|---|
| Composition of S:O:W | 6:1:3 | 7:1:2 |
| Droplet size (nm) | 111.2 ± 27.3 | 697.7 ± 208.3 |
| Size distribution (PI) | 0.434 ± 0.007 | 0.319 ± 0.036 |
| Zeta potential (mV) | - | 15.51 ± 0.24 |
| Viscosity (cP) | 21.86 ± 1.31 | 155.72 ± 2.28 |
| Electronic conductivity (µS/cm) | - | 16.23 ± 0.45 |
| Solubility | Day 0 | Day 14 | Day 30 |
|---|---|---|---|
| mg/mL | 22.78 ± 0.43 | 23.33 ± 0.73 | 23.35 ± 0.35 |
| maximum percentage (%) | 91.3 ± 4.4 | 93.4 ± 2.9 | 93.3 ± 1.4 |
| Parameter (Unit) | DMSO | - | Formulation B | - | |
|---|---|---|---|---|---|
| Administration | Intraperitoneal | Intraperitoneal | p Value | Oral | p Value |
| t1/2ka (h) | 0.06 ± 0.01 | 0.66 ± 0.01 ** | P1 < 0.01 | 0.31 ± 0.10 | P2 < 0.01 |
| t1/2k10 (h) | 0.80 ± 0.20 | 0.69 ± 0.01 | |||
| t1/2Alpha (h) | 0.96 ± 1.00 | ||||
| t1/2Beta (h) | 13.48 ± 4.14 | ||||
| Tmax (h) | 0.24 ± 0.03 | 0.97 ± 0.01 ** | P1 < 0.01 | 0.66 ± 0.15 | P2 < 0.05 |
| Cmax (μg/mL) | 1.61 ± 0.32 | 1.39 ± 0.27 | 0.32 ± 0.11 | P2 < 0.01 | |
| AUC0-t (μg/mL·h) | 2.23 ± 0.26 | 3.66 ± 0.75 * | P1 < 0.05 | 1.50 ± 0.41 | P2 < 0.05 |
| AUMC (μg/mL·h2) | 2.80 ± 0.77 | 7.10 ± 1.53 * | P1 < 0.05 | 23.18 ± 4.06 | P2 < 0.01 |
| MRT (h) | 1.25 ± 0.30 | 1.93 ± 0.02 * | P1 < 0.05 | 13.02 ± 1.14 | P2 < 0.01 |
| Cl/F (mg/kg)/(μg/mL)/h | 13.54 ± 1.56 | 8.43 ± 1.78 * | P1 < 0.05 | 17.37 ± 4.50 | P2 < 0.05 |
| V/F (mg/kg)/(μg/mL) | 53.51 ± 26.39 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.-W.; Chien, C.-C.; Chen, J.-H. CYP3A Excipient-Based Microemulsion Prolongs the Effect of Magnolol on Ischemia Stroke Rats. Pharmaceutics 2020, 12, 737. https://doi.org/10.3390/pharmaceutics12080737
Guo J-W, Chien C-C, Chen J-H. CYP3A Excipient-Based Microemulsion Prolongs the Effect of Magnolol on Ischemia Stroke Rats. Pharmaceutics. 2020; 12(8):737. https://doi.org/10.3390/pharmaceutics12080737
Chicago/Turabian StyleGuo, Jiun-Wen, Chih-Cheng Chien, and Jiann-Hwa Chen. 2020. "CYP3A Excipient-Based Microemulsion Prolongs the Effect of Magnolol on Ischemia Stroke Rats" Pharmaceutics 12, no. 8: 737. https://doi.org/10.3390/pharmaceutics12080737