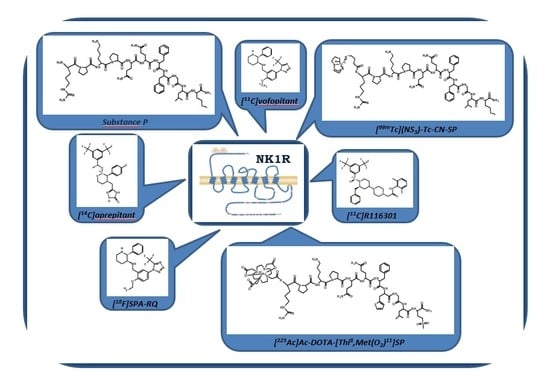
The Significance of NK1 Receptor Ligands and Their Application in Targeted Radionuclide Tumour Therapy
Abstract
:1. Introduction
2. NK1R Ligands in Classical Medicine
2.1. Significance of NK1R Agonists
2.2. Application of NK1R Antagonists
3. NK1R Radioligands in Nuclear Medicine
3.1. Radiolabelled NK1R Agonists for Targeted Radionuclide Tumour Diagnosis
3.2. Radiolabelled NK1R Agonists for Targeted Radionuclide Tumour Therapy
3.3. Antagonist Radioligands of NK1R for Targeted Radionuclide Imaging
4. Conculsions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Muñoz, M.; Coveñas, R. Neurokinin-1 receptor: A new promising target in the treatment of cancer. Discov. Med. 2010, 10, 305–313. [Google Scholar] [PubMed]
- Muñoz, M.; Rosso, M.; Coveñas, R. The NK-1 receptor: A new target in cancer therapy. Curr. Drug Targets 2011, 12, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Recio, S.; Gascón, P. Biological and Pharmacological Aspects of the NK1-Receptor. BioMed Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Chapman, K.; Clark, L.D.; Shao, Z.; Borek, D.; Xu, Q.; Wang, J.; Rosenbaum, D.M. Crystal structure of the human NK1 tachykinin receptor. Proc. Natl. Acad. Sci. USA 2018, 115, 13264–13269. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Geppetti, P. Substance P. Int. J. Biochem. Cell B 2001, 33, 555–576. [Google Scholar] [CrossRef]
- Palma, C. Tachykinins and their receptors in human malignancies. Curr. Drug Targets 2006, 7, 1043–1052. [Google Scholar] [CrossRef]
- Chandrashekar, I.R.; Cowsik, S.M. Three-Dimensional Structure of the Mammalian Tachykinin Peptide Neurokinin A Bound to Lipid Micelles. Biophys. J. 2003, 85, 4002–4011. [Google Scholar] [CrossRef] [Green Version]
- Steinhoff, M.S.; Mentzer, B.; Geppetti, P.; Pothoulakis, C.H.; Bunnett, N.W. Tachykinins and Their Receptors: Contributions to Physiological Control and the Mechanisms of Disease. Physiol. Rev. 2014, 94, 265–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentin-Hansen, L.; Park, M.; Huber, T.; Grunbeck, A.; Naganathan, S.; Schwartz, T.W.; Sakmar, T.P. Mapping Substance P Binding Sites on the Neurokinin-1 Receptor Using Genetic Incorporation of a Photoreactive Amino Acid. J. Biol Chem. 2014, 289, 18045–18054. [Google Scholar] [CrossRef] [Green Version]
- Sachon, E.; Girault-Lagrange, S.; Chassaing, G.; Lavielle, S.; Sagan, S. Analogs of Substance P modified at the C-terminus which are both agonist and antagonist of the NK-1 receptor depending on the second messenger pathway. J. Pept. Res. 2002, 59, 232–240. [Google Scholar] [CrossRef]
- Sagan, S.; Lavielle, S. Internalization of [3H]substance P analogues in NK-1 receptor transfected CHO cells. Biochem. Biophys. Res. Commun. 2001, 282, 958–964. [Google Scholar] [CrossRef]
- Sagan, S.; Quancard, J.; Lequin, O.; Karoyan, P.; Chassaing, G.; Lavielle, S. Conformational Analysis of the C-Terminal Gly-Leu-Met-NH2 Tripeptide of Substance P Bound to the NK-1 Receptor. Chem. Biol. 2005, 12, 555–565. [Google Scholar] [CrossRef]
- Quancard, J.; Karoyan, P.; Sagan, S.; Convert, O.; Lavielle, S.; Chassaing, G.; Lequin, O. Characterization of the bioactive conformation of the C-terminal tripeptide Gly-Leu-Met-NH2 of substance P using [3-prolinoleucine10]SP analogues. Eur. J. Biochem. 2003, 270, 2869–2878. [Google Scholar] [CrossRef]
- Alves, I.D.; Delaroche, D.; Mouillac, B.; Salamon, Z.; Tollin, G.; Hruby, V.J.; Lavielle, S.; Sagan, S. The Two NK-1 Binding Sites Correspond to Distinct, Independent, and Non-Interconvertible Receptor Conformational States as Confirmed by Plasmon-Waveguide Resonance Spectroscopy. Biochemistry 2006, 45, 5309–5318. [Google Scholar] [CrossRef]
- Cordier, D.; Gerber, A.; Kluba, C.H.; Bauman, A.; Hutter, G.; Mindt, T.L.; Mariani, L. Expression of Different Neurokinin-1 Receptor (NK1R) Isoforms in Glioblastoma Multiforme: Potential Implications for Targeted Therapy. Cancer Biother. Radiopharm. 2014, 29, 221–226. [Google Scholar] [CrossRef]
- Berger, M.; Neth, O.; Ilmer, M.; Garnier, A.; Salinas-Martín, M.V.; de Agustín Asencio, J.C.; von Schweinitz, D.; Kappler, R. Hepatoblastoma cells express truncated neurokinin-1 receptor and can be inhibited by aprepitant in vitro and in vivo. J. Hepatol. 2014, 60, 985–994. [Google Scholar] [CrossRef]
- Rosso, M.; Robles-Frías, M.J.; Coveñas, R.; Salinas-Martín, M.V.; Muñoz, M. The NK-1 Receptor Is Expressed in Human Primary Gastric and Colon Adenocarcinomas and Is Involved in the Antitumor Action of L-733,060 and the Mitogenic Action of Substance P on Human Gastrointestinal Cancer Cell Lines. Tumour. Biol. 2008, 29, 245–254. [Google Scholar] [CrossRef]
- Feng, F.; Yang, J.; Tong, L.; Yuan, S.; Tian, Y.; Hong, L.; Wang, W.; Zhang, H. Substance P immunoreactive nerve fibres are related to gastric cancer differentiation status and could promote proliferation and migration of gastric cancer cells. Cell Biol. Int. 2011, 35, 623–629. [Google Scholar] [CrossRef]
- Severini, C.; Improta, G.; Falconieri-Erspamer, G.; Salvadori, S.; Erspamer, V. The Tachykinin Peptide Family. Pharmacol. Rev. 2002, 54, 285–322. [Google Scholar] [CrossRef]
- Łazarczyk, M.; Matyja, E.; Lipkowski, A. Substance P and its receptors—A potential target for novel medicines in malignant brain tumour therapies (mini review). Folia Neuropathol. 2007, 45, 99–107. [Google Scholar]
- Graham, G.J.; Stevens, J.M.; Page, N.M.; Grant, A.D.; Brain, S.D.; Lowry, P.J.; Gibbins, J.M. Tachykinins regulate the function of platelets. Blood 2004, 104, 1058–1065. [Google Scholar] [CrossRef] [Green Version]
- Datar, P.; Srivastava, S.; Coutinho, E.; Govil, G. Substance P: Structure, Function, and Therapeutics. Curr. Top. Med. Chem. 2004, 4, 75–103. [Google Scholar] [CrossRef]
- Page, N.M. New challenges in the study of the mammalian Tachykinins. Peptides 2005, 26, 1356–1368. [Google Scholar] [CrossRef]
- Ho, W.Z.; Douglas, S.D. Substance P and neurokinin-1 receptor modulation of HIV. J. Neuroimmunol. 2004, 157, 48–55. [Google Scholar] [CrossRef]
- Mashaghi, A.; Marmalidou, A.; Tehrani, M.; Grace, P.M.; Pothoulakis, C.H.; Dana, R. Neuropeptide Substance P and the Immune Response. Cell Mol. Life Sci. 2016, 73, 4249–4264. [Google Scholar] [CrossRef]
- Grady, E.F.; Garland, A.M.; Gamp, P.D.; Lovett, M.; Payan, D.G.; Bunnett, N.W. Delineation of the Endocytic Pathway of Substance P and Its Seven-Transmembrane Domain NK1 Receptor. Mol. Biol. Cell 1995, 6, 509–524. [Google Scholar] [CrossRef]
- Muñoz, M.; Martinez-Armesto, J.; Coveñas, R. NK-1 receptor antagonists as antitumor drugs: A survey of the literature from 2000 to 2011. Expert Opin. Ther. 2012, 22, 735–746. [Google Scholar] [CrossRef]
