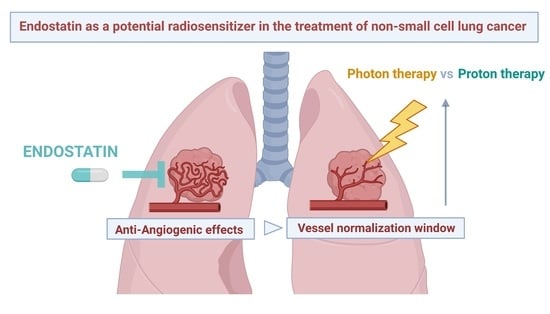
Recombinant Endostatin as a Potential Radiosensitizer in the Treatment of Non-Small Cell Lung Cancer
Abstract
:1. Introduction
1.1. Treatment Landscape of Non-Small Cell Lung Cancer
1.2. The Role of Tumour Angiogenesis in NSCLC
1.3. The Potential of Anti-Angiogenic Drugs in Combination Treatments for NSCLC
2. Endostatin and Its Mechanism of Action
RE Re-Imagined
3. Radiotherapy and Anti-Angiogenic Therapy: A Dilemma
3.1. RE and Vascular Normalization
3.2. Summary of Preclinical Results on RE Combined with RT
| Cancer Type Cell Lines | Endostatin Type Dose | Main Result | RT Type Dose | Year | Reference |
|---|---|---|---|---|---|
| Breast Cancer 4T1 or 4MTMHpc | RE (murine) 0.5, 1, 2, 4 and 8 µg/mL | Inhibits the in vitro growth and potentiates the anti-tumour effects of RT via alteration of the amount of substance P | 60Co γ-rays 45 Gy | 2011 | [138] |
| Human Pulmonary Adenocarcinoma A549 | RE 300 mg/L normoxia; 400 mg/L hypoxia | Radiosensitizing effect under hypoxia, but not under normoxia. RE enhanced radiosensitivity through G2/M arrest | 6 MV X-ray 2 Gy | 2012 | [129] |
| Human ESCC Eca109 and TE3 | RE 25, 50, 100, 200, 400, 600, and 800 µg/mL | Combined treatment inhibited migration, invasion, and vasculogenic mimicry formation, but did not enhance radiosensitivity | 6 MV X-ray 2, 4, 6 or 8 Gy | 2016 | [131] |
| NSCLC Calu-1, A549, 95D, NCI-H292, NCI-H1299 | RE 0, 200, 500, 1000, 2000, and 2500 µg/mL. IC20 of Calu-1 cells: 296.5 μg/mL | Induces apoptosis and enhances radiosensitivity of the VEGFR-2 high-expressing cell line Calu-1, but it has a limited effect on the VEGFR-2 low- expressing cell line A549 | not stated 2, 4, 6 and 8 Gy | 2016 | [132] |
| Breast Cancer 4T1 or 4MTMHpc | RE 0.5, 1, 2, 4 and 8 µg/mL: 4 µg/mL-most cytotoxic | Increase in ADAM10 enzyme activity (4T1 or 4MTMHpc cell line, respectively): RT (55%) vs. RE + RT (74.5%) RE (43.3%) vs. RT (70.9%) vs. RE + RT (72.5%) | 60Co γ-rays 45 Gy | 2016 | [139] |
| Human lung squamous carcinoma H-520 | RE 200 µg/mL | RE significantly enhanced the radiosensitivity by inhibition of cellular proliferation, promotion of cell apoptosis and redistribution of cell cycle, possibly via deactivation of the Akt pathway | 60Co γ-rays 1, 2, 4, 6, 8 and 10 Gy | 2010 | [130] |
| Cancer Type | E/RE Dose | Main Result | RT Type Dose | Year | Reference |
|---|---|---|---|---|---|
| LLC | RE 15 mg/kg | Can promote the normalization of tumour blood vessels and increase the anti-tumour immune-related immune cells infiltrating the tumour post RT | Varian Clinac 600C (energy not specified, 6–10 MV X-rays) 10 Gy | 2020 | [125] |
| EC | E 50 mg/kg | Enhanced the anti-tumour effects of RT and prolonged disease-free survival | Cs137 γ-rays Dose rate 6 Gy/min (dose not specified) | 2007 | [135] |
| ESCC | RE 2.5, 5 and 10 mg/kg | RE promotes the efficacy of RT on esophageal cancer, which may be partly realized by inhibiting the activity of VEGF related signal pathways | 6 MV X-ray 10 Gy | 2016 | [140] |
| NSCLC | RE 0.75 mg/mL for 7 days | RT + weekly RE showed synergistic effects, produced by: RE’s stability, RE’s improvement of tumour hypoxia resulting in increased sensitivity to RT and RE’s inhibition of RT-induced tumour angiogenesis | 6 MV X-ray 10 Gy | 2011 | [144] |
| ESCC | RE 15 mg/kg | RE + RT was more effective at delaying tumour growth than single therapy | RS2000 X-ray irradiator (kV range) 2, 4, 6 or 8 Gy | 2015 | [141] |
| LLC | RE 0, 2.5, 5, 10, and 20 mg/kg | RT + Endo + CP673451 treatment markedly inhibited tumour growth with no improvement in the overall survival and significantly reduced the tumour MVD | Varian Clinac 600C (6–10 MV X-rays) 12 Gy | 2018 | [145] |
| HCC | RE 2, 4, 8, 16, and 32 mg/kg | Combination therapy regulated the expression of genes controlling angiogenesis and cell adhesion. Synergistic effect of RE + RT against HCC in vivo and in vitro | 6 MeV electron beam 10 Gy | 2017 | [142] |
| NPC | RE 20 mg/kg/d | RE normalized tumour vasculature, which alleviated hypoxia and caused significant radiosensitization in human NPC | 160 kV X-ray 6 Gy | 2012 | [127] |
| HNSSC | Endostatin 2.5 mg/kg/day | Endostatin + RT produced an increase in cow pulmonary artery endothelial apoptosis compared with either treatment alone | not stated 15 Gy/day | 2000 | [146] |
| Colorectal cancer | RE 20 mg/kg | The tumour growth inhibition rate in the RT + RE treatment group > single therapy groups | 6 MV X-ray 6 Gy | 2017 | [143] |
| NPC | RE 20 mg/kg | The tumour inhibition rates of RE, RT and RE + RT were 27.12, 60.45 and 86.11%, respectively. Tumour VEGF levels in the RE + RT group < RT only and control groups | 5 MV X-ray 20 Gy | 2012 | [136] |
| NPC/ ung adenocarcinoma | RE 20 mg/kg | RE sensitized anti-tumour/anti-angiogenic RT effects by increasing apoptosis of the endothelial and tumour cells, decreasing hypoxia, and changing proangiogenic factors | 6 MV X-rays 6 Gy per day to 30 Gy, once a day for 1 week | 2009 | [147] |
3.3. Current Status of Clinical Trials in NSCLC Patients Investigating Radiotherapy Combined with RE
| Cancer Type | Phase | E/RE Dose | Year | n | Combined Therapy | Overall Result | Reference |
|---|---|---|---|---|---|---|---|
| NSCLC | Pro cohort | RE 15 mg/day | 2012 | 25 | RT | (+) short term therapeutic effects and local control rates. no severe adverse effects (-) no improvement of 1/3 year OS | [160] |
| NSCLC | n.s. | RE 15mg/day for 10 days | 2013 | RT | (+) decreased hypoxia | [124] | |
| BM of NSCLC | II | RE 7.5 mg/m2/day | 2014 | RT | (+) can relieve brain oedema | [165] NCT01410370 | |
| Stage III NSCLC | SA pro II | RE 7.5 mg/m2/day for 7 days at week 1, 3, 5 and 7 | 2015 | 48 | RT/DOC and CIS | (+) promising survival and local control rates | [166] NCT01218594 |
| Stage IIIA/B NSCLC | SA pro II | E 7.5 mg/m2 on day 1–14, every 3 weeks | 2016 | 19 | RT/TC | (-) did not meet the goal per study design with unacceptable toxicity | [161] NCT01158144 |
| Stage IIIA/B NSCLC | SA retro | RE 7.5 mg/m2/day for 7 days at week 1, 3, 5 and 7 | 2020 | CCRT | Inflammation-based factors as biomarker | [167] | |
| Stage III NSCLC | SA pro II | RE 7.5 mg/m2/day, 14 days/cycle | 2019 | 67 | RT/ ETO-CIS | (-) did not prolong median PFS (+) preferable OS, promising 2-year PFS with tolerable toxicities | [159] HELPER study NCT01733589 |
| Stage III NSCLC | II | RE 7.5 mg/m2/day for seven day | 2020 | 48 | IV RE + RT/DOC/ CIS vs. CIV RE + RT/ ETO-CIS | CIV > IV | [162] |
| Local aLSCC | retro | RE 7.5 mg/m2/day for 14 days (every 3 weeks) | 2020 | 94 | RT/NP | Lipoprotein (a) as biomarker | [168] |
| Stage III NSCLC* | IV | RE 7.5 mg/m2/day, 14 days/cycle | / | / | Durvalumab/ reduced-dose CCRT (50 Gy) | Not yet recruiting | NCT04613284 |
| Stage III NSCLC | Multi-centre, prospective real-world study | RE n.s. | / | / | CCRT | Not yet recruiting | NCT04161352 |
4. Discussion and Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Society of Clinical Oncology, I. Lung Cancer- Non Small-Cell: Statistics. Available online: https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/statistics (accessed on 24 November 2022).
