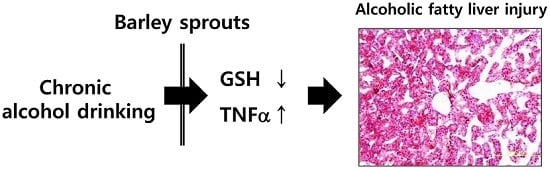
Barley Sprouts Extract Attenuates Alcoholic Fatty Liver Injury in Mice by Reducing Inflammatory Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction
2.2. Analysis of Saponarin Using Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
2.3. Animals and Treatments
2.4. Hematological and Histopathological Evaluation of Liver Injury
2.5. Determination of Hepatic TG Content
2.6. Measurement of Hepatic Glutathione (GSH)
2.7. Cell Culture and Viability Assay
2.8. Determination of Nitric Oxide (NO) Production
2.9. Western Blotting
2.10. Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.11. Statistical Analysis
3. Results
3.1. Analysis of Barley Sprouts Extract Composition
3.2. Preventive Effect of Barley Sprouts on Alcohol-Induced Liver Injury
3.3. Inhibitory Effect of Barley Sprouts Extract on Inflammatory Response-Related Gene Expression in Liver of Alcohol-Treated Mice
3.4. Anti-Inflammatory Effect of Barley Sprouts Extract in LPS-Activated Raw 264.7 Cells
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kumar, R.; Rastogi, A.; Sharma, M.K.; Bhatia, V.; Garg, H.; Bihari, C.; Sarin, S.K. Clinicopathological characteristics and metabolic profiles of non-alcoholic fatty liver disease in indian patients with normal body mass index: Do they differ from obese or overweight non-alcoholic fatty liver disease? Indian J. Endocrinol. Metab. 2013, 17, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Samokhvalov, A.V.; Shield, K.D. Global burden of alcoholic liver diseases. J. Hepatol. 2013, 59, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Suzuki, H.; Sakaguchi, S. Common pathogenic mechanism in development progression of liver injury caused by non-alcoholic or alcoholic steatohepatitis. J. Toxicol. Sci. 2007, 32, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.M.; Mantena, S.K.; Millender-Swain, T.; Cakir, Y.; Jhala, N.C.; Chhieng, D.; Pinkerton, K.E.; Ballinger, S.W. Ethanol and tobacco smoke increase hepatic steatosis and hypoxia in the hypercholesterolemic ApoE(-/-) mouse: Implications for a “multihit” hypothesis of fatty liver disease. Free Radic. Biol. Med. 2009, 46, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Albano, E.; Clot, P.; Morimoto, M.; Tomasi, A.; Ingelman-Sundberg, M.; French, S.W. Role of cytochrome p4502e1-dependent formation of hydroxyethyl free radical in the development of liver damage in rats intragastrically fed with ethanol. Hepatology 1996, 23, 155–163. [Google Scholar] [CrossRef] [PubMed]
- McClain, C.J.; Shedlofsky, S.; Barve, S.; Hill, D.B. Cytokines and alcoholic liver disease. Alcohol. Health Res. World 1997, 21, 317–320. [Google Scholar] [PubMed]
- Tilg, H.; Moschen, A.R.; Kaneider, N.C. Pathways of liver injury in alcoholic liver disease. J. Hepatol. 2011, 55, 1159–1161. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Horiguchi, N.; Jeong, W.I.; Radaeva, S.; Gao, B. Molecular mechanisms of alcoholic liver disease: Innate immunity and cytokines. Alcohol. Clin. Exp. Res. 2011, 35, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Lu, S.C. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001, 15, 1335–1349. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Wang, B.; Wang, J.; Chen, D. Simple steatosis is a more relevant source of serum inflammatory markers than omental adipose tissue. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, M.; Shibamoto, T. Flavonoids with potent antioxidant activity found in young green barley leaves. J. Agric. Food Chem. 2012, 60, 6260–6267. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.M.; Wu, C.H.; Tseng, Y.H.; Tsai, C.E.; Chang, W.C. Antioxidative and hypolipidemic effects of barley leaf essence in a rabbit model of atherosclerosis. Jpn. J. Pharmacol. 