- Joos, G.F.; Germonpre, P.R.; Pauwels, R.A. Role of tachykinins in asthma. Allergy 2000, 55, 321–337. [Google Scholar] [CrossRef]
- Yip, J.; Chahl, L.A. Localization of NK1 and NK3 receptors in guinea-pig brain. Regul. Pept. 2001, 98, 55–62. [Google Scholar] [CrossRef]
- Skidgel, R.A.; Engelbrecht, S.; Johnson, A.R.; Erdös, E.G. Hydrolysis of substance P and neurotensin by converting enzyme and neutral endopeptidase. Peptides 1984, 5, 769–776. [Google Scholar] [CrossRef]
- Skidgel, R.A.; Erdos, E.G. Angiotensin converting enzyme (ACE) and neprilysin hydrolyze neuropeptides: A brief history, the beginning and follow-ups to early studies. Peptides 2004, 25, 521–525. [Google Scholar] [CrossRef]
- Lockridge, O. Substance P hydrolysis by human serum cholinesterase. J. Neurochem. 1982, 39, 106–110. [Google Scholar] [CrossRef]
- Sakurada, C.H.; Watanabe, C.H.; Sakurada, S.; Tan-No, K.; Sakurada, T. Major metabolites of substance P degraded by spinal synaptic membranes antagonize the behavioral response to substance P in rats. J. Pharm. Sci. 1999, 88, 1127–1132. [Google Scholar] [CrossRef]
- Sandberg, B.E.; Lee, C.M.; Hanley, M.R.; Iversen, L.L. Synthesis and biological properties of enzyme-resistant analogues of substance P. Eur. J. Biochem. 1981, 114, 329–337. [Google Scholar] [CrossRef]
- Wagner, E.; Partsch, G.; Dunky, A. Substance P and its cleavage products: Effects on interleukin-1 secretion of rheumatoid arthritis monocytes/macrophages. Arthritis Res. 2001, 3, P019. [Google Scholar] [CrossRef]
- Skidgel, R.A.; Jackman, H.L.; Erdos, E.G. Metabolism of substance P and bradykinin by human neutrophils. Biochem. Pharmacol. 1991, 41, 1335–1344. [Google Scholar] [CrossRef]
- Chubb, I.W.; Hodgson, A.J.; White, G.H. Acetylocholinoesterase hydrolyzes substance P. Neuroscience 1980, 5, 2065–2072. [Google Scholar] [CrossRef]
- Mantyh, P.W. Neurobiology of substance P and the NK1 receptor. J. Clin. Psychiatry 2002, 63, 6–10. [Google Scholar]
- Gesztesi, Z.; Scuderi, P.E.; White, P.F.; Wright, W.; Wender, R.H.; D’Angelo, R.; Black, L.S.; Dalby, P.L.; MacLean, D. Substance P (Neurokinin-1) antagonist prevents postoperative vomiting after abdominal hysterectomy procedures. Anesthesiology 2000, 93, 931–937. [Google Scholar] [CrossRef]
- Aapro, M.S.; Walko, C.M. Aprepitant: Drug–drug interactions in perspective. Ann. Oncol. 2010, 21, 2316–2323. [Google Scholar] [CrossRef]
- Schmidt, P.T.; Lordal, M.; Gazelius, B.; Hellstrom, P.M. Tachykinins potently stimulate human small bowel blood flow: A laser Doppler flowmetry study in humans. Gut 2003, 52, 53–56. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Robert, M.E.; Eysselein, V.E. Rectal substance P concentrations are increased in ulcerative colitis but not in Crohn’s disease. Am. J. Gastroenterol. 1993, 88, 908–913. [Google Scholar]
- Goode, T.; O’Connell, J.; Anton, P.; Wong, H.; Reeve, J.; O’Sullivan, G.C.; Collins, J.K.; Shanahan, F. Neurokinin-1 receptor expression in inflammatory bowel disease: Molecular quantitation and localisation. Gut 2000, 47, 387–396. [Google Scholar] [CrossRef]
- Evangelista, S. Involvement of tachykinins in intestinal inflammation. Curr. Pharm Des. 2001, 7, 19–30. [Google Scholar] [CrossRef]
- McMahona, S.B.; Cafferty, W.B.J.; Marchand, F. Review, Immune and glial cell factors as pain mediators and modulators. Exp. Neurol. 2005, 192, 444–462. [Google Scholar] [CrossRef]
- El-Raziky, M.S.; Gohar, N.; El-Raziky, M. Study of substance P, renine and aldosterone in chronic liver disease in Egyptian children. J. Top. Pediatr. 2005, 51, 320–323. [Google Scholar] [CrossRef]
- Goto, T.; Tanaka, T. Tachykinins and tachykinin receptors in bone. Microsc. Res. Tech. 2002, 58, 91–97. [Google Scholar] [CrossRef]
- Lorente, L. New prognostic biomarkers of mortality in patients undergoing liver transplantation for hepatocellular carcinoma. J. Neurochem. 2018, 24, 4230–4242. [Google Scholar] [CrossRef]
- Goto, T.; Nakao, K.; Gunjigake, K.K.; Kido, M.A.; Kobayashi, S.; Tanaka, T. Substance P stimulates late-stage rat osteoblastic bone formation through neurokinin-1 receptors. Neuropeptides 2007, 41, 25–31. [Google Scholar] [CrossRef]
- Nowicki, M.; Ostalska-Nowicka, D.; Konwerska, A.; Miskowiak, B. The predicting role of substance P in the neoplastic transformation of the hypoplastic bone marrow. J. Clin. Pathol. 2006, 59, 935–941. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Chikama, T.; Nishida, T. Synergistic effect with Phe-Gly-Leu-Met-NH2 of the C-terminal of substance P and insulin-like growth factor-1 on epithelial wound healing of rabbit cornea. Br. J. Pharmacol. 1999, 127, 489–497. [Google Scholar] [CrossRef]
- Fan, T.P.; Hu, D.E.; Guard, S.; Gresham, G.A.; Watling, K.J. Stimulation of angiogenesis by substance P and interleukin-1 in the rat and its inhibition by NK1 or interleukin-1 receptor antagonists. Br. J. Pharmacol. 1993, 110, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Merlo, A.; Mäcke, H.; Reubi, J.C.; Good, S. Radiolabeled Conjugates Based on Substance P and the Uses. Thereof. Patent No. WO 2004/082722, 30 September 2004. [Google Scholar]
- Hoover, D.B.; Chang, Y.; Hancock, J.C.; Zhang, L. Action of Tachykinins Within the Heart and Their Relevance to Cardiovascular Disease. Jpn. J. Pharmacol. 2000, 84, 367–373. [Google Scholar] [CrossRef]
- Cordier, D.; Forrer, F.; Bruchertseifer, F.; Morgenstern, A.; Apostolidis, C.; Good, S.; Müller-Brand, J.; Mäcke, H.; Reubi, J.C.; Merlo, A. Targeted alpha-radionuclide therapy of functionally critically located gliomas with 213Bi-DOTA-[Thi8,Met(O2)11]-substance P: A pilot trial. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1335–1344. [Google Scholar] [CrossRef]
- Juszczak, M.; Stempniak, B. Melatonin inhibits the substance P-induced secretion of vasopressin and oxytocin from the rat hypothalamo-neurohypophysial system: In vitro studies. Brain Res. Bull. 2003, 59, 393–397. [Google Scholar] [CrossRef]
- Juszczak, M.; Boczek-Leszczyk, E.; Stempniak, B. Effect of melatonin on the vasopressin secretion as influenced by tachykinin NK-1 receptor agonist and antagonist: In vivo and in vitro studies. J. Physiol. Pharmacol. 2007, 58, 829–843. [Google Scholar]
- Feng, Z.; Xu, B. Inspiration from the mirror: D-amino acid containing peptides in biomedical approaches. Biomol. Concepts 2016, 7, 179–187. [Google Scholar] [CrossRef]
- Kasheverov, I.E.; Utkin, Y.N.; Franke, P.; Tsetlin, V.I. Substance P derivatives with photoactivatable labels in the N-terminal part of the molecule. J. Pept. Res. 1997, 50, 408–414. [Google Scholar] [CrossRef]
- Pradier, L.; Menager, J.; Le Guern, J.; Bock, M.D.; Heuillet, F.; Fardin, V.; Garret, C.; Doble, A.; Mayaux, J.F. Septide: An agonist for the NK1 receptor acting at a site distinct from substance P. Mol. Pharmmacol. 1994, 45, 287–293. [Google Scholar]
