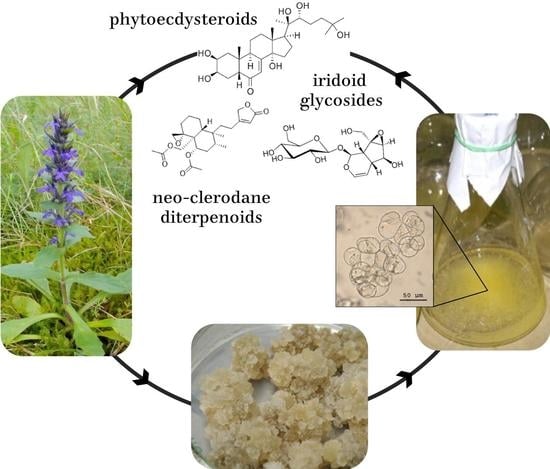
Sustainable Production of Ajuga Bioactive Metabolites Using Cell Culture Technologies: A Review
Abstract
:1. Introduction
2. Biologically Active Compounds of Genus Ajuga and the Need for Their Biotechnological Production
2.1. Phytoecdysteroids and Their Role in Plants and Beyond
2.2. Other Biologically Active Compounds of Ajuga Genus
2.3. Cell Culture as an Alternative Option for Sustainable Production of Phytochemicals
3. Cell Culture Establishment in Different Ajuga Species
4. Phytoecdysteroids in Ajuga Cell Cultures and Strategies for Their Enhanced Production
4.1. Nutrient Medium Composition
4.2. Growth Regulators in Culture Medium
4.3. Elicitation and Precursor Feeding
4.4. Induced Mutagenesis
4.5. Phytoecdysteroid Production and Culture Growth Phases
4.6. Stability of Phytoecdysteroid Content in Cell Cultures during Continuous Cultivation
5. Anthocyanin Production in Ajuga Cell Cultures
5.1. Anthocyanins in Ajuga Cell Cultures–General Considerations
5.2. Effect of Carbohydrate Source on Anthocyanin Production
5.3. Effect of Growth Regulators on Anthocyanin Production
5.4. Stability of Cell-Culture-Produced Anthocyanins
6. Other Biologically Active Compounds Produced in Ajuga Cell Cultures
7. Bioreactor Cultivation of Ajuga Cell Suspensions
8. Biological Activities of Cell Culture Extracts and Individual Compounds
8.1. Antioxidant Activity
8.2. Antimicrobial Activity
8.3. Biological Activities on the In Vitro and In Vivo Models
8.4. Other Uses of Ajuga Cell Cultures
9. Comparison of Cell Culture Production Systems with Plants and Differentiated Tissue Cultures
9.1. Cell Culture Production System Compared to Wild and Greenhouse-Grown Plants
9.2. Cell Culture Production System Compared to In Vitro-Grown Shoots
9.3. Cell Cultures Production System Compared to Hairy and Adventitious Root Cultures
10. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Flora Online. Available online: http://www.worldfloraonline.org/ (accessed on 4 October 2022).
