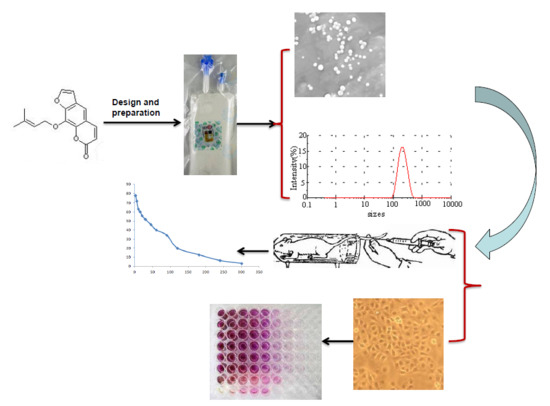
1. Introduction
Imperatorin is a chemical compound belonging to the linear furanocoumarins and it is mainly extracted and isolated from the traditional herbal medicine of
Angelica dahurica. Modern pharmaceutical studies have identified that imperatorin has good biological activity, including analgesic, antibacterial, anti-inflammatory, vessel dilating, and CYP450-inhibiting effects [
1]. Many studies have suggested that imperatorin had certain antitumor efficacies, such as an inhibiting effect on a human hepatocellular carcinoma cell line, a human breast cancer cell line, a human cervical carcinoma cell line, a human osteosarcoma cell line, and other metastatic tumor cells. The mechanism mainly included a downregulating effect on mitochondrial MCl-1 protein expression [
2,
3,
4,
5]. Jakubowicz-Gil showed that imperatorin combined with quercetin could effectively inhibit the proliferation of tumor cells and induce the cells’ apoptosis [
6,
7,
8]. These findings suggested that imperatorin is a potential anticancer drug with a good application prospect. So, it is obvious that the compound has great potential to be developed as a pharmaceutical formulation for subsequent clinical assessment. But, Imperatorin belongs the BCS II classfication system. It shows a relatively low bioavailability because of its poor water solubility [
9]. So, it is difficult to prepare an ideal oral pharmaceutical preparation [
10]. It is more difficult to be developed as an injection. Research reports ultradeformable liposomes as a novel vesicular carrier could increase skin permeation efficiency of imperatorin [
11], and Jingjing Pan prepared imperatorin sustained-release tablets to lower blood pressure [
12]. There is no injection of imperatorin reported. In this study, we took advantage of imperatorin’s characteristics of poor solubility in water and good fat solubility and dissolved it in an oil-based injection, selected lecithin as an emulsifier, and used nanoemulsification technology to prepare imperatorin lipid microspheres.
Lipid microsphere (LM) has been recently used as intravenous (i.v.) carriers for drugs, especially for those drugs that have enough solubility in oil while have poor solubility in water. It is difficult for this kind of drugs to prepare injection preparations. The LM, with a diameter of 0.2 microns, is composed of soybean oil and lecithin, can carry lipophilic or hydrophilic drugs, drugs are usually incorporated into the oil phase or into the interface of oil phase in LM, so they are presumed to avoid or reduce local or blood vessel irritation. As a lipophilic drug carrier, it has the following advantages of targeting positioning, improving the solubility and stability of drugs, reducing the adverse reactions, etc. [
13,
14].
Uniform design and orthogonal design are two kinds of experimental design methods that are widely used in the research of pharmaceutical preparations of Chinese herbal medicines [
15]. However, the uniform design and orthogonal design optimization method is constrained by the linear model, as it can only point out the direction of factor values and is unable to find extremes, and the deviation between the measured and predicted values is larger under the optimum preparation conditions [
16]. Response surface methodology (RSM) is a combination of mathematical and statistical techniques, which has the characteristics of requiring fewer tests and having higher test accuracy. It is also more simplified and comprehensive than orthogonal design and uniform design. In the process of optimization, practical research mainly focuses on central composite design (CCD) under RSM [
17]. Because CCD is very practically suitable for comparing experimental methodologies with theoretical models [
18], and it includes not only the effects of interaction of the variables but also the overall effects of the parameters in the process [
19], it is often used in the optimization method for the preparation of new technologies.
Breast cancer is one of the most common female cancers in the world. It is still associated with high morbidity and mortality. At present, chemotherapy and surgery are the most important methods of treating breast cancer. Imperatorin is a single component of some traditional Chinese medicines, which has the characteristics of high efficacy and low toxicity. Previous studies have showed that imperatorin has an antitumor effect [
20,
21], and it has a strong inhibitory effect on MDA-MB-231 cells [
22]. However, because of its physical and chemical properties, its druggability is very low. In order to increase its druggability and to exert its antitumor effect, the optimization of the preparation and formulation of imperatorin lipid microspheres was accomplished; furthermore, the pharmacokinetics of imperatorin in rats was investigated, and the respective effects of imperatorin and imperatorin lipid microspheres on MDA-MB-231 cell proliferation were also compared in the study.
4. Conclusions
The lipid microsphere is a good candidate for drug loading because of its safety, stability, and good biocompatibility, especially for those drugs with low solubility.
The central composite design–response surface method is an optimal design method that is used in the optimization of formulations due to its relatively small number of experiments required and high precision. According to the surface change, the three-dimensional effect of the surface chart could directly respond to the influence of factors on the survey index. Based on the overlying of better conditions chosen by multiple effects, the range of better conditions can be further reduced.
The optimal formulation was: egg lecithin—13.9 g, poloxamer 188—2.1 g, and soybean oil—105.7 g. The particle size was 168 ± 1.73 nm, polydispersity index (PDI) was 0.138 ± 0.02, zeta potential was −43.5 ± 0.5 mV, drug loading was 0.833 ± 0.027 mg/mL, and the encapsulation efficiency was 90 ± 1.27%. The results showed that imperatorin lipid microspheres could significantly inhibit MDA-MB-231 cell proliferation. However, pharmacokinetic study of the imperatorin lipid microspheres showed that the half-time of imperatorin was very short. Thus, further studies should be conducted on how to increase its half-life duration and improve the residence time in blood.