- Sakurada, C.; Watanabe, C.; Inoue, M.; Tan-No, K.; Ando, R.; Kisara, K.; Sakurada, T. Spinal actions of GR73632, a novel tachykinin NK1 receptor agonist. Peptides 1999, 20, 301–304. [Google Scholar] [CrossRef]
- Lazarus, L.H.; Diaugustine, R.P.; Soldato, C.M. A Substance with Immunoreactivity to the Peptide Physalaemin in Mammalian Respiratory Tissue. J. Exp. Lung Res. 1982, 3, 329–341. [Google Scholar] [CrossRef]
- Champagne, D.; Ribeiro, E.J.M. Sialokinin I and II: Vasodilatory tachykinins from the yellow fever mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 1994, 91, 138–142. [Google Scholar] [CrossRef]
- Schwyzer, R. Membrane-assisted molecular mechanism of neurokinin receptor subtype selection. EMBO J. 1987, 6, 2255–2259. [Google Scholar] [CrossRef]
- Iwabuchi, Y.; Aoki, C.H.; Masuhara, T. Effects of Tachykinins on the Secretion of Fluid and Glycoproteins from the Submandibular Glands of Rat, Mouse, Hamster and Guinea Pig. Jpn. J. Pharmacol. 1989, 51, 428–431. [Google Scholar] [CrossRef]
- Majumdar, A.K.; Howard, L.; Goldberg, M.R.; Hickey, L.; Constanzer, M.; Rothenberg, P.L.; Crumley, T.M.; Panebianco, D.; Bradstreet, T.E.; Bergman, A.J.; et al. Pharmacokinetics of Aprepitant After Single and Multiple Oral Doses in Healthy Volunteers. J. Clin. Pharmacol. 2006, 46, 291–300. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, X.; Guan, Y.; Gu, D.; Shen, Y.; Xu, Z.; Wei, X.; Chen, J. Efficacy and safety of aprepitant in the prevention of chemotherapy-induced nausea and vomiting: A pooled analysis. Support. Care Cancer 2012, 20, 1815–1822. [Google Scholar] [CrossRef]
- Aapro, M.S.; Schmoll, H.J.; Jahn, F.; Carides, A.D.; Webb, R.T. Review of the efficacy of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in a range of tumor types. Cancer Treat. Rev. 2013, 39, 113–117. [Google Scholar] [CrossRef]
- Aoki, S.; Iihara, H.; Nishigaki, M.; Imanishi, Y.; Yamauchi, K.; Ishihara, M.; Kitaichi, K.; Itoh, Y. Difference in the emetic control among highly emetogenic chemotherapy regimens: Implementation for appropriate use of aprepitant. Mol. Clin. Oncol. 2013, 1, 41–46. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R. Safety of neurokinin-1 receptor antagonists. Expert Opin. Drug Saf. 2013, 12, 673–685. [Google Scholar] [CrossRef]
- Varuby Product Characteristics-EMEA/H/C/004196-T/0015. Available online: Ema.europa.eu/en/documents/product-information/varuby-epar-product-information_en.pdf (accessed on 15 May 2019).
- Cerenia Product Characteristics-EMEA/V/C/000106-IB/0035/G. Available online: Ema.europa.eu/en/documents/product-information/cerenia-epar-product-information_en.pdf (accessed on 15 May 2019).
- Akynzeo Product Characteristics-EMEA/H/C/003728-N/0022. Available online: Ema.europa.eu/en/documents/product-information/akynzeo-epar-product-information_en.pdf (accessed on 15 May 2019).
- Zunrisa Withdrawal Report EMEA/H/C/1040. Available online: Ema.europa.eu/en/documents/withdrawal-report/withdrawal-assessment-report-zunrisa_en.pdf (accessed on 15 May 2019).
- Quartara, L.; Altamura, M.; Evangelista, S.; Maggi, C.A. Tachykinin receptor antagonists in clinical trials. Expert Opin. Investig. Drugs 2009, 18, 1843–1864. [Google Scholar] [CrossRef]
- Keller, M.; Montgomery, S.; Ball, W.; Morrison, M.; Snavely, D.; Liu, G.; Hargreaves, R.; Hietala, J.; Lines, C.; Beebe, K.; et al. Lack of Efficacy of the Substance P (Neurokinin1 Receptor) Antagonist Aprepitant in the Treatment of Major Depressive Disorder. Biol. Psychiatry 2006, 59, 216–223. [Google Scholar] [CrossRef]
- Huang, S.C.; Korlipara, V.L. Neurokinin-1 receptor antagonists: A comprehensive patent survey. Expert Opin. Ther. Pat. 2010, 20, 1019–1045. [Google Scholar] [CrossRef]
- Michelson, D.; Hargreaves, R.; Alexander, R.; Ceesay, P.; Hietala, J.; Lines, C.; Reines, S. Lack of efficacy of L-759274, a novel neurokinin 1 (substance P) receptor antagonist, for the treatment of generalized anxiety disorder. Int. J. Neuropsychopharmacol. 2013, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.; Coveñas, R. Involvement of substance P and the NK-1 receptor in human pathology. Amino Acids 2014, 46, 1727–1750. [Google Scholar] [CrossRef]
- George, D.T.; Gilman, J.; Hersh, J.; Thorsell, A.; Herion, D.; Geyer, C.; Peng, X.; Kielbasa, W.; Rawlings, R.; Brandt, J.E.; et al. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science 2008, 319, 1536–1539. [Google Scholar] [CrossRef]
- Torres, T.; Fernandes, I.; Selores, M.; Alves, R.; Lima, M. Aprepitant: Evidence of its effectiveness in patients with refractory pruritus continues. J. Am. Acad. Dermatol. 2012, 68, e14–e15. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Ständer, S.; Kerby, M.B.; Larrick, J.W.; Perlman, A.J.; Schnipper, E.F.; Zhang, X.; Tang, J.Y.; Luger, T.; Steinhoff, M. Serlopitant for the treatment of chronic pruritus: Results of a randomized, multicenter, placebo-controlled phase 2 clinical trial. J. Am. Acad. Dermatol. 2018, 78, 882–891. [Google Scholar] [CrossRef]
- Muñoz, M.; Rosso, M.; Pérez, A.; Coveñas, R.; Rosso, R.; Zamarriego, C.; Piruat, J.I. The NK1 receptor is involved in the antitumoural action of L-733,060 and the mitogenic action of substance P on neuroblastoma and glioma cell lines. Neuropeptides 2005, 39, 427–432. [Google Scholar] [CrossRef]
- Muñoz, M.; Rosso, M.; Pérez, A.; Coveñas, R.; Rosso, R.; Zamarriego, C.; Soult, J.A.; Montero, I. Antitumoral action of the neurokinin-1-receptor antagonist L-733,060 and mitogenic action of substance P on human retinoblastoma cell lines. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2567–2570. [Google Scholar] [CrossRef]
- Muñoz, M.; Rosso, M.; Coveñas, R. The NK-1 receptor is involved in the antitumoural action of L-733,060 and in the mitogenic action of substance P on human pancreatic cancer cell lines. Lett. Drug Des. Discov. 2006, 3, 323–329. [Google Scholar] [CrossRef]
- Muñoz, M.; Rosso, M.; Aguilar, F.J.; González-Moles, M.A.; Redondo, M.; Esteban, F. NK-1 receptor antagonists induce apoptosis and counteract substance P-related mitogenesis in human laryngeal cancer cell line HEp-2. Investig. New Drugs 2008, 26, 111–118. [Google Scholar] [CrossRef]
- Muñoz, M.; Rosso, M.; Coveñas, R. A new frontier in the treatment of cancer: NK-1 receptor antagonists. Curr. Med. Chem. 2010, 17, 504–516. [Google Scholar] [CrossRef]
- Muñoz, M.; Rosso, M.; Robles-Frías, M.J.; Salinas-Martín, M.V.; Coveñas, R. The NK-1 receptor is expressed in human melanoma and is involved in the antitumor action of the NK-1 receptor antagonist aprepitant on melanoma cell lines. Lab. Investig. 2010, 90, 1259–1269. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.; Rosso, M. The NK-1 receptor antagonist aprepitant as a broad-spectrum antitumor drug. Investig. New Drugs 2010, 28, 187–193. [Google Scholar] [CrossRef]