- Qing, X.; Yan, H.M.; Ni, Z.Y.; Vavrickaa, C.J.; Zhang, M.L.; Shi, Q.W.; Gu, Y.C.; Kiyota, H. Chemical and pharmacological research on the plants from genus Ajuga. Heterocycl. Commun. 2017, 23, 245–268. [Google Scholar] [CrossRef] [Green Version]
- Ni, B.; Dong, X.; Fu, J.; Yin, X.; Lin, L.; Xia, Z.; Zhao, Y.; Xue, D.; Yang, C.; Ni, J. Phytochemical and biological properties of Ajuga decumbens (Labiatae): A review. Trop. J. Pharm. Res. 2015, 14, 1525–1536. [Google Scholar] [CrossRef] [Green Version]
- Ali, T.; Zahida, S.; Basher, R. A review on phytochemical and ethnopharmacological studies of Ajuga bracteosa Wall. Ex Benth. J. Drug Deliv. Ther. 2019, 9, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Nagarkoti, K.; Kanyal, J.; Prakash, O.; Kumar, R.; Rawat, D.S.; Pant, A.K. Ajuga L.: A systematic review on chemical composition, phytopharmacological and biological potential. Curr. Bioact. Comp. 2021, 17, e010621189843. [Google Scholar] [CrossRef]
- Hussain, M.; Bibi, Y.; Raja, N.I.; Iqbal, M.; Aslam, S.; Tahir, N.; Imran, M.; Iftikhar, A. A review of therapeutic potential of Ajuga bracteosa: A critically endangered plant from Himalaya. J. Coast. Life Med. 2016, 4, 918–924. [Google Scholar] [CrossRef]
- Mamarasulov, B.; Davranov, K.; Jabborova, D. Phytochemical, pharmacological and biological properties of Ajuga turkestanica (Rgl.) Brig (Lamiaceae). Ann. Phytomed. 2020, 9, 44–57. [Google Scholar] [CrossRef]
- Rana, S.; Narinderpal, K.; Poonam, D.A. Review on pharmacognostic profile of Ajuga reptans. J. Pharmacogn. 2020, 7, 9–18. [Google Scholar]
- Syrov, V.N. Mechanism of the anabolic action of phytoecdysteroids in mammals. Biol. Nauk (Moscow) 1984, 11, 16–20. [Google Scholar]
- Volodin, V.V. Phytoecdysteroids; Nauka: St.-Peterburg, Russia, 2003. (In Russian) [Google Scholar]
- Lafont, R.; Dinan, L. Practical uses for ecdysteroids in mammals including humans: An update. J. Insect Sci. 2003, 3, 7. [Google Scholar] [CrossRef]
- Parr, M.K.; Botrè, F.; Na, A.; Hengevoss, J.; Diel, P.; Wolber, G. Ecdysteroids: A novel class of anabolic agents? Biol. Sport. 2015, 32, 169–173. [Google Scholar] [CrossRef] [Green Version]
- Volodin, V.V.; Volodina, S.O. Phytoecdysteroids and adaptogens. New ecdysteroid-containing substance Serpisten. Pharm. Bull. 2015, 3–4, 69–83. (In Russian) [Google Scholar]
- Isenmann, E.; Ambrosio, G.; Joseph, J.F.; Mazzarino, M.; de la Torre, X.; Zimmer, P.; Kazlauskas, R.; Goebel, C.; Botrè, F.; Diel, P.; et al. Ecdysteroids as non-conventional anabolic agent: Performance enhancement by ecdysterone supplementation in humans. Arch. Toxicol. 2019, 93, 1807–1816. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Bajguz, A.; Hayat, S. Phytoecdysteroids: Distribution, structural diversity, biosynthesis, activity, and crosstalk with phytohormones. Int. J. Mol. Sci. 2022, 23, 8664. [Google Scholar] [CrossRef]
- Dinan, L.; Harmatha, J.; Volodin, V.; Lafont, R. Phytoecdysteroids: Diversity, biosynthesis and distribution. In Ecdysone: Structures and Functions, 1st ed.; Smagghe, G., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 3–45. [Google Scholar] [CrossRef]