2002, 89, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.D.; Yuk, H.J.; Curtis-Long, M.J.; Jang, K.C.; Lee, J.H.; Han, S.I.; Kang, H.W.; Nam, M.H.; Lee, S.J.; Lee, J.H.; et al. Effect of the growth stage and cultivar on policosanol profiles of barley sproutss and their adenosine 5’-monophosphate-activated protein kinase activation. J. Agric. Food Chem. 2013, 61, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.H.; Park, M.J.; Ra, J.E.; Han, S.I.; Nam, M.H.; Kim, J.H.; Lee, J.H.; Seo, W.D. Saponarin from barley sproutss inhibits NF-kappab and mapk on LPS-induced raw 264.7 cells. Food Funct. 2014, 5, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Vitcheva, V.; Simeonova, R.; Krasteva, I.; Yotova, M.; Nikolov, S.; Mitcheva, M. Hepatoprotective effects of saponarin, isolated from gypsophila trichotoma wend. on cocaine-induced oxidative stress in rats. Redox Rep. 2011, 16, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, R.; Vitcheva, V.; Kondeva-Burdina, M.; Krasteva, I.; Manov, V.; Mitcheva, M. Hepatoprotective and antioxidant effects of saponarin, isolated from gypsophila trichotoma wend. on paracetamol-induced liver damage in rats. Biomed. Res. Int. 2013, 2013, 757126. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Roll, F.J. Glutathione measurement by high-performance liquid chromatography separation and fluorometric detection of the glutathione-orthophthalaldehyde adduct. Anal. Biochem. 1989, 179, 236–241. [Google Scholar] [CrossRef]
- Arteel, G.E. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology 2003, 124, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Colell, A.; Garcia-Ruiz, C.; Miranda, M.; Ardite, E.; Mari, M.; Morales, A.; Corrales, F.; Kaplowitz, N.; Fernandez-Checa, J.C. Selective glutathione depletion of mitochondria by ethanol sensitizes hepatocytes to tumor necrosis factor. Gastroenterology 1998, 115, 1541–1551. [Google Scholar] [CrossRef]
- Bode, C.; Bode, J.C. Activation of the innate immune system and alcoholic liver disease: Effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol. Clin. Exp. Res. 2005, 29, 166S–171S. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Checa, J.C.; Kaplowitz, N.; Garcia-Ruiz, C.; Colell, A.; Miranda, M.; Mari, M.; Ardite, E.; Morales, A. GSH transport in mitochondria: Defense against TNF-induced oxidative stress and alcohol-induced defect. Am. J. Physiol. 1997, 273, G7–G17. [Google Scholar] [PubMed]
- Wheeler, M.D.; Kono, H.; Yin, M.; Rusyn, I.; Froh, M.; Connor, H.D.; Mason, R.P.; Samulski, R.J.; Thurman, R.G. Delivery of the Cu/Zn-superoxide dismutase gene with adenovirus reduces early alcohol-induced liver injury in rats. Gastroenterology 2001, 120, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.D.; Nakagami, M.; Bradford, B.U.; Uesugi, T.; Mason, R.P.; Connor, H.D.; Dikalova, A.; Kadiiska, M.; Thurman, R.G. Overexpression of manganese superoxide dismutase prevents alcohol-induced liver injury in the rat. J. Biol. Chem. 2001, 276, 36664–36672. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, J.W.; Jung, Y.S.; Kwon do, Y.; Park, H.K.; Ryu, C.S.; Kim, S.K.; Oh, G.T.; Kim, Y.C. Ethanol-induced liver injury and changes in sulfur amino acid metabolomics in glutathione peroxidase and catalase double knockout mice. J. Hepatol. 2009, 50, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Rusyn, I.; Bradford, B.U.; Connor, H.D.; Mason, R.P.; Thurman, R.G. Allopurinol prevents early alcohol-induced liver injury in rats. J. Pharmacol. Exp. Ther. 2000, 293, 296–303. [Google Scholar] [PubMed]
- Kono, H.; Arteel, G.E.; Rusyn, I.; Sies, H.; Thurman, R.G. Ebselen prevents early alcohol-induced liver injury in rats. Free Radic. Biol. Med. 2001, 30, 403–411. [Google Scholar] [CrossRef]
- Kono, H.; Rusyn, I.; Uesugi, T.; Yamashina, S.; Connor, H.D.; Dikalova, A.; Mason, R.P.; Thurman, R.G. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G1005–G1012. [Google Scholar] [PubMed]
- McKim, S.E.; Konno, A.; Gabele, E.; Uesugi, T.; Froh, M.; Sies, H.; Thurman, R.G.; Arteel, G.E. Cocoa extract protects against early alcohol-induced liver injury in the rat. Arch. Biochem. Biophys. 2002, 406, 40–46. [Google Scholar] [CrossRef]