- Wang, M.; Herbst, R.S.; Boshoff, C. Toward Personalized Treatment Approaches for Non-Small-Cell Lung Cancer Meina. Nat. Med. 2021, 27. [Google Scholar] [CrossRef]
- Houston, K.A.; Henley, S.J.; Li, J.; White, M.C.; Richards, T.B. Patterns in Lung Cancer Incidence Rates and Trends by Histologic Type in the United States, 2004–2009. Lung Cancer 2014, 86, 22–28. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society, Lung Cancer Early Detection, Diagnosis, and Staging. Available online: https://www.cancer.org/content/dam/CRC/PDF/Public/8661.00.pdf (accessed on 24 November 2022).
- Qiang, H.; Chang, Q.; Xu, J.; Qian, J.; Zhang, Y.; Lei, Y.; Han, B. New Advances in Antiangiogenic Combination Therapeutic Strategies for Advanced Non - Small Cell Lung Cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 631–645. [Google Scholar] [CrossRef]
- Hong, Y.; Park, S.; Lee, M.K. The Prognosis of Non-Small Cell Lung Cancer Patients According to Endobronchial Metastatic Lesion. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Alexander, M.; Kim, S.Y.; Cheng, H. Update 2020: Management of Non-Small Cell Lung Cancer. Lung 2020, 198, 897–907. [Google Scholar] [CrossRef]
- Sandler, A.; Gray, R.; Perry, M.C.; Brahmer, J.; Schiller, J.H.; Dowlati, A.; Lilenbaum, R.; Johnson, D.H. Paclitaxel-Carboplatin Alone or with Bevacizumab for Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2006, 355, 2542–2550. [Google Scholar] [CrossRef] [Green Version]
- Manzo, A.; Montanino, A.; Carillio, G.; Costanzo, R.; Sandomenico, C.; Normanno, N.; Piccirillo, M.C.; Daniele, G.; Perrone, F.; Rocco, G.; et al. Angiogenesis Inhibitors in NSCLC. Int. J. Mol. Sci. 2017, 18, 2021. [Google Scholar] [CrossRef] [Green Version]
- Yadav, L.; Puri, N.; Rastogi, V.; Satpute, P.; Sharma, V. Tumour Angiogenesis and Angiogenic Inhibitors: A Review. J. Clin. Diagnostic Res. 2015, 9, XE01–XE05. [Google Scholar] [CrossRef]
- Baeriswyl, V.; Christofori, G. The Angiogenic Switch in Carcinogenesis. Semin. Cancer Biol. 2009, 19, 329–337. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Jain, R.K. Normalizing Tumor Vasculature with Anti-Angiogenic Therapy: A New Paradigm for Combination Therapy. Nat. Med. 2001, 7, 987–989. [Google Scholar] [CrossRef]
- Bishop-Bailey, D. Tumour Vascularisation: A Druggable Target. Curr. Opin. Pharmacol. 2009, 9, 96–101. [Google Scholar] [CrossRef]
- Poto, R.; Cristinziano, L.; Modestino, L.; de Paulis, A.; Marone, G.; Loffredo, S.; Galdiero, M.R.; Varricchi, G. Neutrophil Extracellular Traps, Angiogenesis and Cancer. Biomedicines 2022, 10, 431. [Google Scholar] [CrossRef]
- Hwang, I.; Kim, J.W.; Ylaya, K.; Chung, E.J.; Kitano, H.; Perry, C.; Hanaoka, J.; Fukuoka, J.; Chung, J.Y.; Hewitt, S.M. Tumor-Associated Macrophage, Angiogenesis and Lymphangiogenesis Markers Predict Prognosis of Non-Small Cell Lung Cancer Patients. J. Transl. Med. 2020, 18, 1–15. [Google Scholar] [CrossRef]
- Sammarco, G.; Varricchi, G.; Ferraro, V.; Ammendola, M.; De Fazio, M.; Altomare, D.F.; Luposella, M.; Maltese, L.; Currò, G.; Marone, G.; et al. Mast Cells, Angiogenesis and Lymphangiogenesis in Human Gastric Cancer. Int. J. Mol. Sci. 2019, 20, 2106. [Google Scholar] [CrossRef] [Green Version]
- Loizzi, V.; Del Vecchio, V.; Giulio, G.; De Liso, M.; Kardashi, A.; Naglieri, E.; Resta, L.; Cicinelli, E.; Cormio, G. Biological Pathways Involved in Tumor Angiogenesis and Bevacizumab Based Anti-Angiogenic Therapy with Special References to Ovarian Cancer. Int. J. Mol. Sci. 2017, 18, 1967. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, T.; Kudo, M. Signaling Pathways Governing Tumor Angiogenesis. Oncology 2011, 81 (Suppl. 1), 24–29. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The Biology of VEGF and Its Receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Huang, Z.; Bao, S.D. Roles of Main Pro- and Anti-Angiogenic Factors in Tumor Angiogenesis. World J. Gastroenterol. 2004, 10, 463–470. [Google Scholar] [CrossRef]
- Jia, T.; Jacquet, T.; Dalonneau, F.; Coudert, P.; Vaganay, E.; Exbrayat-Héritier, C.; Vollaire, J.; Josserand, V.; Ruggiero, F.; Coll, J.-L.; et al. FGF-2 Promotes Angiogenesis through a SRSF1/SRSF3/SRPK1-Dependent Axis That Controls VEGFR1 Splicing in Endothelial Cells. BMC Biol. 2021, 19, 173. [Google Scholar] [CrossRef]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef] [Green Version]
- Thurston, G.; Kitajewski, J. VEGF and Delta-Notch: Interacting Signalling Pathways in Tumour Angiogenesis. Br. J. Cancer 2008, 99, 1204–1209. [Google Scholar] [CrossRef] [Green Version]
- Cross, M.J.; Claesson-Welsh, L. FGF and VEGF Function in Angiogenesis: Signalling Pathways, Biological Responses and Therapeutic Inhibition. Trends Pharmacol. Sci. 2001, 22, 201–207. [Google Scholar] [CrossRef]
- Sullivan, D.C.; Bicknell, R. New Molecular Pathways in Angiogenesis. Br. J. Cancer 2003, 89, 228–231. [Google Scholar] [CrossRef] [Green Version]
- Farzaneh, Z.; Vosough, M.; Agarwal, T.; Farzaneh, M. Critical Signaling Pathways Governing Hepatocellular Carcinoma Behavior; Small Molecule-Based Approaches. Cancer Cell Int. 2021, 21. [Google Scholar] [CrossRef]
- Akil, A.; Gutiérrez-García, A.K.; Guenter, R.; Rose, J.B.; Beck, A.W.; Chen, H.; Ren, B. Notch Signaling in Vascular Endothelial Cells, Angiogenesis, and Tumor Progression: An Update and Prospective. Front. Cell Dev. Biol. 2021, 9, 1–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Oliveira, R.H.M.; Zhao, C.; Popel, A.S. Systems Biology of Angiogenesis Signaling: Computational Models and Omics. WIREs Mech. Dis. 2021, 1–37. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Koukourakis, M.I.; Sivridis, E.; Turley, H.; Talks, K.; Pezzella, F.; Gatter, K.C.; Harris, A.L. Relation of Hypoxia Inducible Factor 1 Alpha and 2 Alpha in Operable Non-Small Cell Lung Cancer to Angiogenic/Molecular Profile of Tumours and Survival. Br. J. Cancer 2001, 85, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Jackson, A.L.; Zhou, B.; Kim, W.Y. HIF, Hypoxia and the Role of Angiogenesis in Non-Small Cell Lung Cancer. Expert Opin. Ther. Targets 2010, 14, 1047–1057. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Liu, T.; Lee, M.; Yang, S.; Tsao, T.C. Independent Prognostic Value of Hypoxia-Inducible Factor 1-Alpha Expression in Small Cell Lung Cancer. Int. J. Med. Sci. 2017, 14, 785–790. [Google Scholar] [CrossRef]
- Sardari Nia, P.; Colpaert, C.; Blyweert, B.; Kui, B.; Vermeulen, P.; Ferguson, M.; Hendriks, J.; Weyler, J.; Pezzella, F.; Van Marck, E.; et al. Prognostic Value of Nonangiogenic and Angiogenic Growth Patterns in Non-Small-Cell Lung Cancer. Br. J. Cancer 2004, 91, 1293–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, A.L.; Gomes, M.P.; Catarino, R.J.; Rolfo, C.; Lopes, A.M.; Medeiros, R.M.; Araújo, A.M. Angiogenesis in NSCLC: Is Vessel Co-Option the Trunk That Sustains the Branches? Oncotarget 2017, 8, 39795–39804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardari Nia, P.; Colpaert, C.; Vermeulen, P.; Weyler, J.; Pezzella, F.; Van Schil, P.; Van Marck, E. Different Growth Patterns of Non-Small Cell Lung Cancer Represent Distinct Biologic Subtypes. Ann. Thorac. Surg. 2008, 85, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, A.; Truong, A.K.; Becker, L.M.; Saavedra-garc, P. Tumor Vessel Co-Option: The Past & the Future. Front. Oncol. 2022, 1–20. [Google Scholar] [CrossRef]