- Muñoz, M.; Bernabeu-Wittel, J.; Coveñas, R. NK-1 as a melanoma target. Expert Opin. Ther. Targets 2011, 15, 889–897. [Google Scholar] [CrossRef]
- Muñoz, M.; González-Ortega, A.; Coveñas, R. The NK-1 receptor is expressed in human leukemia and is involved in the antitumor action of aprepitant and other NK-1 receptor antagonists on acute lymphoblastic leukemia cell lines. Investig. New Drugs 2012, 30, 529–540. [Google Scholar] [CrossRef]
- Muñoz, M.; González-Ortega, A.; Rosso, M.; Robles-Frías, M.J.; Carranza, A.; Salinas-Martín, M.V.; Coveñas, R. The substance P/neurokinin-1 receptor system in lung cancer: Focus on the antitumor action of neurokinin-1 receptor antagonists. Peptides 2012, 38, 318–325. [Google Scholar] [CrossRef]
- Muñoz, M.; Rosso, M.; Coveñas, R. The NK-1 receptor antagonist L-732,138 induces apoptosis in human gastrointestinal cancer cell lines. Pharm. Rep. 2017, 69, 696–701. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R.; Esteban, F.; Redondo, M. The substance P/NK-1 receptor system: NK-1 receptor antagonists as anti-cancer drugs. J. Biosci. 2015, 40, 441–463. [Google Scholar] [CrossRef]
- Muñoz, M.; Berger, M.; Rosso, M.; Gonzalez-Ortega, A.; Carranza, A.; Coveñas, R. Antitumor activity of neurokinin-1 receptor antagonists in MG-63 human osteosarcoma xenografts. Int. J. Oncol. 2014, 44, 137–146. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R. Involvement of substance P and the NK-1 receptor in cancer progression. Peptides 2013, 48, 1–9. [Google Scholar] [CrossRef]
- Coveñas, R.; Muñoz, M. Cancer progression and substance P. Histol. Histopathol. 2014, 29, 881–890. [Google Scholar] [CrossRef]
- Muñoz, M.; González-Ortega, A.; Salinas-Martín, M.V.; Carranza, A.; Garcia-Recio, S.; Almendro, V.; Coveñas, R. The neurokinin-1 receptor antagonist aprepitant is a promising candidate for the treatment of breast cancer. Int. J. Oncol. 2014, 45, 1658–1672. [Google Scholar] [CrossRef]
- Kast, R.E.; Boockvar, J.A.; Brüning, A.; Cappello, F.; Chang, W.W.; Cvek, B.; Dou, Q.P.; Duenas-Gonzalez, A.; Efferth, T.; Focosi, D.; et al. A conceptually new treatment approach for relapsed glioblastoma: Coordinated undermining of survival paths with nine repurposed drugs (CUSP9) by the International Initiative for Accelerated Improvement of Glioblastoma Care. Oncotarget 2013, 4, 502–530. [Google Scholar] [CrossRef]
- Muñoz, M.; Rosso, M.; González, A.; Saenz, J.; Coveñas, R. The broad-spectrum antitumor action of cyclosporin A is due to its tachykinin receptor antagonist pharmacological profile. Peptides 2010, 31, 1643–1648. [Google Scholar] [CrossRef]
- González-Ortega, A.; Sánchez-Valderrábanos, E.; Ramiro-Fuentes, S.; Salinas-Martín, M.V.; Carranza, A.; Coveñas, R.; Muñoz, M. Uveal melanoma expresses NK-1 receptors and cyclosporin A induces apoptosis in human melanoma cell lines overexpressing the NK-1 receptor. Peptides 2014, 55, 1–12. [Google Scholar] [CrossRef]
- Baum, R.P. Therapeutic Nuclear Medicine; Springer Publisher: Heidelberg, Germany, 2014. [Google Scholar]
- International Atomic Energy Agency, Nuclear Data Services, Live Chart of Nuclides, Nuclear Structure and Decay Data. Available online: https://www-nds.iaea.org/relnsd/vcharthtml/VChartHTML.html (accessed on 28 July 2019).
- Sjödin, L. Binding and internalization of 125I-Bolton-Hunter-substance-P by pancreatic acinar cells. Biochem. Biophys. Res. Commun. 1984, 124, 578–584. [Google Scholar] [CrossRef]
- Larsen, P.J.; Mikkelsen, J.D.; Saermark, T. Binding of a iodinated substance P analog to a NK-1 receptor on isolated cell membranes from rat anterior pituitary. Endocrinology 1989, 124, 2548–2557. [Google Scholar] [CrossRef]
- Larsen, P.J.; Mikkelsen, J.D.; Mau, S.; Saermark, T. Binding and internalization of a iodinated substance P analog by cultured anterior pituitary cells. Mol. Cell Endocrinol. 1989, 65, 91–101. [Google Scholar] [CrossRef]
- Larsen, P.J.; Saermark, T.; Mau, S.E. Binding of an iodinated substance P analogue to cultured anterior pituitary prolactin- and luteinizing hormone-containing cells. J. Histochem. Cytochem. 1992, 40, 487–493. [Google Scholar] [CrossRef]
- Shigematsu, K.; Saavedra, J.M.; Kurihara, M. Specific substance P binding sites in rat thymus and spleen: In vitro autoradiographic study. Regul. Pept. 1986, 16, 147–156. [Google Scholar] [CrossRef]
- Beaujouan, J.C.; Torrens, Y.; Saffroy, M.; Glowinski, J. Quantitative autoradiographic analysis of the distribution of binding sites for [125I]Bolton Hunter derivatives of eledoisin and substance P in the rat brain. Neuroscience 1986, 18, 857–875. [Google Scholar] [CrossRef]
- Geraghty, D.P.; Maguire, C.M. Reduced [125I]-Bolton Hunter substance P binding (NK1 receptors) in the basal forebrain nuclei of aged rats. Clin. Exp. Pharmacol. Physiol. 2002, 29, 1112–1115. [Google Scholar] [CrossRef]
- Charlton, C.G.; Helke, C.J. Characterization and segmental distribution of 125I-Bolton-Hunter-labeled substance P binding sites in rat spinal cord. J. Neurosci. 1985, 5, 1293–1299. [Google Scholar] [CrossRef]
- Cridland, R.A.; Yashpal, K.; Romita, V.V.; Gauthier, S.; Henry, J.L. Distribution of label after intrathecal administration of 125I-substance P in the rat. Peptides 1987, 8, 213–221. [Google Scholar] [CrossRef]
- Aanonsen, L.M.; Kajander, K.C.; Bennett, G.J.; Seybold, V.S. Autoradiographic analysis of 125I-substance P binding in rat spinal cord following chronic constriction injury of the sciatic nerve. Brain Res. 1992, 596, 259–268. [Google Scholar] [CrossRef]
- Stucky, C.L.; Galeazza, M.T.; Seybold, V.S. Time-dependent changes in Bolton-Hunter-labelled 125I-substance P binding in rat spinal cord following unilateral adjuvant-induced peripheral inflammation. Neuroscience 1993, 57, 397–409. [Google Scholar] [CrossRef]
- Maguire, C.M.; Geraghty, D.P. Comparison of [125I]-bolton-hunter substance P binding in young and aged rat spinal cord. Brain Res. 1998, 786, 263–266. [Google Scholar] [CrossRef]
- Liu, L.; Burcher, E. Radioligand binding and functional characterisation of tachykinin receptors in chicken small intestine. Naunyn Schmiedebergs Arch. Pharmacol. 2001, 364, 305–313. [Google Scholar] [CrossRef]
- Garland, A.M.; Grady, E.F.; Payan, D.G.; Vigna, S.R.; Bunnett, N.W. Agonist-induced internalization of the substance P (NK1) receptor expressed in epithelial cells. Biochem. J. 1994, 303, 177–186. [Google Scholar] [CrossRef]