- Kotenko, L.D.; Yakubova, M.R.; Mamatkhanov, A.U.; Saatov, Z.; Turakhozhaev, M.T. Iridoids of Ajuga turkestanica and their quantitative determination. Chem. Nat. Comp. 1993, 29, 606–607. [Google Scholar] [CrossRef]
- Alekseeva, L. Ecdysone 20-monooxygenase activity of cytochrome P450 in Ajuga reptans L. plants and cell culture. Appl. Biochem. Microbiol. 2004, 40, 135–139. [Google Scholar] [CrossRef]
- Tomás, J.; Camps, F.; Claveria, E.; Coll, J.; Melé, E.; Messeguer, J. Composition and location of phytoecdysteroids in Ajuga reptans in vivo and In vitro cultures. Phytochemistry 1992, 31, 1585–1591. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, D.H.; Sivanesan, I. Micropropagation of Ajuga species: A mini review. Biotechnol. Lett. 2017, 39, 1291–1298. [Google Scholar] [CrossRef]
- Thiem, B.; Kikowska, M.; Maliński, M.P.; Kruszka, D.; Napierała, M.; Florek, E. Ecdysteroids: Production in plant In vitro cultures. Phytochem. Rev. Proc. Phytochem. Soc. Eur. 2017, 16, 603–622. [Google Scholar] [CrossRef] [Green Version]
- Nosov, A.M. Application of cell technologies for production of plant-derived bioactive substances of plant origin. Appl. Biochem. Microbiol. 2012, 48, 609–624. [Google Scholar] [CrossRef]
- Krasteva, G.; Georgiev, V.; Pavlov, A. Recent applications of plant cell culture technology in cosmetics and foods. Eng. Life Sci. 2021, 21, 68–76. [Google Scholar] [CrossRef]
- Murthy, H.N.; Dandin, V.S.; Zhong, J.J.; Paek, K.Y. Strategies for enhanced production of plant secondary metabolites from cell and organ cultures. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology, 3rd ed.; Paek, K.Y., Murthy, H.N., Zhong, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 471–508. [Google Scholar] [CrossRef]
- Yue, W.; Ming, Q.L.; Lin, B.; Rahman, K.; Zheng, C.J.; Han, T.; Qin, L.P. Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Kayani, W.K.; Erum, D.; Tanveer, A.; Hammad, I.; Bushra, M. Evaluation of Ajuga bracteosa for antioxidant, anti-inflammatory, analgesic, antidepressant and anticoagulant activities. BMC Complem. Altern. Med. 2016, 16, 375. [Google Scholar] [CrossRef] [Green Version]
- Tahan, A.; Jafari, M.; Razmjoue, D.; Javadi, S. Relationship among some ecological factors and chemical composition of Ajuga chamaecistus Ging. plant species. Acta Ecol. Sin. 2020, 40, 268–276. [Google Scholar] [CrossRef]
- Kaithwas, G.; Gautam, R.; Jachak, S.M.; Saklani, A. Antiarthritic effects of Ajuga bracteosa wall ex Benth. in acute and chronic models of arthritis in albino rats. Asian Pac. J. Trop. Biomed. 2012, 2, 185–188. [Google Scholar] [CrossRef] [Green Version]
- Yousaf, T.; Rafique, S.; Wahid, F.; Rehman, S.; Nazira, A.; Rafique, J.; Aslam, K.; Shabir, G.; Shah, S.M. Phytochemical profiling and antiviral activity of Ajuga bracteosa, Ajuga parviflora, Berberis lycium and Citrus lemon against Hepatitis C Virus. Microb. Pathogen. 2018, 118, 154–158. [Google Scholar] [CrossRef]
- Vohra, A.; Kaur, H. Chemical investigation of medicinal plant Ajuga bracteosa. J. Nat. Prod. Plant Resour. 2011, 1, 37–45. [Google Scholar]