- Liao, Y.; Wu, X.; Wu, M.; Fang, Y.; Li, J.; Tang, W. Non-Coding RNAs in Lung Cancer: Emerging Regulators of Angiogenesis. J. Transl. Med. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.; Boehm, T.; Shing, Y.; Flynn, E.; Birkhead, J.; Bjor, R.; Folkman, J. Endostatin, a Endogenous Inhibitor of Angiogenesis and Tumor Growth. Cell Press 1997, 88, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Abdelrahim, M.; Konduri, S.; Basha, R.; Philip, P.A.; Baker, C.H. Angiogenesis: An Update and Potential Drug Approaches (Review). Int. J. Oncol. 2010, 36, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a Therapeutic Target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef]
- Jain, R.K. Antiangiogenesis Strategies Revisited: From Starving Tumors to Alleviating Hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef] [Green Version]
- Berry, M.R.; Fan, T.M. Target-Based Radiosensitization Strategies: Concepts and Companion Animal Model Outlook. Front. Oncol. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Yang, T.; Xiao, H.; Liu, X.; Wang, Z.; Zhang, Q.; Wei, N. Vascular Normalization: A New Window Opened for Cancer Therapies. Front. Oncol. 2021, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Goedegebuure, R.S.A.; de Klerk, L.K.; Bass, A.J.; Derks, S.; Thijssen, V.L.J.L. Combining Radiotherapy With Anti-Angiogenic Therapy and Immunotherapy; A Therapeutic Triad for Cancer? Front. Immunol. 2018, 9, 3107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing Cancer Immunotherapy Using Antiangiogenics: Opportunities and Challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Cui, J. Anti-Angiogenesis: Opening a New Window for Immunotherapy. Life Sci. 2020, 258, 118163. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Zhai, Y.; Hui, Z. Application Basis of Combining Antiangiogenic Therapy with Radiotherapy and Immunotherapy in Cancer Treatment. Front. Oncol. 2022, 12, 1–8. [Google Scholar] [CrossRef]
- Fontanini, G.; Lucchi, M.; Vignati, S.; Mussi, A.; Ciardiello, F.; De Laurentiis, M.; De Placido, S.; Basolo, F.; Angeletti, C.A.; Bevilacqua, G. Angiogenesis as a Prognostic Indicator of Survival in Non-Small-Cell Lung Carcinoma: A Prospective Study. J. Natl. Cancer Inst. 1997, 89, 881–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbst, R.S.; Onn, A.; Sandler, A. Angiogenesis and Lung Cancer: Prognostic and Therapeutic Implications. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 3243–3256. [Google Scholar] [CrossRef]
- Daum, S.; Hagen, H.; Naismith, E.; Wolf, D.; Pircher, A. The Role of Anti-Angiogenesis in the Treatment Landscape of Non-Small Cell Lung Cancer – New Combinational Approaches and Strategies of Neovessel Inhibition. Front. Cell Dev. Biol. 2021, 8, 1–17. [Google Scholar] [CrossRef]
- Takeda, M.; Nakagawa, K. First- and Second-Generation EGFR-TKIs Are All Replaced to Osimertinib in Chemo-Naive EGFR Mutation-Positive Non-Small Cell Lung Cancer? Int. J. Mol. Sci. 2019, 20, 146. [Google Scholar] [CrossRef] [Green Version]
- Teuwen, L.-A.; De Rooij, L.P.M.H.; Cuypers, A.; Rohlenova, K.; Dumas, S.J.; García-Caballero, M.; Meta, E.; Amersfoort, J.; Taverna, F.; Becker, L.M.; et al. Tumor Vessel Co-Option Probed by Single-Cell Analysis. Cell Rep. 2021, 35, 109253. [Google Scholar] [CrossRef]
- Fernández-Cortés, M.; Delgado-Bellido, D.; Javier Oliver, F. Vasculogenic Mimicry: Become an Endothelial Cell “But Not so Much”. Front. Oncol. 2019, 9, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergers, G.; Hanahan, D. Modes of Resistance to Anti-Angiogenic Therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Tang, H.; Huang, Y.; Song, N.; Luo, Y. Critical Review Unraveling the Mysteries of Endostatin. IUBMB Life 2009, 61, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Antiangiogenesis in Cancer Therapy—Endostatin and Its Mechanisms of Action. Exp. Cell Res. 2006, 312, 594–607. [Google Scholar] [CrossRef]
- Su, Y.; Zhu, J.S. Canstatin, a endogenous inhibitor of angiogenesis and tumor growth. Chin. J. Cancer Res. 2004, 16, 229–234. [Google Scholar] [CrossRef]
- Li, K.; Shi, M.; Qin, S. Current Status and Study Progress of Recombinant Human Endostatin in Cancer Treatment. Oncol. Ther. 2018, 6, 21–43. [Google Scholar] [CrossRef] [Green Version]
- Shu, H.; Dong, Y.; Xu, Z.; Luo, W.; Xu, L.; Zhu, H.; Cheng, L.; Lv, Y. The Efficacy and Safety of Continuous Intravenous Endostar Treatment Combined With Concurrent Chemoradiotherapy in Patients With Locally Advanced Cervical Squamous Cell Carcinoma: A Randomized Controlled Trial. Front. Oncol. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Lv, W.; Pei, X.; Zhao, W.; Cong, Y.; Wei, Y.; Li, T.; Zhang, H.; Lin, Z.; Saito, Y.; Kim, J.J.; et al. Safety and Efficacy of Nivolumab plus Recombinant Human Endostatin in Previously Treated Advanced Non-Small-Cell Lung Cancer. Transl. Lung Cancer Res. 2022, 11, 201–212. [Google Scholar] [CrossRef]
- Ma, H.; Peng, F.; Xu, Y.; Bao, Y.; Hu, X.; Wang, J.; Fang, M.; Kong, Y.; Dong, B.; Chen, M. Five-Year Survival Rate Analysis: The Combination of Fortnightly-Administration of Endostar and Concurrent Chemoradiotherapy versus Concurrent Chemoradiotherapy in the Treatment of Inoperable Locally Advanced Non-Small Cell Lung Cancer. Ann. Palliat. Med. 2021, 10, 7560–7570. [Google Scholar] [CrossRef]
- Bodzioch, M.; Bajger, P.; Foryś, U. Angiogenesis and Chemotherapy Resistance: Optimizing Chemotherapy Scheduling Using Mathematical Modeling. J. Cancer Res. Clin. Oncol. 2021, 147, 2281–2299. [Google Scholar] [CrossRef]
- Telarovic, I.; Wenger, R.H.; Pruschy, M. Interfering with Tumor Hypoxia for Radiotherapy Optimization. J. Exp. Clin. Cancer Res. 2021, 40, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Yu, J.; Zheng, J. Intraperitoneal Infusion of Recombinant Human Endostatin Improves Prognosis in Gastric Cancer Ascites. Futur. Oncol. 2022, 18, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jin, F.; Jiang, S.; Cao, J.; Meng, Y.; Xu, Y.; Yong, C. Rh-Endostatin Combined with Chemotherapy in Patients with Advanced or Recurrent Mucosal Melanoma: Retrospective Analysis of Real - World Data. Inverstigational New Drugs 2022, 40, 453–460. [Google Scholar] [CrossRef]
- Zhang, S.L.; Han, C.B.; Sun, L.; Huang, L.T.; Ma, J.T. Efficacy and Safety of Recombinant Human Endostatin Combined with Radiotherapy or Chemoradiotherapy in Patients with Locally Advanced Non-Small Cell Lung Cancer: A Pooled Analysis. Radiat. Oncol. 2020, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Felbor, U.; Dreier, L.; Bryant, R.A.R.; Ploegh, H.L.; Olsen, B.R.; Mothes, W. Secreted Cathepsin L Generates Endostatin from Collagen XVIII. EMBO J. 2000, 19, 1187–1194. [Google Scholar] [CrossRef]