- Beaujouan, J.C.; Torrens, Y.; Herbet, A.; Daguet, M.C.; Glowinski, J.; Prochiantz, A. Specific binding of an immunoreactive and biologically active 125I-labeled substance P derivative to mouse mesencephalic cells in primary culture. Mol. Pharmacol. 1982, 22, 48–55. [Google Scholar]
- Liang, T.; Cascieri, M.A. Substance P receptor on parotid cell membranes. J. Neurosci. 1981, 1, 1133–1141. [Google Scholar] [CrossRef] [Green Version]
- Kieselbach, G.F.; Ragaut, R.; Knaus, H.G.; König, P.; Wiedermann, C.J. Autoradiographic analysis of binding sites for 125I-Bolton-Hunter-substance P in the human eye. Peptides 1990, 11, 655–659. [Google Scholar] [CrossRef]
- Buffington, C.A.; Wolfe, S.A. High affinity binding sites for [3H]substance P in urinary bladders of cats with interstitial cystitis. J. Urol. 1998, 160, 605–611. [Google Scholar] [CrossRef]
- Breeman, W.A.P.; Van Hagen, M.P.; Visser-Wisselaar, H.A.; van der Pluijm, M.E.; Koper, J.W.; Setyono-Han, B.; Bakker, W.H.; Kwekkeboom, D.J.; Hazenberg, M.P.; Lamberts, S.W.J.; et al. In Vitro and In Vivo Studies of Substance Receptor Expression in Rats with the New Analog [Indium-111-DTPA-Arg1]Substance P. J. Nucl. Med. 1996, 37, 108–117. [Google Scholar]
- van Hagen, P.M.; Breeman, W.A.; Reubi, J.C.; Postema, P.T.; van den Anker-Lugtenburg, P.J.; Kwekkeboom, D.J.; Laissue, J.; Waser, B.; Lamberts, S.W.; Visser, T.J.; et al. Visualization of the thymus by substance P receptor scintigraphy in man. Eur. J. Nucl. Med. 1996, 23, 1508–1513. [Google Scholar] [CrossRef] [Green Version]
- Ozker, S.K.; Hellman, R.S.; Krasnow, A.Z. Preparation of 99mTc labeled substance P (SP). Appl. Radiat. Isot. 2002, 57, 729–732. [Google Scholar] [CrossRef]
- Gniazdowska, E.; Koźmiński, P.; Fuks, L. Synthesis, radiochemistry and stability of the conjugates of technetium-99m complexes with Substance P. J. Radioanal. Nucl. Chem. 2013, 298, 1171–1177. [Google Scholar] [CrossRef]
- Smilkov, K.; Janevik, E.; Guerrini, R.; Pasquali, M.; Boschi, A.; Uccelli, L.; Duatti, G.D.A. Preparation and first biological evaluation of novel Re-188/Tc-99m peptide conjugates with substance-P. Appl. Radiat. Isot. 2014, 92, 25–31. [Google Scholar] [CrossRef]
- Królicki, L.; Bruchertseifer, F.; Kunikowska, J.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Apostolidis, C.; Mirzadeh, S.; Rola, R.; et al. Prolonged survival in secondary glioblastoma following local injection of targeted alpha therapy with 213Bi-substance P analogue. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1636–1644. [Google Scholar] [CrossRef] [Green Version]
- Mozaffari, S.; Erfani, M.; Beiki, D.; Johari Daha, F.; Kobarfard, F.; Balalaie, S.; Fallahi, B. Synthesis and preliminary evaluation of a new 99mTc labeled Substance P analogue as a potential tumor imaging agent. Iranian J. Pharm. Res. 2015, 14, 97–110. [Google Scholar]
- Lew, R.; Geraghty, D.P.; Drapeau, G.; Regoli, D.; Burcher, E. Binding characteristics of [125I]Bolton-Hunter [Sar9,Met(O2)11]substance P, a new selective radioligand for the NK1 receptor. Eur. J. Pharmacol. 1990, 184, 97–108. [Google Scholar] [CrossRef]
- Tousignant, C.; Guillemette, G.; Drapeau, G.; Télémaque, S.; Dion, S.; Regoli, D. 125I-BH[Sar9, Met(O2)11]-SP, a new selective ligand for the NK-1 receptor in the central nervous system. Brain Res. 1990, 524, 263–270. [Google Scholar] [CrossRef]
- Dam, T.V.; Martinelli, B.; Quirion, R. Autoradiographic distribution of brain neurokinin-1/substance P receptors using a highly selective ligand [3H]-[Sar9, Met(O2)11]-substance P. Brain Res. 1990, 531, 333–337. [Google Scholar] [CrossRef]
- Oyen, W.J.G.; Bodei, L.; Giammarile, F.; Maecke, H.R.; Tenvall, J.; Luster, M.; Brans, B. Targeted therapy in nuclear medicine–current status and future prospects. Ann. Oncol. 2007, 18, 1782–1792. [Google Scholar] [CrossRef]
- De Araújo, E.B.; Pujatti, P.B.; Barrio, O.; Caldeira, J.S.; Suzuki, M.F.; Mengatti, J. Radiolabeling of Substance P with Lutetium-177 and biodistribution study in AR42J pancreatic tumor xenografted Nude mice. J. Nucl. Med. 2008, 49, 6. [Google Scholar]
- Kneifel, S.; Cordier, D.; Good, S.; Ionescu, M.C.S.; Ghaffari, A.; Hofer, S.; Kretzschmar, M.; Tolnay, M.; Apostolidis, C.; Waser, B.; et al. Local Targeting of Malignant Gliomas by the Diffusible Peptidic Vector 1,4,7,10-Tetraazacyclododecane-1-Glutaric Acid-4,7,10-Triacetic Acid-Substance P. Clin. Cancer Res. 2006, 12, 3843–3850. [Google Scholar] [CrossRef]
- Cordier, D.; Forrer, F.; Kneifel, S.; Sailer, M.; Mariani, L.; Maecke, H.; Muller-Brand, J.; Merlo, A. Neoadjuvant targeting of glioblastoma multiforme with radiolabeled DOTAGA–substance P—Results from a phase I study. J. Neurooncol. 2010, 100, 129–136. [Google Scholar] [CrossRef]
- Królicki, L.; Bruchertseifer, F.; Kunikowska, J.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Apostolidis, C.; Mirzadeh, S.; Rola, R.; et al. Safety and efficacy of targeted alpha therapy with 213Bi-DOTA-substance P in recurrent glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 614–622. [Google Scholar] [CrossRef]
- Ohgaki, H.; Dessen, P.; Jourde, B.; Horstmann, S.; Nishikawa, T.; Di Patre, P.L.; Burkhard, C.; Schüler, D.; Probst-Hensch, N.M.; Maiorka, P.C.; et al. Genetic pathways to glioblastoma: A population-based study. Cancer Res. 2004, 64, 6892–6899. [Google Scholar] [CrossRef]
- Park, J.K.; Hodges, T.; Arko, L.; Shen, M.; Dello Iacono, D.; McNabb, A.; Olsen Bailey, N.; Kreisl, T.N.; Iwamoto, F.M.; Sul, J.; et al. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J. Clin. Oncol. 2010, 28, 3838–3843. [Google Scholar] [CrossRef]
- Majkowska-Pilip, A.; Rius, M.; Bruchertseifer, F.; Apostolidis, C.; Weis, M.; Bonelli, M.; Laurenza, M.; Królicki, L.; Morgenstern, A. In vitro evaluation of 225Ac-DOTA-substanceP for targeted alpha therapy of glioblastoma multiforme. Chem. Biol. Drug Des. 2018, 92, 1344–1356. [Google Scholar] [CrossRef]
- Song, H.; Guerrero-Cazares, H.; Horti, A.; Wahl, R.L.; Quinones-Hinojosa, A.; Sgouros, G. Synthesis and Biodistribution of 225Ac-substance P for Intracavitary Radiopharmaceutical Therapy of High-grade Recurrent Glioma. In Cancer Research, Proceedings of the AACR 104th Annual Meeting, Washington, DC/Philadelphia, PA, USA, 6–10 April 2013; AACR: Washington, DC, USA; Philadelphia, PA, USA, 2013; Volume 73, p. 4533. [Google Scholar]
- Jordan, C.T.; Guzman, M.L.; Noble, M. Cancer stem cells. N. Eng. J. Med. 2006, 355, 1253–1261. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, X.; Zeng, Z.; Tunici, P.; Ng, H.; Abdulkadir, I.R.; Lu, L.; Irvin, D.; Black, K.L.; Yu, J.S. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol. Cancer 2006, 5, 67–79. [Google Scholar] [CrossRef]