- Yin, M.; Wheeler, M.D.; Kono, H.; Bradford, B.U.; Gallucci, R.M.; Luster, M.I.; Thurman, R.G. Essential role of tumor necrosis factor α in alcohol-induced liver injury in mice. Gastroenterology 1999, 117, 942–952. [Google Scholar] [CrossRef]
- Rao, R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 2009, 50, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Deng, Q.; Kaplowitz, N. Role of TNF-α in ethanol-induced hyperhomocysteinemia and murine alcoholic liver injury. Hepatology 2004, 40, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Grunfeld, C. Tumor necrosis factor-α stimulates hepatic lipogenesis in the rat in vivo. J. Clin. Investig. 1987, 80, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Hardardottir, I.; Doerrler, W.; Feingold, K.R.; Grunfeld, C. Cytokines stimulate lipolysis and decrease lipoprotein lipase activity in cultured fat cells by a prostaglandin independent mechanism. Biochem. Biophys. Res. Commun. 1992, 186, 237–243. [Google Scholar] [CrossRef]
- Nachiappan, V.; Curtiss, D.; Corkey, B.E.; Kilpatrick, L. Cytokines inhibit fatty acid oxidation in isolated rat hepatocytes: Synergy among TNF, IL-6, and IL-1. Shock 1994, 1, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Masaki, T.; Seike, M.; Yoshimatsu, H. TNF-α induces hepatic steatosis in mice by enhancing gene expression of sterol regulatory element binding protein-1c (srebp-1c). Exp. Biol. Med. 2007, 232, 614–621. [Google Scholar]








| HPLC Condition | ||
|---|---|---|
| Column | Luna C18 RP column (2.0 × 150 mm, 5 μm) | |
| Flow rate | 0.3 mL/min | |
| Injection volume | 5 μL | |
| Column temperature | 40 °C | |
| Autosampler temperature | 4 °C | |
| Mass Condition | ||
| Ion source | Turbo spray (Negative) | |
| Curtain Gas | 10 psi | |
| Collision Gas | N2 (Medium) | |
| Ion spray Voltage | −4.2 kV | |
| Source temperature | 400 °C | |
| Gas 1 | 40 psi | |
| Gas 2 | 50 psi | |
| Component | Standard Diet | Alcohol Diet | ||
|---|---|---|---|---|
| g/L | kcal/L | g/L | kcal/L | |
| Casein | 41.4 | 176.778 | 41.4 | 176.778 |
| l-Cystine | 0.5 | 2 | 0.5 | 2 |
| dl-Methionine | 0.3 | 1.2 | 0.3 | 1.2 |
| Corn oil | 8.5 | 75.14 | 8.5 | 75.14 |
| Olive oil | 28.4 | 251.056 | 28.4 | 251.056 |
| Safflower oil | 2.7 | 23.868 | 2.7 | 23.868 |
| Dextrin maltose | 115.2 | 456.192 | 24.72 | 97.89 |
| Choline barbiturate | 0.53 | 0 | 0.53 | 0 |
| Fiber | 10.0 | 0 | 10.0 | 0 |
| Xanthan gum | 3.0 | 0 | 3.0 | 0 |
| mineral | 8.75 | 4.1125 | 8.75 | 4.1125 |
| vitamin | 2.5 | 9.5 | 2.5 | 9.5 |
| Ethanol | 0 | 0 | 51.3 | 358.46 |
| Total energy | 1000 kcal/L | 1000 kcal/L | ||
| Genes | Primer Sequences | |
|---|---|---|
| TNF-α | F: GGCCTCTCTACCTTGTTGCC | R: CAGCCTGGTCACCAAATCAG |
| IL-1β | F: TTCACCATGGAATCCGTGTC | R: GTCTTGGCCGAGGACTAAGG |
| IL-6 | F: TTGCCTTCTTGGGACTGATG | R: CCACGATTTCCCAGAGAACA |
| CD14 | F: AAACTCGCTCAATCTGTCTTTCACT | R: TCCTATCCAGCCTGTTGTAACTGA |
| iNOS | F: CGAAACGCTTCACTTCCAA | R: TGAGCCTATATTGCTGTGGCT |
| COX2 | F: GCATTCTTTGCCCAGCACTT | R: AGACCAGGCACCAGACCAAAG |
| 18S | F: CAGCCACCCGAGATTGAGCA | R: TAGTAGCGACGGGCGGTGTG |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-H.; Kim, J.-H.; Kim, S.H.; Oh, J.Y.; Seo, W.D.; Kim, K.-M.; Jung, J.-C.; Jung, Y.-S. Barley Sprouts Extract Attenuates Alcoholic Fatty Liver Injury in Mice by Reducing Inflammatory Response. Nutrients 2016, 8, 440. https://doi.org/10.3390/nu8070440
Lee Y-H, Kim J-H, Kim SH, Oh JY, Seo WD, Kim K-M, Jung J-C, Jung Y-S. Barley Sprouts Extract Attenuates Alcoholic Fatty Liver Injury in Mice by Reducing Inflammatory Response. Nutrients. 2016; 8(7):440. https://doi.org/10.3390/nu8070440
Chicago/Turabian StyleLee, Yun-Hee, Joung-Hee Kim, Sou Hyun Kim, Ji Youn Oh, Woo Duck Seo, Kyung-Mi Kim, Jae-Chul Jung, and Young-Suk Jung. 2016. "Barley Sprouts Extract Attenuates Alcoholic Fatty Liver Injury in Mice by Reducing Inflammatory Response" Nutrients 8, no. 7: 440. https://doi.org/10.3390/nu8070440