- Yoon, S.S.; Eto, H.; Lin, C.M.; Nakamura, H.; Pawlik, T.M.; Song, S.U.; Tanabe, K.K. Mouse Endostatin Inhibits the Formation of Lung and Liver Metastases. Cancer Res. 1999, 59, 6251–6256. [Google Scholar]
- Kisker, O.; Becker, C.M.; Prox, D.; Fannon, M.; D’Amato, R.; Flynn, E.; Fogler, W.E.; Sim, B.K.; Allred, E.N.; Pirie-Shepherd, S.R.; et al. Continuous Administration of Endostatin by Intraperitoneally Implanted Osmotic Pump Improves the Efficacy and Potency of Therapy in a Mouse Xenograft Tumor Model. Cancer Res. 2001, 61, 7669–7674. [Google Scholar] [PubMed]
- Fu, Y.; Chen, Y.; Luo, X.; Liang, Y.; Shi, H.; Gao, L.; Zhan, S.; Zhou, D.; Luo, Y. The Heparin Binding Motif of Endostatin Mediates Its Interaction with Receptor. Biochemistry 2009, 11655–11663. [Google Scholar] [CrossRef]
- Shi, H.; Huang, Y.; Zhou, H.; Song, X.; Yuan, S.; Fu, Y.; Luo, Y. Nucleolin Is a Receptor That Mediates Antiangiogenic and Antitumor Activity of Endostatin. Blood 2007, 110, 2899–2906. [Google Scholar] [CrossRef]
- Bager, C.L.; Karsdal, M.A. Type XVIII Collagen; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128098998. [Google Scholar]
- Poluzzi, C.; Iozzo, R.V.; Schaefer, L. Endostatin and Endorepellin: A Common Route of Action for Similar Angiostatic Cancer Avengers. Adv. Drug Deliv. Rev. 2016, 97, 156–173. [Google Scholar] [CrossRef] [Green Version]
- Mutgan, A.C.; Jandl, K.; Kwapiszewska, G. Endothelial Basement Membrane Components and Their Products, Matrikines: Active Drivers of Pulmonary Hypertension? Cells 2020, 9, 2029. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, A.; Sugimoto, H.; Yang, C.; Lively, J.; Zeisberg, M.; Kalluri, R. Human Tumstatin and Human Endostatin Exhibit Distinct Antiangiogenic Activities Mediated by Avβ and A5β1 Integrins. Proc. Natl. Acad. Sci. USA 2003, 100, 4766–4771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, Y.; Yang, Y.; Lu, N.; You, Q.; Wang, S.; Gao, Y.; Chen, Y.; Guo, Q. Endostar, a Novel Recombinant Human Endostatin, Exerts Antiangiogenic Effect via Blocking VEGF-Induced Tyrosine Phosphorylation of KDR/Flk-1 of Endothelial Cells. Biochem. Biophys. Res. Commun. 2007, 361, 79–84. [Google Scholar] [CrossRef]
- Moreau, C.; Chautard, E.; Jetne, R.; Fukai, N.; Ruggiero, F.; Humphries, M.J.; Olsen, B.R.; Ricard-blum, S. Molecular interplay between endostatin, integrins, and heparan sulfate. J. Biol. Chem. 2009, 284, 22029–22040. [Google Scholar] [CrossRef] [Green Version]
- Wickström, S.A.; Veikkola, T.; Rehn, M.; Pihlajaniemi, T.; Alitalo, K.; Keski-Oja, J. Endostatin-Induced Modulation of Plasminogen Activation with Concomitant Loss of Focal Adhesions and Actin Stress Fibers in Cultured Human Endothelial Cells. Cancer Res. 2001, 61, 6511–6516. [Google Scholar] [PubMed]
- Dixelius, J.; Cross, M.; Matsumoto, T.; Sasaki, T.; Timpl, R.; Claesson-Welsh, L. Endostatin Regulates Endothelial Cell Adhesion and Cytoskeletal Organization. Cancer Res. 2002, 62, 1944–1947. [Google Scholar]
- Wang, S.; Lu, X.-A.; Liu, P.; Fu, Y.; Jia, L.; Zhan, S.; Luo, Y. Endostatin Has ATPase Activity, Which Mediates Its Antiangiogenic and Antitumor Activities. Mol. Cancer Ther. 2015, 14, 1192–1201. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Zhang, S.; Jia, L.; Wang, S.; Liu, J.; Ma, X.; Wang, C.; Fu, Y.; Luo, Y. E-M, an Engineered Endostatin with High ATPase Activity, Inhibits the Recruitment and Alternative Activation of Macrophages in Non-Small Cell Lung Cancer. Front. Pharmacol. 2017, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Jeung, I.C.; Park, T.W.; Lee, K.; Lee, D.G.; Cho, Y.; Lee, T.S.; Na, H.; Lee, H.G.; Jeong, M.S.; et al. Extension of the in Vivo Half-Life of Endostatin and Its Improved Anti-Tumor Activities upon Fusion to a Humanized Antibody against Tumor-Associated Glycoprotein 72 in a Mouse Model of Human Colorectal Carcinoma. Oncotarget 2015, 6. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Tjin Tham Sjin, R.M.; Movahedi, S.; Ahmed, B.; Pravda, E.A.; Lo, K.-M.; Gillies, S.D.; Folkman, J.; Javaherian, K. Linking Antibody Fc Domain to Endostatin Significantly Improves Endostatin Half-Life and Efficacy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 1487–1493. [Google Scholar] [CrossRef] [Green Version]
- Hai-Tao, Z.; Hui-Cheng, L.; Zheng-Wu, L.; Chang-Hong, G. A Tumor-Penetrating Peptide Modification Enhances the Antitumor Activity of Endostatin in Vivo. Anticancer. Drugs 2011, 22, 409–415. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Ramakrishnan, S. Addition of Integrin Binding Sequence to a Mutant Human Endostatin Improves Inhibition of Tumor Growth. Int. J. Cancer 2004, 111, 839–848. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, X.; Wang, X.; Chen, J. Preparation and Stability of N-Terminal Mono-PEGylated Recombinant Human Endostatin. Bioconjug. Chem. 2006, 17, 995–999. [Google Scholar] [CrossRef]
- Li, H.L.; Li, S.; Shao, J.Y.; Lin, X.B.; Cao, Y.; Jiang, W.Q.; Liu, R.Y.; Zhao, P.; Zhu, X.F.; Zeng, M.S.; et al. Pharmacokinetic and Pharmacodynamic Study of Intratumoral Injection of an Adenovirus Encoding Endostatin in Patients with Advanced Tumors. Gene Ther. 2008, 15, 247–256. [Google Scholar] [CrossRef]
- Jin, X.; Bookstein, R.; Wills, K.; Avanzini, J.; Tsai, V.; LaFace, D.; Terracina, G.; Shi, B.; Nielsen, L.L. Evaluation of Endostatin Antiangiogenesis Gene Therapy in Vitro and in Vivo. Cancer Gene Ther. 2001, 8, 982–989. [Google Scholar] [CrossRef] [Green Version]
- Adeyemi, S.A.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Pillay, V. Design and Characterization of Endostatin-Loaded Nanoparticles for in Vitro Antiangiogenesis in Squamous Cell Carcinoma. J. Nanomater. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Adeyemi, S.A.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Marimuthu, T.; Kondiah, P.P.D.; Pillay, V. Folate-Decorated, Endostatin-Loaded, Nanoparticles for Anti-Proliferative Chemotherapy in Esophaegeal Squamous Cell Carcinoma. Biomed. Pharmacother. 2019, 119, 109450. [Google Scholar] [CrossRef]
- Adeyemi, S.A.; Choonara, Y.E. In Vitro and In Vivo Evaluation of a Cyclic LyP-1-Modified Nanosystem for Targeted Endostatin Delivery in a KYSE-30 Cell Xenograft Athymic Nude Mice Model. Pharmaceuticals 2022, 15, 353. [Google Scholar] [CrossRef]