- Krolicki, L.; Bruchertseifer, F.; Morgenstern, A.; Kunikowska, J.; Koziara, H.; Krolicki, B.; Jakucinski, M.; Pawlak, D.; Apostolidis, C.; Rola, R.; et al. Safety and Therapeutic Efficacy of 225Ac-DOTA-SubstanceP for Therapy of Brain Tumors. J. Med. Imaging Radiat. Sci. 2019, 50, S22. [Google Scholar] [CrossRef]
- Majkowska-Pilip, A.; Koźmiński, P.; Wawrzynowska, A.; Budlewski, T.; Kostkiewicz, B.; Gniazdowska, E. Application of Neurokinin-1 Receptor in Targeted Strategies for Glioma Treatment. Part I: Synthesis and Evaluation of Substance P Fragments Labeled with 99mTc and 177Lu as Potential Receptor Radiopharmaceuticals. Molecules 2018, 23, 2542. [Google Scholar] [CrossRef]
- Del Rosario, R.B.; Managner, T.J.; Gildersleeve, D.L.; Shreve, P.D.; Wieland, D.M.; Lowe III, J.A.; Drozda, S.E.; Snider, R.M. Synthesis of a Nonpeptide Carbon-11 Labeled Substance P Antagonist for PET Studies. Nucl. Med. Biol. 1993, 20, 545–547. [Google Scholar] [CrossRef]
- Livni, E.; Babich, J.W.; Desa, M.C.; Godek, D.M.; Wilkinson, R.A.; Rubin, R.H.; Fischman, A.J. Synthesis of a 11 C-labeled NK1 Receptor Ligand for PET Studies. Nucl. Med. Biol. 1995, 22, 31–36. [Google Scholar] [CrossRef]
- Bender, D.; Olsen, A.K.; Marthi, M.K.; Smith, D.F.; Cumming, P. PET evaluation of the uptake of N-[11C]methyl CP-643,051, an NK1 receptor antagonist, in the living porcine brain. Nucl. Med. Biol. 2004, 31, 699–704. [Google Scholar] [CrossRef]
- Bergström, M.; Fasth, K.J.; Kilpatrick, G.; Ward, P.; Cable, K.M.; Wipperman, M.D.; Sutherland, D.R.; Langström, B. Brain uptake and receptor binding of two [11C]labelled selective high affinity NK1-antagonists, GR203040 and GR205171-PET studies in rhesus monkey. Neuropharmacology 2000, 39, 664–670. [Google Scholar] [CrossRef]
- Nyman, M.J.; Eskola, O.; Kajander, J.; Vahlberg, T.; Sanabria, S.; Burns, D.; Hargreaves, R.; Solin, O.; Hietala, J. Gender and age affect NK1 receptors in the human brain—A positron emission tomography study with [18F]SPA-RQ. Int. J. Neuropsychopharmacol. 2007, 10, 219–229. [Google Scholar] [CrossRef]
- Engman, J.; Åhs, F.; Furmark, T.; Linnman, C.; Pissiota, A.; Appel, L.; Frans, Ö.; Långström, B.; Fredrikson, M. Age, sex and NK1 receptors in the human brain—A positron emission tomography study with [11C]GR205171. Eur. Neuropsychopharmacol. 2012, 22, 562–568. [Google Scholar] [CrossRef]
- Hietala, J.; Nyman, M.J.; Eskola, O.; Laakso, A.; Grönroos, T.; Oikonen, V.; Bergman, J.; Haaparanta, M.; Forsback, S.; Marjamäki, P.; et al. Visualization and Quantification of Neurokinin-1 (NK1) Receptors in the Human Brain. Mol. Imaging Biol. 2005, 7, 262–272. [Google Scholar] [CrossRef]
- Haneda, E.; Higuchi, M.; Maeda, J.; Inaji, M.; Okauchi, T.; Ando, K.; Obayashi, S.; Nagai, Y.; Narazaki, M.; Ikehira, H.; et al. In Vivo Mapping of Substance P Receptors in Brains of Laboratory Animals by High-Resolution Imaging Systems. Synapse 2007, 61, 205–215. [Google Scholar] [CrossRef]
- Okumura, M.; Arakawa, R.; Ito, H.; Seki, C.; Takahashi, H.; Takano, H.; Haneda, E.; Nakao, R.; Suzuki, H.; Suzuki, K.; et al. Quantitative Analysis of NK1 Receptor in the Human Brain Using PET with 18F-FE-SPA-RQ. J. Nucl. Med. 2008, 49, 1749–1755. [Google Scholar] [CrossRef]
- Danfors, T.; Åhs, F.; Appel, L.; Linnman, C.; Fredrikson, M.; Furmark, T.; Kumlien, E. Increased neurokinin-1 receptor availability in temporal lobe epilepsy: A positron emission tomography study using [11C]GR205171. Epilepsy Res. 2011, 97, 183–189. [Google Scholar] [CrossRef]
- Frick, A.; Åhs, F.; Linnman, C.; Jonasson, M.; Appel, L.; Lubberink, M.; Långström, B.; Fredrikson, M.; Furmark, T. Increased neurokinin-1 receptor availability in the amygdala in social anxiety disorder: A positron emission tomography study with [11C]GR205171. Transl. Psychiatry 2015, 5, e597. [Google Scholar] [CrossRef]
- Michelgård, Å.; Appel, L.; Pissiota, A.; Frans, Ö.; Långström, B.; Bergström, M.; Fredrikson, M. Symptom Provocation in Specific Phobia Affects the Substance P Neurokinin-1 Receptor System. Biol. Psychiatry 2007, 61, 1002–1006. [Google Scholar] [CrossRef]
- Fujimura, Y.; Yasuno, F.; Farris, A.; Liow, J.S.; Geraci, M.; Drevets, W.; Pine, D.S.; Ghose, S.; Lerner, A.; Hargreaves, R.; et al. Decreased Neurokinin-1 (Substance P) Receptor Binding in Patients with Panic Disorder: Positron Emission Tomographic Study with [18F]SPA-RQ. Biol. Psychiatry 2009, 66, 94–97. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov Identifier: NCT00088738. Available online: https://clinicaltrials.gov/ct2/show/NCT00088738 (accessed on 15 May 2019).
- Jarcho, J.M.; Mandelkern, M.; Ebrat, B.; Smith, S.R.; Naliboff, B.D.; Labus, J.S.; Tillisch, K.; Mayer, E.A. Reduced Neurokinin-1 (Substance P) Receptor Binding in Patients With Irritable Bowel Syndrome: A Positron Emission Tomography Study With [18f]SPA-RQ. Gastroenterology 2010, 138, S372. [Google Scholar] [CrossRef]
- ClinicalTrials.gov Identifier: NCT00102102. Available online: https://clinicaltrials.gov/ct2/show/NCT00102102 (accessed on 15 May 2019).
- ClinicalTrials.gov Identifier: NCT00547612. Available online: https://clinicaltrials.gov/ct2/show/NCT00547612 (accessed on 15 May 2019).
- Bergström, M.; Hargreaves, R.J.; Burns, H.D.; Goldberg, M.R.; Sciberras, D.; Reines, S.A.; Petty, K.J.; Ögren, M.; Antoni, G.; Långström, B.; et al. Human Positron Emission Tomography Studies of Brain Neurokinin 1 Receptor Occupancy by Aprepitant. Biol. Psychiatry 2004, 55, 1007–1012. [Google Scholar] [CrossRef]
- Zamuner, S.; Rabiner, E.A.; Fernandes, S.A.; Bani, M.; Gunn, R.N.; Gomeni, R.; Ratti, E.; Cunningham, V.J. A pharmacokinetic PET study of NK1 receptor occupancy. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 226–235. [Google Scholar] [CrossRef]
- Ranga, K.; Krishnan, R. Clinical experience with substance P receptor (NK1) antagonists in depression. J. Clin. Psychiatry 2002, 63, 25–29. [Google Scholar]
- Ratti, E.; Bellew, K.; Bettica, P.; Bryson, H.; Zamuner, S.; Archer, G.; Squassante, L.; Bye, A.; Trist, D.; Krishnan, R.; et al. Results From 2 Randomized, Double-Blind, Placebo-Controlled Studies of the Novel NK1 Receptor Antagonist Casopitant in Patients with Major Depressive Disorder. J. Clin. Psychopharmacol. 2011, 31, 727–733. [Google Scholar] [CrossRef]
- Poma, A.; Christensen, J.; Davis, J.; Kansra, V.; Martell, R.E.; Hedley, M.L. Phase 1 positron emission tomography (PET) study of the receptor occupancy of rolapitant, a novel NK-1 receptor antagonist. J. Clin. Oncol. 2017, 32. [Google Scholar] [CrossRef]
- ClinicalTrials.gov Identifier: NCT01381419. Available online: https://clinicaltrials.gov/ct2/show/NCT01381419 (accessed on 15 May 2019).
- ClinicalTrials.gov Identifier: NCT01059578. Available online: https://clinicaltrials.gov/ct2/show/NCT01059578 (accessed on 15 May 2019).