- Hsieh, W.T.; Liu, Y.T.; Lin, W.C. Anti-inflammatory properties of Ajuga bracteosa in vivo and In vitro study and their effects on mouse model of liver fibrosis. J. Ethnopharmacol. 2011, 135, 116–125. [Google Scholar] [CrossRef]
- Imran, M.; Jan, H.; Faisal, S.; Shah, S.A.; Shah, S.; Khan, M.N.; Akbar, M.T.; Rizwan, M.; Jan, F.; Syed, S. In vitro examination of anti-parasitic, anti-Alzheimer, insecticidal and cytotoxic potential of Ajuga bracteosa Wallich leaves extracts. Saudi J. Biol. Sci. 2021, 28, 3031–3036. [Google Scholar] [CrossRef]
- Pal, A.; Pawar, R.S. A study on Ajuga bracteosa wall ex. Benth for analgesic activity. Int. J. Cur. Bio. Med. Sci. 2011, 1, 12–14. [Google Scholar]
- Zahra, S.S.; Ahmed, M.; Qasim, M.; Gul, B.; Zia, M.; Bushra, M.; Haq, I. Polarity based characterization of biologically active extracts of Ajuga bracteosa Wall. ex Benth. and RP-HPLC analysis. BMC Complement. Altern. Med. 2017, 17, 443. [Google Scholar] [CrossRef] [Green Version]
- Ganaie, H.A.; Ali, M.N.; Ganai, B.A.; Meraj, M.; Ahmad, M. Antibacterial activity of 14, 15-dihydroajugapitin and 8-o-acetylharpagide isolated from Ajuga bracteosa wall ex. Benth against human pathogenic bacteria. Microb. Path. 2017, 103, 114–118. [Google Scholar] [CrossRef]
- Castro, A.; Coll, J.; Arfan, M. Neo-clerodane diterpenoids from Ajuga bracteosa. J. Nat. Prod. 2011, 74, 1036–1041. [Google Scholar] [CrossRef]
- Ono, Y.; Fukaya, Y.; Imai, S.; Yamakuni, T. Beneficial effects of Ajuga decumbens on osteoporosis and arthritis. Biol. Pharm. Bull. 2008, 31, 1199–1204. [Google Scholar] [CrossRef] [Green Version]
- Gautam, R.; Jachak, S.M.; Saklani, A. Anti-inflammatory effect of Ajuga bracteosa Wall ex Benth. mediated through cyclooxygenase (COX) inhibition. J. Ethnopharmacol. 2011, 133, 928–930. [Google Scholar] [CrossRef]
- Nissar, A.; Akhtar, N.; Hassan, A.; Banday, T.; Wani, B.; Zargar, M.A. Effect of Ajuga bracteosa on systemic T-cell Immunity in Balb/C mice: Dual Th1/Th2 immunostimulatory effects. Am. J. Chin. Med. 2014, 42, 375–392. [Google Scholar] [CrossRef]
- Rubnawaz, S.; Okla, M.K.; Akhtar, N.; Khan, I.U.; Bhatti, M.Z.; Duong, H.Q.; El-Tayeb, M.A.; Elbadawi, Y.B.; Almaary, K.S.; Moussa, I.M.; et al. Antibacterial, antihemolytic, cytotoxic, anticancer, and antileishmanial effects of Ajuga bracteosa transgenic plants. Plants 2021, 10, 1894. [Google Scholar] [CrossRef]
- Shaukat, B.; Mehmood, M.H.; Murtaza, B.; Javaid, F.; Khan, M.T.; Farrukh, M.; Rana, R.; Shahzad, M. Ajuga bracteosa exerts antihypertensive activity in l-name-induced hypertension possibly through modulation of oxidative stress, proinflammatory cytokines, and the nitric oxide/cyclic guanosine monophosphate pathway. ACS Omega 2022, 7, 33307–33319. [Google Scholar] [CrossRef]
- Nazer, S.; Andleeb, S.; Ali, S.; Gulzar, N.; Raza, A.; Khan, H.; Akhtar, K.; Ahmed, M.N. Cytotoxicity, anti-diabetic, and hepato-protective potential of Ajuga bracteosa-conjugated silver nanoparticles in balb/c mice. Curr. Pharm. Biotechnol. 2022, 23, 318–336. [Google Scholar] [CrossRef]