- Lu, L.; Chen, H.; Wang, L.; Zhao, L.; Cheng, Y.; Wang, A.; Wang, F.; Zhang, X. A Dual Receptor Targeting-and Bbb Penetrating-Peptide Functionalized Polyethyleneimine Nanocomplex for Secretory Endostatin Gene Delivery to Malignant Glioma. Int. J. Nanomedicine 2020, 15, 8875–8892. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Du, B.; Li, X.; Liu, S.; Yang, X.-Y.; Ding, H.; Yang, W.; Pan, F.; Wu, X.; et al. Gold Nanoparticle–Mediated Targeted Delivery of Recombinant Human Endostatin Normalizes Tumour Vasculature and Improves Cancer Therapy. Sci. Rep. 2016, 6, 30619. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, N.; Mehrnejad, F.; Vaezi, Z.; Sedghi, M.; Asghari, S.M.; Naderi-Manesh, H. Encapsulation of an Endostatin Peptide in Liposomes: Stability, Release, and Cytotoxicity Study. Colloids Surf. B Biointerfaces 2020, 185, 110552. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, H.; Zheng, B.; Liu, J.; Huang, Y.; Wang, H.; Zheng, D.; Mao, N.; Meng, J.; Zhou, S.; Zhong, L.; et al. Efficient Targeted Tumor Imaging and Secreted Endostatin Gene Delivery by Anti-CD105 Immunoliposomes. J. Exp. Clin. Cancer Res. 2018, 37, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Gao, Q.; Tang, J.; Jiang, Y.Q.; Yang, L.S.; Shi, X.X.; Chen, Y.; Zhang, Y.; Fu, S.Z.; Lin, S. Anti-Tumor Effect of Local Injectable Hydrogel-Loaded Endostatin Alone and in Combination with Radiotherapy for Lung Cancer. Drug Deliv. 2021, 28, 183–194. [Google Scholar] [CrossRef]
- de la Torre, P.; Pérez-Lorenzo, M.J.; Alcázar-Garrido, Á.; Flores, A.I. Cell-Based Nanoparticles Delivery Systems for Targeted Cancer Therapy: Lessons from Anti-Angiogenesis Treatments. Molecules 2020, 25, 715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouleftour, W.; Rowinski, E.; Louati, S.; Sotton, S.; Wozny, A.-S.; Moreno-Acosta, P.; Mery, B.; Rodriguez-Lafrasse, C.; Magne, N. A Review of the Role of Hypoxia in Radioresistance in Cancer Therapy. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e934116. [Google Scholar] [CrossRef] [PubMed]
- Rakotomalala, A.; Escande, A.; Furlan, A.; Meignan, S.; Lartigau, E. Hypoxia in Solid Tumors: How Low Oxygenation Impacts the “Six Rs” of Radiotherapy. Front. Endocrinol. 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Steel, G.G.; McMillan, T.J.; Peacock, J.H. The 5Rs of Radiobiology. Int. J. Radiat. Biol. 1989, 56, 1045–1048. [Google Scholar] [CrossRef] [Green Version]
- Van Den Heuvel, F.; Vella, A.; Fiorini, F.; Brooke, M.; Hill, M.A.; Maughan, T. Incorporating Oxygenation Levels in Analytical DNA-Damage Models - Quantifying the Oxygen Fixation Mechanism. Phys. Med. Biol. 2021, 66. [Google Scholar] [CrossRef]
- Wu, J.; Tang, Y.; Liang, X. Targeting VEGF Pathway to Normalize the Vasculature: An Emerging Insight in Cancer Therapy. Onco. Targets. Ther. 2018, 11, 6901–6909. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Li, W.; Quan, Y.-Y.; Yong, L.; Lu, L.; Cui, M. Monitoring of Tumor Vascular Normalization: The Key Points from Basic Research to Clinical Application. Cancer Manag. Res. 2018, 10, 4163–4172. [Google Scholar] [CrossRef]
- Alaoui-lasmaili, K.E.; Faivre, B. Critical Reviews in Oncology/Hematology Antiangiogenic Therapy: Markers of Response, “Normalization” and Resistance. Crit. Rev. Oncol./Hematol. 2018, 128, 118–129. [Google Scholar] [CrossRef]
- Lee, C.G.; Heijn, M.; di Tomaso, E.; Griffon-Etienne, G.; Ancukiewicz, M.; Koike, C.; Park, K.R.; Ferrara, N.; Jain, R.K.; Suit, H.D.; et al. Anti-Vascular Endothelial Growth Factor Treatment Augments Tumor Radiation Response under Normoxic or Hypoxic Conditions. Cancer Res. 2000, 60, 5565–5570. [Google Scholar]
- Park, J.-S.; Park, I.; Koh, G.Y. Benefits and Pitfalls of Tumor Vessel Normalization. In Tumor Angiogenesis: A Key Target for Cancer Therapy; Marmé, D., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 51–71. ISBN 978-3-319-33673-2. [Google Scholar]
- Lupo, G.; Caporarello, N.; Olivieri, M.; Cristaldi, M.; Motta, C.; Bramanti, V.; Avola, R.; Salmeri, M.; Nicoletti, F.; Anfuso, C.D. Anti-Angiogenic Therapy in Cancer: Downsides and New Pivots for Precision Medicine. Front. Pharmacol. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, Q.; Hong, Y. Tumor Vessel Normalization: A Window to Enhancing Cancer Immunotherapy. Technol. Cancer Res. Treat. 2020, 19, 1533033820980116. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, A.L.; Mills, I.G. Vascular Normalisation as the Stepping Stone into Tumour Microenvironment Transformation. Br. J. Cancer 2021. [Google Scholar] [CrossRef]
- Durante, M.; Orecchia, R.; Loeffler, J.S. Charged-Particle Therapy in Cancer: Clinical Uses and Future Perspectives. Nat. Rev. Clin. Oncol. 2017, 14, 483–495. [Google Scholar] [CrossRef]
- Grabham, P.; Sharma, P. The Effects of Radiation on Angiogenesis. Vasc. Cell 2013, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Durante, M.; Debus, J.; Loeffler, J.S. Physics and Biomedical Challenges of Cancer Therapy with Accelerated Heavy Ions. Nat. Rev. Phys. 2021, 3, 777–790. [Google Scholar] [CrossRef]
- Girdhani, S.; Sachs, R.; Hlatky, L. Biological Effects of Proton Radiation: An Update. Radiat. Prot. Dosimetry 2015, 166. [Google Scholar] [CrossRef]
- Tinganelli, W.; Durante, M. Carbon Ion Radiobiology. Cancers 2020, 12, 3022. [Google Scholar] [CrossRef]
- Girdhani, S.; Lamont, C.; Hahnfeldt, P.; Abdollahi, A.; Hlatky, L. Proton Irradiation Suppresses Angiogenic Genes and Impairs Cell Invasion and Tumor Growth. Radiat. Res. 2012, 178, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Kamlah, F.; Hanze, J.; Arenz, A.; Seay, U.; Hasan, D.; Juricko, J.; Bischoff, B.; Gottschald, O.; Fournier, C.; Taucher-Scholz, G.; et al. Comparison of the Effects of Carbon Ion and Photon Irradiation on the Angiogenic Response in Human Lung Adenocarcinoma Cells. Int. J. Radiation Oncol. Biol. Phys. 2011, 80, 1541–1549. [Google Scholar] [CrossRef]
- Takahashi, Y.; Teshima, T.; Kawaguchi, N.; Hamada, Y.; Mori, S.; Madachi, A.; Ikeda, S.; Mizuno, H.; Ogata, T.; Nojima, K.; et al. Heavy Ion Irradiation Inhibits in Vitro Angiogenesis Even at Sublethal Dose. Cancer Res. 2003, 63, 4253–4257. [Google Scholar]
- Girdhani, S.; Lamont, C.; Peluso, M.; Sun, M.; Hlatky, L. 56Fe Ion Irradiation Enhances Angiogenesis and Other Inter-Cellular Determinants of Carcinogenesis Risk. J. Radiat. Res. 2014, 55, i124–i126. [Google Scholar] [CrossRef] [Green Version]
- He, L. Normalization Time Window of Recombinant Endostatin: An Overview. Cancer Cell Res. 2019, 21, 558–564. [Google Scholar]
- Guo, L.; Chen, Y.; He, T.; Qi, F.; Liu, G.; Fu, Y.A.N. Nuclear-Translocated Endostatin Downregulates Hypoxia Inducible Factor-1 α Activation through Interfering with Zn ( II ) Homeostasis. Mol. Med. Rep. 2015, 3473–3480. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Zheng, D.; Wei, X.; Jin, Z. Effects of Recombinant Human Endostatin and Its Synergy with Cisplatin on Circulating Endothelial Cells and Tumor Vascular Normalization in A549 Xenograft Murine Model. J. Cancer Res. Clin. Oncol. 2012, 1131–1144. [Google Scholar] [CrossRef]
- Peng, Q.; Li, M.; Wang, Z.; Jiang, M.; Yan, X.; Lei, S.; Zhang, H.; Zhang, W.; Liu, Y.Y.; Luo, F. Polarization of Tumor-Associated Macrophage Is Associated with Tumor Vascular Normalization by Endostatin. Thorac. Cancer 2013, 4, 295–305. [Google Scholar] [CrossRef]
- Meng, M.-B.; Jiang, X.-D.; Deng, L.; Na, F.-F.; He, J.-Z.; Xue, J.-X.; Guo, W.-H.; Wen, Q.-L.; Lan, J.; Mo, X.-M.; et al. Enhanced Radioresponse with a Novel Recombinant Human Endostatin Protein via Tumor Vasculature Remodeling: Experimental and Clinical Evidence. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2013, 106, 130–137. [Google Scholar] [CrossRef]