- Van der Mey, M.; Janssen, C.G.M.; Janssens, F.E.; Jurzak, M.; Langlois, X.; Sommen, F.M.; Verreet, B.; Windhorst, A.D.; Leysen, J.E.; Herscheid, J.D.M. Synthesis and biodistribution of [11C]R116301, a promising PET ligand for central NK1 receptors. Bioorg. Med. Chem. 2005, 13, 1579–1586. [Google Scholar] [CrossRef]
- Wolfensberger, S.P.A.; van Berckel, B.N.M.; Airaksinen, A.J.; Maruyama, K.; Lubberink, M.; Boellaard, R.; Carey, W.D.H.; Reddingius, W.; Veltman, D.J.; Windhorst, A.D.; et al. First Evaluation of [11C]R116301 as an In Vivo Tracer of NK1 Receptors in Man. Mol. Imaging Biol. 2009, 11, 241–245. [Google Scholar] [CrossRef] [Green Version]
- Wolfensberger, S.P.; Maruyama, K.; van Berckel, B.N.; Lubberink, M.; Airaksinen, A.J.; Boellaard, R.; Luurtsema, G.; Reddingius, W.; Janssens, F.E.; Veltman, D.J.; et al. Quantification of the NK1 Receptor ligand [11C]R116301. Nucl. Med. Commun. 2011, 32, 896–902. [Google Scholar] [CrossRef]
- Huskey, S.E.W.; Dean, B.J.; Doss, G.A.; Wang, Z.; Hop, C.E.C.A.; Anari, R.; Finke, P.E.; Robichaud, A.J.; Zhang, M.; Wang, B.; et al. The metabolic disposition of aprepitant, a Substance P receptor antagonist, in rats and dogs. Drug Metab. Dispos. 2004, 32, 246–258. [Google Scholar] [CrossRef]
- Miraglia, L.; Pagliarusco, S.; Bordini, E.; Martinucci, S.; Pellegatti, M. Metabolic disposition of casopitant, a potent NK1 receptor antagonist, in mice, rats and dogs. Drug Metab. Dispos. 2010, 38, 1876–1891. [Google Scholar] [CrossRef]
- Pellegatti, M.; Bordini, E.; Fizzotti, P.; Roberts, A.; Johnson, B.M. Disposition and Metabolism of Radiolabeled Casopitant in Humans. Drug Metab. Dispos. 2009, 37, 1635–1645. [Google Scholar] [CrossRef] [Green Version]
- Spinelli, T.; Calcagnile, S.; Giuliano, C.; Rossi, G.; Lanzarotti, C.; Mair, S.; Stevens, L.; Nisbet, I. Netupitant PET Imaging and ADME Studies in Humans. J. Clin. Pharmacol. 2014, 54, 97–108. [Google Scholar] [CrossRef]









| NK1R ligands | Ligand Biological Properties and Applications | References |
|---|---|---|
| Mammalian NK1R ligands | ||
| • Phosphatidylinositol signal pathway activation and intracellular calcium concentration increase; | [20] | |
| • Treatment of depression and associated anxiety; | [38] | |
| • Prevention of vomiting after anaesthesia or chemotherapy; | [39,40] | |
| Substance P (SP, SP(1–11), [Arg1]SP) | • Increase of endothelial ion transport and permeability of vessels in tissue inflammation states; | [41,42,43,44] |
| • Neuropathic pain modulation; | [45] | |
| • Liver cirrhosis biomarker; | [46,47,48] | |
| • Bone tissue metabolism modulator, especially of osteoblast activity at a later stage of bone formation; | [47,48] | |
| Arg1-Pro2-Lys3-Pro4-Gln5-Gln6- Phe7-Phe8-Gly9-Leu10-Met11-NH2 | • Cancer growth promotor (astrocytoma, melanoma, neuroblastoma, pancreatic cancer), angiogenesis, migration and metastasis; | [5,49,50] |
| (Figure 2) | • Study of the synergistic effect of SP and insulin-like growth factor 1 (IGF-1) on corneal epithelial wound healing – synergistic effect possible only in the presence of the SP fragment containing minimum C-terminus 4 amino acids, SP(8–11); | [51] |
| [Thi8,Met(O2)11]SP Pro4-Gln5-Gln6-Phe7-Thi8-Gly9-Leu10-Met(O2)11-NH2 | Treatment of recurrent and critically located glioblastoma multiforme; | [55] |
| [Sar9,Met(O2)11]SP(1–11) and (Sendide) [Tyr6,D-Phe7,D-His9]SP(6–11) | Studies of the role of NK1R in regulation and release of vasopressin peptide; | [56,57] |
| (X)Arg1-Pro2-Lys3-Pro4-Gln5-Gln6-Phe7-Phe8-Gly9-Leu10-Met11-NH2 (1) or Arg1-Pro2-(X)Lys3-Pro4-Gln5-Gln6-Phe7-Phe8-Gly9-Leu10-Met11-NH2 (1) | Studies of photoactivatable SP derivatives; | [59] |
| Bapa0[(pBzl)PheX]SP(2) Bapa0[Pro9,(pBzl)Hcy(O2)11]SP(2) Bapa0[Hcy(ethylaminodansyl)11]SP | Studies of activation of different second messenger pathways as a result of ligand binding to various NK1Rs sites; studies of dual behaviour of the tested SP derivatives: as antagonists at the NK-1M binding site activating AC pathway or agonists at the NK-1m binding site activating PLC pathway; | [10] |
| Septide [pGlu6,Pro9]SP(6–11) pGlu6-Phe7-Phe8-Pro9-Leu10-Met11-NH2 | Agonist as potent as SP in eliciting smooth muscle contraction, however poor competitor of SP due to interaction with another binding site of NK1R (NK-1m, so-called ‘septide-sensitive’); | [7,60] |
| GR 73,632 NH2(CH2)4C(O)-Phe7-Phe8-Pro9-(Me)Leu10-Met11-NH2 | Approximately 200-fold more potent than SP in inducing the characteristic behavioural response in murine models. | [61] |
| Non-mammalian NK1R ligands | ||
| Physalaemin Pyr1-Ala2-Asp(OH)3-Pro4-Asp(NH2)5-Lys6-Phe7-Tyr8-Gly9-Leu10-Met11-NH2 | Stimulation of extravascular smooth muscles, component of eye drops for Sjögren syndrome treatment and other forms of keratoconjunctivitis sicca; | [3,19,62,63] |
| Eledoisin pGlu1-Pro2-Ser3-Lys4-Asp5-Ala6-Phe7-Ile8-Gly9-Leu10-Met11-NH2 | Similar biological activities as Physalaemin but slightly less active and more stable in vivo; clinical trials for limb arteriosclerosis treatment; component of eye drops for Sjögren syndrome; | [19,63] |
| Sialokinin I Asn2-Thr3-Gly4-Asp5-Lys6-Phe7-Tyr8-Gly9-Leu10-Met11-NH2 Sialokinin II, Asp2-Thr3-Gly4-Asp5-Lys6-Phe7-Tyr8-Gly9-Leu10-Met11-NH2 | Vasodilation, effect on salivation, influence on the acinar cells of the submandibular glands. | [3,64,65] |
| Substance | IC50 ± SEM [nM] |
|---|---|
| Substance P | 2.7 ± 0.22 |
| 111In-DOTAGA-Substance P | 1.1 |
| 111In-DOTAGA-[Met(O2)11]-Substance P | 9.8 ± 1.00 |
| 111In-DOTA-[Met(O2)11]-Substance P | 3.55 ± 0.45 |
| 111In-DOTA-[Sar9]-Substance P | 3.20 ± 0.30 |
| 111In-DOTA-[Thi8]-Substance P | 7.30 ± 2.00 |
| 111In-DOTA-[Thi7]-Substance P | 9.40 ± 1.60 |
| 111In-DOTA-[Sar9,Met(O2)11]-Substance P | 2.00 ± 0.00 |
| 111In-DOTA-[Thi8,Met(O2)11]-Substance P | 0.78 ± 0.03 |
| 111In-DOTA-[Thi8,Sar9]-Substance P | 3.40 ± 0.40 |
| 111In-DOTA-[Thi7,Thi8]-Substance P | 7.70 ± 0.70 |
| NK1R Antagonists | Anticancer Effect | References |
|---|---|---|
| Aprepitant | Tumour cell growth inhibition | [2,16,17,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98] |
| Tumour cell migration and proliferation inhibition | [2,16,17,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98] | |
| L-733,060 | Apoptotic action on cells | [2,16,17,85,86,87,88,89,90,91,92,93,94,95,96,97,98] |
| Tumour size/volume decrease | [2,86,91,93,94,95,96] | |
| L-732,138 | Inflammation state inhibition | [2,93,95,96] |
| Angiogenesis decrease | [2,16,86,91,93,95,96] | |
| (Figure 3) | Antiproliferative effect | [2,16,86,91,93,94,95,96] |
| Metastases prevention | [2,86,91,93,95,96] |
| NK1R Radioligand Molecules | Biological Properties and Potential Applications | References |
|---|---|---|
| [125I]I-BH-[Tyr8]SP | • Animal tests: high affinity to pancreatic acinar cells isolated from guinea pigs; | [104] |
| • Animal or human tests: used as a radiotracer for determination of specific binding and/or internalisation in various organs, tissues and cells, namely in: | ||
| ▪ anterior pituitary cells | [104,105,106] | |
| ▪ in rat thymus, spleen | [105,106,107] | |
| ▪ brain | [108] | |
| ▪ spinal cord | [109,110] | |
| ▪ chicken small intestine | [111,112,113,114,115] | |
| ▪ epithelial cells | [116] | |
| ▪ mesencephalic primary cultures prepared from embryonic mouse brain | [117,118] | |
| ▪ rat parotid membranes | [119] | |
| ▪ human eyes; | [120] | |
| [3H]H-SP | Animal tests: specificity for imaging of cat inflamed bladder tissue; | [121] |
| [111In]In-DTPA-[Arg1]SP | • Animal tests: imaging of SP receptor-positive (SPR+) immunologic disorders; high affinity to NK1R presented in parotid gland and brain cortex membranes; rapid enzymatic degradation; high uptake in pancreatic tumour (CA20948), salivary glands, kidneys and arthritic hind leg joints; unable to cross the intact blood–brain barrier; | [122] |
| • Clinical trials: used in scintigraphy of immune-mediated diseases; | [123] | |
| [99mTc]Tc-IMB-SP (1) | Animal tests: significant uptake in the salivary glands; | [124] |
| [99mTc]Tc-Hynic-SP (2) [99mTc](NS3)-Tc-CN-SP and [99mTc]((NS3)-Tc-CN)2-SP (3) | In vitro study: high stability in biological fluids; relationship between molecular structure and physicochemical properties; | [125] |
| [99mTc][Tc(N)(Cys-Cys-SP)(PCN)] (4) [188Re][Re(N)(Cys-Cys-SP)(PCN)] | • In vitro study: application of theranostic pair 99mTc and 188Re; affinity studies using U87MG cell line expressing NK1R and negative control cell line L-929; • Animal tests: accumulation in salivary glands, kidneys and thymus; | [126] |
| [111In]In-DOTA-[Thi8,Met(O2)11]SP [68Ga]Ga-DOTA-[Thi8,Met(O2)11]SP | Clinical trials: used for visualisation of NK1R expression and control of radiocompound distribution at the target site and whole body; administrated simultaneously with therapeutic radiopharmaceutical [213Bi]Bi-DOTA-[Thi8,Met(O2)11]SP; | [55,127] |
| [99mTc][Tc-Hynic-[Tyr8,Met(O)11]SP (5) | Animal tests: specific uptake in the tumour; stable in HS; internalisation studies on U373 MG astrocytoma cell line; significant accumulation in kidneys; | [128] |
| [3H]H-[Pro9]SP and [3H]H-propionyl-[Met(O2)11]SP(7–11) | In vitro study: applied for the studies of different NK1R binding sites: NK-1M (majority) and NK-1m (minority); | [11] |
| [125I]I-BH-[Sar9,Met(O2)11]SP | • Animal tests: comparison of uptake in submandibular gland and in several regions of rat brain of the tested radiocompound and [125I]I-BH-SP; • In vitro study: comparison of physicochemical properties of the tested radiocompound and [125I]I-BH-SP. | [129,130,131] |
| NK1R Radioligand Molecules | Biological Properties and Potential Applications | Reference |
|---|---|---|
| [177Lu]Lu-DOTA-SP | • Animal tests: biodistribution studies on mice bearing AR42J pancreatic tumour, high uptake in kidneys, satisfactory uptake in tumour, significant uptake in intestine and stomach; • In vitro study: high specific uptake and internalisation using LN319 cells isolated directly from the tumours; | [133] |
| [177Lu]Lu-DOTAGA-SP [90Y]Y-DOTAGA-SP [213Bi]Bi-DOTAGA-SP | Medical experiments: well tolerated therapy of critically located gliomas, low toxicity; | [134] |
| [90Y]Y-DOTAGA-SP | Medical experiments: recorded completed encapsulation of the tumour in patients administered with the highest dose; | [135] |
| [213Bi]Bi-DOTA-[Thi8,Met(O2)11]SP [111In]In-DOTA-[Thi8,Met(O2)11]SP | Medical experiments: treatment of critically located gliomas; well tolerated and safe for patients; complete necrosis of small tumours and necrosis only in the nearness of the implanted catheters in the case of large tumours; | [55] |
| [213Bi]Bi-DOTA-[Thi8,Met(O2)11]SP [68Ga]Ga-DOTA-[Thi8,Met(O2)11]SP | Medical experiments: treatment of patients with secondary GBM (after surgery, chemo- and radiotherapy); very low accumulation in kidneys, urine, bladder and blood; no side effects, necrosis and demarcation of the tumours; | [127] |
| [213Bi]Bi-DOTA-[Thi8,Met(O2)11]SP | Medical experiments: higher efficiency of radiolabelled NK1R ligands application and local brain tumours treatment in patients suffering from secondary GBM compared to standard treatment options; | [136] |
| [225Ac]Ac-DOTA-[Thi8,Met(O2)11]SP | • In vitro study: high affinity to glioblastoma cancer cells: T98G, U87MG, U138MG and glioblastoma stem cells (GSC); significant reduction in glioblastoma cell viability in comparison to the conventional treatment with temozolomide; high cytotoxicity towards GBM stem cells; | [139] |
| • Medical experiments: safe and well-tolerated therapy without side effects; | [144] | |
| [177Lu]Lu-DOTA-SP(4–11) [177Lu]Lu-DOTA-SP(5–11) [177Lu]Lu-DOTA-[Thi8,Met(O2)11]SP(5–11) | In vitro study: radiobioconjugates characterized with higher lipophilicity and lower molecular weight than those based on analogue [Thi8,Met(O2)11]SP—changes in physicochemical properties of radiobioconjugates leading to their deeper diffusion into the cavity walls after surgical resection of the tumour. | [145] |
| Radiotracer | Structure | Application | Reference |
|---|---|---|---|
| [11C]CP-96,345 1 | Investigational “lead structure” compounds | Preclinical tests in animal models | [146] |
| [11C]CP-99,994 1 | [147] | ||
| [11C]CP-643,051 1 | [148] | ||
| [11C]GR205171([11C]vofopitant) 2 | Optimized radiotracers | Pharmacodynamics and pharmacokinetics studies, receptor occupancy imaging in clinical trials | [149,151,155,156,157,164,165,166,167,168,169] |
| [18F]SPA-RQ ([18F]L-829,165) 2 | [76,78,150,152,158,159,160,161,162,163] | ||
| [18F]FE-SPA-RQ | [153,154] | ||
| [11C]R116301 3 | [170,171,172] | ||
| [14C]aprepitant 3,4 | Well known high-selective antagonists indicated in CINV | ADME investigations in animals and men | [173] |
| [14C]casopitant 4 | [174,175] | ||
| [14C]netupitnat 4 | [176] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majkowska-Pilip, A.; Halik, P.K.; Gniazdowska, E. The Significance of NK1 Receptor Ligands and Their Application in Targeted Radionuclide Tumour Therapy. Pharmaceutics 2019, 11, 443. https://doi.org/10.3390/pharmaceutics11090443
Majkowska-Pilip A, Halik PK, Gniazdowska E. The Significance of NK1 Receptor Ligands and Their Application in Targeted Radionuclide Tumour Therapy. Pharmaceutics. 2019; 11(9):443. https://doi.org/10.3390/pharmaceutics11090443
Chicago/Turabian StyleMajkowska-Pilip, Agnieszka, Paweł Krzysztof Halik, and Ewa Gniazdowska. 2019. "The Significance of NK1 Receptor Ligands and Their Application in Targeted Radionuclide Tumour Therapy" Pharmaceutics 11, no. 9: 443. https://doi.org/10.3390/pharmaceutics11090443