- Peng, L.; Wang, Y.; Fei, S.; Wei, C.; Tong, F.; Wu, G.; Ma, H.; Dong, X. The Effect of Combining Endostar with Radiotherapy on Blood Vessels, Tumor-Associated Macrophages, and T Cells in Brain Metastases of Lewis Lung Cancer. Transl. Lung Cancer Res. 2020, 9, 745–760. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhu, S.; Huang, J.; Liang, J.; Zhang, D.; Zhao, X.; Ding, H.; Qin, L.; Shi, C.; Luo, L.; et al. Monitoring the Process of Endostar-Induced Tumor Vascular Normalization by Non-Contrast Intravoxel Incoherent Motion. Front. Oncol. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, F.; Xu, Z.; Wang, J.; Chen, Y.; Li, Q.; Zuo, Y.; Chen, J.; Hu, X.; Zhou, Q.; Wang, Y.; et al. Recombinant Human Endostatin Normalizes Tumor Vasculature and Enhances Radiation Response in Xenografted Human Nasopharyngeal Carcinoma Models. PLoS ONE 2012, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chu, X.-D.; Bao, H.; Lin, Y.-J.; Chen, R.-X.; Zhang, Y.-R.; Huang, T.; He, J.-S.; Huangfu, S.-C.; Pan, Y.-L.; Ding, H. Endostatin Induces Normalization of Blood Vessels in Colorectal Cancer and Promotes Infiltration of CD8+ T Cells to Improve Anti-PD-L1 Immunotherapy. Front. Immunol. 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Jiang, X.D.; Qiao, Y.; Dai, P.; Chen, Q.; Wu, J.; Song, D.A.; Li, S.Q. Enhancement of Recombinant Human Endostatin on the Radiosensitivity of Human Pulmonary Adenocarcinoma A549 Cells and Its Mechanism. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef]
- You, Z.Y.; Zhao, Y.; Liu, F.; Zhang, Y.D.; Wang, J.J. The Radiosensitization Effects of Endostar on Human Lung Squamous Cancer Cells H-520. Cancer Cell Int. 2010, 10, 1–10. [Google Scholar]
- Chen, X.; Zhang, H.; Zhu, H.; Yang, X.; Yang, Y.; Yang, Y.; Min, H.; Chen, G.; Lu, J.; Cheng, H.; et al. Endostatin Combined with Radiotherapy Suppresses Vasculogenic Mimicry Formation through Inhibition of Epithelial – Mesenchymal Transition in Esophageal Cancer. Tumor Biol. 2016, 37, 4679–4688. [Google Scholar] [CrossRef]
- Liu, L.; Qiao, Y.; Hu, C.; Liu, Y.; Xia, Y.; Wang, L.; Liu, B.; Chen, H.; Jiang, X. Endostatin Exerts Radiosensitizing Effect in Non-Small Cell Lung Cancer Cells by Inhibiting VEGFR2 Expression. Clin. Transl. Oncol. 2016, 18–26. [Google Scholar] [CrossRef]
- Ling, C.; Ji, C.; Chen, Y.; Fu, J.; Zhou, J.; Chen, W.; Yang, J.; Su, L. Combined effects of endostatin gene transfer and ionizing radiation on lung adenocarcinoma model of A549-cells. Zhonghua Jie He He Hu Xi Za Zhi = Zhonghua Jiehe He Huxi Zazhi = Chin. J. Tuberc. Respir. Dis. 2004, 27, 683–686. [Google Scholar]
- Wu, D.S.; Wu, C.M.; Huang, T.H.; Xie, Q.D. Combined Effects of Radiotherapy and Endostatin Gene Therapy in Melanoma Tumor Model. Radiat. Environ. Biophys. 2008, 47, 285–291. [Google Scholar] [CrossRef]
- Itasaka, S.; Komaki, R.; Herbst, R.S.; Shibuya, K.; Shintani, T.; Hunter, N.R.; Onn, A.; Bucan, C.D.; Milas, L.; Kian Ang, K.; et al. Endostatin Improves Radioresponse and Blocks Tumor Revascularization after Radiation Therapy for A431 Xenografts in Mice. Int. J. Radiation Oncol. Biol. Phys. 2007, 67, 870–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Wang, L.; Xu, X.; Tu, Y.; Qin, S.; Yin, Y. Antitumor Activity of Endostar Combined with Radiation against Human Nasopharyngeal Carcinoma in Mouse Xenograft Models. Oncol. Lett. 2012, 4, 976–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Pan, L.K.; Qi, D.; Xin, L.; Cui, Y.; An, G. Recombinant Human Endostatin Combined with TP Regimen as Postoperative Adjuvant Treatment for Non-Small-Cell Lung Cancer: Efficacy Analysis. Med. J. Chinese People’s Lib. Army 2012, 37, 49–53. [Google Scholar]
- Aydemir, E.A.; Oz, E.C.E.S.; Korcum, A.F.; Fiskin, K. Endostatin Enhances Radioresponse in Breast Cancer Cells via Alteration of Substance P Levels. Oncol. Lett. 2011, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Aydemir, E.A.; Şimşek, E.C.E.; Korcum, A.F.; Fişkin, K. Endostatin and Irradiation Modifies the Activity of ADAM10 and Neprilysin in Breast Cancer Cells. Mol. Med. Rep. 2016, 2343–2351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Chang, H.; Li, B.; Zhang, Y.; Li, D.; Liu, Y.; Yang, Y. Effect of Recombinant Human Endostatin Onradiotherapy for Esophagus Cancer. Asian Pac. J. Trop. Med. 2016, 9, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Yang, X.; Ding, Y.; Liu, J.; Lu, J.; Zhan, L.; Qin, Q.; Zhang, H.; Chen, X.; Yang, Y.; et al. Recombinant Human Endostatin Enhances the Radioresponse in Esophageal Squamous Cell Carcinoma by Normalizing Tumor Vasculature and Reducing Hypoxia. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Luo, W.; Qin, S.; Wu, Q.; Wang, X.; Yin, X.; Sun, X.; Qu, W.; Ye, Q. Synergistic Effects of the Combination of Endostar and Radiotherapy against Hepatocellular Carcinoma in a Mouse Model. Int. J. Clin. Exp. Med. 2017, 10, 10066–10078. [Google Scholar]
- Zhang, K.; Wang, Y.; Yu, X.; Shi, Y.; Yao, Y.; Wei, X.; Ma, X. Recombinant Human Endostatin Combined with Radiotherapy Inhibits Colorectal Cancer Growth. BMC Cancer 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Jiang, X.; Dai, P.; Wu, J.; Song, D.; Yu, J. Lung Cancer Inhibitory Effect of Radiotherapy Combined with Weekly Recombinant Human Endostatin on the Human Pulmonary Adenocarcinoma A549 Xenografts in Nude Mice. Lung Cancer 2011, 72, 165–171. [Google Scholar] [CrossRef]
- Yin, L.; He, J.; Xue, J.; Na, F.; Tong, R.; Wang, J.; Gao, H.; Tang, F.; Mo, X.; Deng, L.; et al. PDGFR- β Inhibitor Slows Tumor Growth but Increases Metastasis in Combined Radiotherapy and Endostar Therapy. Biomed. Pharmacother. 2018, 99, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.N.; Seetharam, S.; Mauceri, H.J.; Beckett, M.A.; Jaskowiak, N.T.; Salloum, R.M.; Hari, D.; Dhanabal, M.; Ramchandran, R.; Kalluri, R.; et al. Antitumor Interaction of Short-Course Endostatin and Ionizing Radiation. Cancer J. 2000, 6, 287–293. [Google Scholar]
- Wen, Q.L.; Meng, M.B.; Yang, B.; Tu, L.L.; Jia, L.; Zhou, L.; Xu, Y.; Lu, Y. Endostar, a Recombined Humanized Endostatin, Enhances the Radioresponse for Human Nasopharyngeal Carcinoma and Human Lung Adenocarcinoma Xenografts in Mice. Cancer Sci. 2009, 100, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, J.W.; Liu, Y.Y.; Yu, Q.T.; Zhang, Y.P.; Li, K.; Xu, L.Y.; Luo, S.X.; Qin, F.Z.; Chen, Z.T.; et al. Long-Term Results of a Randomized, Double-Blind, and Placebo-Controlled Phase III Trial: Endostar (Rh-Endostatin) versus Placebo in Combination with Vinorelbine and Cisplatin in Advanced Non-Small Cell Lung Cancer. Thorac. Cancer 2013, 4, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.; Zhai, Y.; Men, Y.; Wang, J.; Deng, L.; Wang, W.; Bao, Y.; Yang, X.; Sun, S.; Ma, Z.; et al. Endostar (Rh-Endostatin) Improves Efficacy of Concurrent Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Thorac. Cancer 2021, 12, 3208–3215. [Google Scholar] [CrossRef]
- Jiang, X.; Guan, W.; Li, M.; Liang, W.; Qing, Y.; Dai, N.; Zhang, S.; Deng, Y.; Meng, H.; Yang, Y.; et al. Endostatin Combined with Platinum-Based Chemo-Radiotherapy for Advanced Non-Small Cell Lung Cancer. Cell Biochem. Biophys. 2015, 71, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhou, F.; Wei, Q.; Zou, L.; Qin, B.; Peng, X. Phase II Study of Cisplatin/Etoposide and Endostar for Extensive-Stage Small-Cell Lung Cancer. Cancer Chemother. Pharmacol. 2011, 68, 1027–1032. [Google Scholar] [CrossRef]
- Jianhua, C.; Yongzhong, L.U.O.; Wenwei, Z.; Hui, Z.; Wei, W. Clinical Observation of Recombinant Human Endostatin Combined with Carboplatin and Etoposide for Advanced Small-Cell Lung Cancer. J. Clin. Med. Pract. 2013, 26–28. [Google Scholar] [CrossRef]
- Li, N.; Jin, Z.; Liu, Z.; Wang, J.; Li, K. Efficacy of endostar combined with chemotherapy in multi-cycle treatment of patients with advanced non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi 2011, 33, 937–942. [Google Scholar]
- Wang, J.; Sun, Y.; Liu, Y.; Yu, Q.; Zhang, Y.; Li, K.; Zhu, Y.; Zhou, Q.; Hou, M.; Guan, Z.; et al. Results of randomized, multicenter, double-blind phase III trial of rh-endostatin (YH-16) in treatment of advanced non-small cell lung cancer patients. Zhongguo Fei Ai Za Zhi 2005, 8, 283–290. [Google Scholar] [CrossRef]
- Ge, W.; Cao, D.; Wang, H.; Jie, F.; Zheng, Y.; Chen, Y. Endostar Combined with Chemotherapy versus Chemotherapy Alone for Advanced NSCLCs: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2011, 12, 2705–2711. [Google Scholar] [PubMed]
- Rong, B.; Yang, S.; Li, W.; Zhang, W.; Ming, Z. Systematic Review and Meta-Analysis of Endostar (Rh-Endostatin) Combined with Chemotherapy versus Chemotherapy Alone for Treating Advanced Non-Small Cell Lung Cancer. World J. Surg. Oncol. 2012, 10, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Wang, Z.-Y.; Li, Y.-H.; Lu, Y.-H.; Wang, S.; Yu, W.-X.; Zhao, H. Usefulness of Dynamic Contrast-Enhanced Magnetic Resonance Imaging for Predicting Treatment Response to Vinorelbine-Cisplatin with or without Recombinant Human Endostatin in Bone Metastasis of Non-Small Cell Lung Cancer. Am. J. Cancer Res. 2016, 6, 2890–2900. [Google Scholar] [CrossRef]
- Han, B.; Xiu, Q.; Wang, H.; Shen, J.; Gu, A.; Luo, Y.; Bai, C.; Guo, S.; Liu, W.; Zhuang, Z.; et al. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy of Paclitaxel-Carboplatin Alone or with Endostar for Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2011, 6, 1104–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Y.; Ma, H.; Hui, Z.; Zhao, L.; Li, D.; Liang, J.; Wang, X.; Xu, L.; Chen, B.; Tang, Y.; et al. HELPER Study: A Phase II Trial of Continuous Infusion of Endostar Combined with Concurrent Etoposide plus Cisplatin and Radiotherapy for Treatment of Unresectable Stage III Non-Small-Cell Lung Cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019, 131, 27–34. [Google Scholar] [CrossRef]
- Jiang, X.-D.; Dai, P.; Wu, J.; Song, D.-A.; Yu, J.-M. Effect of Recombinant Human Endostatin on Radiosensitivity in Patients with Non-Small-Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1272–1277. [Google Scholar] [CrossRef]
- Sun, X.-J.; Deng, Q.-H.; Yu, X.-M.; Ji, Y.-L.; Zheng, Y.-D.; Jiang, H.; Xu, Y.-P.; Ma, S.-L. A Phase II Study of Endostatin in Combination with Paclitaxel, Carboplatin, and Radiotherapy in Patients with Unresectable Locally Advanced Non-Small Cell Lung Cancer. BMC Cancer 2016, 16, 266. [Google Scholar] [CrossRef] [Green Version]
- Honglian, M.; Zhouguang, H.; Fang, P.; Lujun, Z.; Dongming, L.; Yujin, X.; Yong, B.; Liming, X.; Yirui, Z.; Xiao, H.; et al. Different Administration Routes of Recombinant Human Endostatin Combined with Concurrent Chemoradiotherapy Might Lead to Different Efficacy and Safety Profile in Unresectable Stage III Non-Small Cell Lung Cancer: Updated Follow-up Results from Two Phase. Thorac. Cancer 2020, 11, 898–906. [Google Scholar] [CrossRef]
- Wang, B.; Xu, L.; Li, Q.; Man, S.; Jin, C.; Liu, L.; Zhan, S.; Ning, Y. Endostar Continuous versus Intermittent Intravenous Infusion Combined with Chemotherapy for Advanced NSCLC: A Systematic Review and Meta-Analysis Including Non-Randomized Studies. BMC Cancer 2020, 20, 1021. [Google Scholar] [CrossRef]
- Chen, L.; Tong, F.; Peng, L.; Huang, Y.; Yin, P.; Feng, Y.; Cheng, S.; Wang, J.; Dong, X. Efficacy and Safety of Recombinant Human Endostatin Combined with Whole-Brain Radiation Therapy in Patients with Brain Metastases from Non-Small Cell Lung Cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2022, 174, 44–51. [Google Scholar] [CrossRef]
- Jiang, X.D.; Ding, M.H.; Qiao, Y.; Liu, Y.; Liu, L. Study on Lung Cancer Cells Expressing Vegfr2 and the Impact on the Effect of RHES Combined with Radiotherapy in the Treatment of Brain Metastases. Clin. Lung Cancer 2014, 15, e23–e29. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Peng, F.; Zhou, Q.C.; Yu, Z.H.; Li, J.C.; Cheng, Z.B.; Chen, L.; Hu, X.; Chen, Y.Y.; Wang, J.; et al. Phase II Trial of Recombinant Human Endostatin in Combination with Concurrent Chemoradiotherapy in Patients with Stage III Non-Small-Cell Lung Cancer. Radiother. Oncol. 2015, 114, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Ma, H.; Peng, F.; Bao, Y.; Hu, X.; Wang, J.; Xu, Y.; Chen, M. Prognostic Performance of Inflammation-Based Prognostic Indices in Locally Advanced Non-Small-Lung Cancer Treated with Endostar and Concurrent Chemoradiotherapy. Mol. Clin. Oncol. 2016, 4, 801–806. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Lv, D.; Meng, Y.; Wang, M.; Wang, W.; Zhou, C.; Zhou, S.; Chen, X.; Yang, H. Endostar Improved Efficacy of Concurrent Chemoradiotherapy with Vinorelbine plus Carboplatin in Locally Advanced Lung Squamous Cell Carcinoma Patients with High Serum Lp(a) Concentration. Ann. Palliat. Med. 2020, 9, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Valable, S.; Gérault, A.N.; Lambert, G.; Leblond, M.M.; Anfray, C.; Toutain, J.; Bordji, K.; Petit, E.; Bernaudin, M.; Pérès, E.A. Impact of Hypoxia on Carbon Ion Therapy in Glioblastoma Cells: Modulation by LET and Hypoxia-Dependent Genes. Cancers 2020, 12, 2019. [Google Scholar] [CrossRef]
- Césaire, M.; Montanari, J.; Curcio, H.; Lerouge, D.; Gervais, R.; Demontrond, P.; Balosso, J.; Chevalier, F. Radioresistance of Non-Small Cell Lung Cancers and Therapeutic Perspectives. Cancers 2022, 14, 2829. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Annese, T.; Ruggieri, S.; Tamma, R.; Crivellato, E. Limitations of Anti-Angiogenic Treatment of Tumors. Transl. Oncol. 2019, 12, 981–986. [Google Scholar] [CrossRef]
- Ribatti, D. Antiangiogenic Therapy Accelerates Tumor Metastasis. Leuk. Res. 2011, 35, 24–26. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Iwamoto, H.; Hosaka, K.; Seki, T.; Andersson, P.; Lim, S.; Fischer, C.; Nakamura, M.; Abe, M.; et al. Discontinuation of Anti-VEGF Cancer Therapy Promotes Metastasis through a Liver Revascularization Mechanism. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Mukwaya, A.; Jensen, L.; Lagali, N. Relapse of Pathological Angiogenesis: Functional Role of the Basement Membrane and Potential Treatment Strategies. Exp. Mol. Med. 2021, 53, 189–201. [Google Scholar] [CrossRef]
- Van Beijnum, J.R.; Nowak-Sliwinska, P.; Huijbers, E.J.M.; Thijssen, V.L.; Griffioen, A.W. The Great Escape; the Hallmarks of Resistance to Antiangiogenic Therapy. Pharmacol. Rev. 2015, 67, 441–461. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H. Relative Biological Effectiveness (RBE) Values for Proton Beam Therapy. Variations as a Function of Biological Endpoint, Dose, and Linear Energy Transfer. Phys. Med. Biol. 2014, 59, R419–R472. [Google Scholar] [CrossRef] [PubMed]
- Vanderwaeren, L.; Dok, R.; Verstrepen, K.; Nuyts, S. Clinical Progress in Proton Radiotherapy: Biological Unknowns. Cancers 2021, 13, 604. [Google Scholar] [CrossRef]
- Mohan, R. A Review of Proton Therapy – Current Status and Future Directions. Precis. Radiat. Oncol. 2022, 6, 164–176. [Google Scholar] [CrossRef]
- Kim, H.; Pyo, H.; Noh, J.M.; Lee, W.; Park, B.; Park, H.Y.; Yoo, H. Preliminary Result of Definitive Radiotherapy in Patients with Non-Small Cell Lung Cancer Who Have Underlying Idiopathic Pulmonary Fibrosis: Comparison between X-Ray and Proton Therapy. Radiat. Oncol. 2019, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Lee, J.J.; Komaki, R.; Gomez, D.R.; O’Reilly, M.S.; Fossella, F.V.; Blumenschein, G.R.J.; Heymach, J.V.; Vaporciyan, A.A.; Swisher, S.G.; et al. Bayesian Adaptive Randomization Trial of Passive Scattering Proton Therapy and Intensity-Modulated Photon Radiotherapy for Locally Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Han, Y. Current Status of Proton Therapy Techniques for Lung Cancer. Radiat. Oncol. J. 2019, 37, 232–248. [Google Scholar] [CrossRef]
- Gjyshi, O.; Liao, Z. Proton Therapy for Locally Advanced Non-Small Cell Lung Cancer. Br. J. Radiol. 2020, 93, 20190378. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Doi, R.; Obata, T.; Hatachi, G.; Nagayasu, T. Lung Microvascular Niche, Repair, and Engineering. Front. Bioeng. Biotechnol. 2020, 8, 1–19. [Google Scholar] [CrossRef]
- Onoi, K.; Chihara, Y.; Uchino, J.; Shimamoto, T.; Morimoto, Y.; Iwasaku, M.; Kaneko, Y.; Yamada, T.; Takayama, K. Immune Checkpoint Inhibitors for Lung Cancer Treatment: A Review. J. Clin. Med. 2020, 9. [Google Scholar] [CrossRef]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of Anti-Angiogenic Therapy and Immune Checkpoint Blockade Normalizes Vascular-Immune Crosstalk to Potentiate Cancer Immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-J.; Wang, J.; Wei, X.-Y.; Chen, P.; Wang, L.-C.; Lin, L.; Sun, B.-C.; Li, K. Predictive Value of Circulating Endothelial Cells for Efficacy of Chemotherapy with Rh-Endostatin in Non-Small Cell Lung Cancer. J. Cancer Res. Clin. Oncol. 2012, 138, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Su, Y.; You, J.; Gong, L.; Zhang, Z.; Wang, M.; Zhao, Z.; Zhang, Z.; Li, X.; Wang, C. Combining Antiangiogenic Therapy with Neoadjuvant Chemotherapy Increases Treatment Efficacy in Stage IIIA (N2) Non-Small Cell Lung Cancer without Increasing Adverse Effects. Oncotarget 2016, 7, 62619–62626. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wang, J.W.; Cui, C.X.; Hang, J.; Zang, H.P.; Li, S.T.; Sun, Y. Rh-Endostatin (YH-16) in Combination with Vinorelbine and Cisplatin for Advanced Non-Small Cell Lung Cancer: A Multicenter Phase II Trial. Chin. J. New Drugs 2005, 14, 204–207. [Google Scholar]
- Zhao, X.; Mei, K.; Cai, X.; Chen, J.; Yu, J.; Zhou, C.; Li, Q. A Randomized Phase II Study of Recombinant Human Endostatin plus Gemcitabine/Cisplatin Compared with Gemcitabine/Cisplatin Alone as First-Line Therapy in Advanced Non-Small-Cell Lung Cancer. Investig. New Drugs 2012, 30, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, K.; Sun, T.; Zhang, M.; Li, W.; Yao, Q.; Liu, W.; Ding, C.; He, Z.; Mao, W.; et al. Efficacy and safety of rh-endostatin combined with docetaxel in second-line or intolerant toxicity for first-line treatment in patients with advanced non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi 2013, 35, 618–622. [Google Scholar]
- Zhang, F.-L.; Gao, E.-Y.; Shu, R.-B.; Wang, H.; Zhang, Y.; Sun, P.; Li, M.; Tang, W.; Jiang, B.-Q.; Chen, S.-Q.; et al. Human Recombinant Endostatin Combined with Cisplatin Based Doublets in Treating Patients with Advanced NSCLC and Evaluation by CT Perfusion Imaging. Asian Pac. J. Cancer Prev. 2015, 16, 6765–6768. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Yu, H.; Han, T.; Wang, W.; Tong, W.; Zhu, X. A Study on the Efficacy of Recombinant Human Endostatin Combined with Apatinib Mesylate in Patients with Middle and Advanced Stage Non-Small Cell Lung Cancer. J. BUON. 2019, 24, 2267–2272. [Google Scholar]
- Yu, X.; Zhang, L.; Chen, J. Effectiveness of Treatment with Endostatin in Combination with Emcitabine, Carboplatin, and Gemcitabine in Patients with Advanced Non-Small Cell Lung Cancer: A Retrospective Study. Open Med. 2018, 13, 142–147. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Zhou, C.; Long, X.; Guan, R.; Yang, N.; Zhang, Y. Real-World Outcomes of Various Regimens of Recombinant Human Endostatin Combined with Chemotherapy in Non-Driver Gene Mutation Advanced Non-Small Cell Lung Cancer. Cancer Med. 2019, 8, 1434–1441. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Li, L.; Luo, Y.; Zhang, L.; Wu, G.; Chen, Z.; Huang, C.; Guo, S.; Zhang, Y.; Song, X.; et al. A Multicenter, Open-Label, Randomized Phase II Controlled Study of Rh-Endostatin (Endostar) in Combination with Chemotherapy in Previously Untreated Extensive-Stage Small-Cell Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2015, 10, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Zuo, L.; He, X.; Pi, J.; Jin, J.; Shi, Y. Efficacy and Safety of Rh-Endostatin (Endostar) Combined with Pemetrexed/Cisplatin Followed by Rh-Endostatin plus Pemetrexed Maintenance in Non-Small Cell Lung Cancer: A Retrospective Comparison with Standard Chemotherapy. Thorac. Cancer 2018, 9, 1354–1360. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunningham, C.; Bolcaen, J.; Bisio, A.; Genis, A.; Strijdom, H.; Vandevoorde, C. Recombinant Endostatin as a Potential Radiosensitizer in the Treatment of Non-Small Cell Lung Cancer. Pharmaceuticals 2023, 16, 219. https://doi.org/10.3390/ph16020219
Cunningham C, Bolcaen J, Bisio A, Genis A, Strijdom H, Vandevoorde C. Recombinant Endostatin as a Potential Radiosensitizer in the Treatment of Non-Small Cell Lung Cancer. Pharmaceuticals. 2023; 16(2):219. https://doi.org/10.3390/ph16020219
Chicago/Turabian StyleCunningham, Charnay, Julie Bolcaen, Alessandra Bisio, Amanda Genis, Hans Strijdom, and Charlot Vandevoorde. 2023. "Recombinant Endostatin as a Potential Radiosensitizer in the Treatment of Non-Small Cell Lung Cancer" Pharmaceuticals 16, no. 2: 219. https://doi.org/10.3390/ph16020219