- Rauca, V.-F.; Vlase, L.; Casian, T.; Sesarman, A.; Gheldiu, A.-M.; Mocan, A.; Banciu, M.; Toiu, A. Biologically active species extracts modulate supportive processes for cancer cell development. Front. Pharmacol. 2019, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Toiu, A.; Mocan, A.; Vlase, L.; Pârvu, A.A.; Vodnar, D.C.; Gheldiu, A.M.; Moldovan, C.; Oniga, I. Comparative phytochemical profile, antioxidant, antimicrobial and in vivo anti-inflammatory activity of different extracts of traditionally used Romanian Ajuga genevensis L. and A. reptans L. (Lamiaceae). Molecules 2019, 24, 1597. [Google Scholar] [CrossRef] [Green Version]
- Göger, G.; Köse, Y.B.; Demirci, F.; Göger, F. Phytochemical characterization of phenolic compounds by LC-MS/MS and biological activities of Ajuga reptans L., Ajuga salicifolia (L.) Schreber and Ajuga genevensis L. from Turkey. Turk. J. Pharm. Sci. 2021, 18, 616–627. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Riccardelli, M.; Foddai, S.; Nicoletti, M.; Serafini, M.; Bianco, A. Unusual molecular pattern in Ajugoideae subfamily: The case of Ajuga genevensis L. from Dolomites. Nat. Prod. Res. 2016, 30, 1098–1102. [Google Scholar] [CrossRef]
- Malakov, P.Y.; Papanov, G.Y.; De La Torre, M.C.; Rodríguez, B. Neo-clerodane diterpenoids from Ajuga genevensis. Phytochemistry 1991, 30, 4083–4085. [Google Scholar] [CrossRef]
- Păduraru, A.; Cioancă, O.; Mircea, C.; Trifan, A.; Aprotosoaie, A.C.; Miron, A.; Gille, E.; Hritcu, L.; Hăncianu, M. Bioactive extracts from cultivated Ajuga genevensis L. and A. reptans L.: In vitro/in vivo pharmacological effects. Farmacia 2019, 67, 603–609. [Google Scholar] [CrossRef]
- Boneva, I.; Malakov, P.; Papano, G. Ajugapyrin A, a neo-clerodane diterpene from Ajuga pyramidalis. Phytochemistry 1998, 47, 303–305. [Google Scholar] [CrossRef]
- Akkol, E.; Mert, I.; Büşra, K.; Hakkı, T.; Sobarzo-Sánchez, E.; Haroon, K. Beneficial effects of Ajuga chamaepitys (L.) Schreber subsp. chia (Schreber) and its iridoids on the colitis model: Histopathological and biochemical evidence. Food Chem. Toxicol. 2020, 144, 111589. [Google Scholar] [CrossRef]
- Delazar, A.; Delnavazi, M.; Yassa, N.; Parkhideh, S.; Delazar, N.; Nahar, L.; Satyajit, D.S. Essential oil composition and isolation of free- radical-scavenging phenolic glycosides from the aerial parts of Ajuga chamaepitys growing in Iran. Braz. J. Pharmacogn. 2012, 22, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Venditti, A.; Frezza, C.; Maggi, F.; Lupidi, G.; Bramucci, M.; Quassinti, L.; Giuliani, C.; Cianfaglione, K.; Papa, F.; Serafini, M.; et al. Phytochemistry, micromorphology and bioactivities of Ajuga chamaepitys (L.) Schreb. (Lamiaceae, Ajugoideae): Two new harpagide derivatives and an unusual iridoid glycosides pattern. Fitoterapia 2016, 113, 35–43. [Google Scholar] [CrossRef]
- Jakovljević, D.Z.; Sava, M.V.; Stanković, M.S.; Čomić, L.R.; Topuzović, M.D. Secondary metabolite content and In vitro biological effects of Ajuga chamaepitys (L.) Schreb. subsp. chamaepitys. Arch. Biol. Sci. 2015, 67, 1195–1202. [Google Scholar] [CrossRef]
- Azizan, J.; Fallah-Bagher-Shaidaei, H.; Kefayati, H. Chemical constituents of the essential oil of Ajuga chamaepitys growing in Iran. J. Essent. Oil Res. 2002, 14, 344–345. [Google Scholar] [CrossRef]
- Taha-Salaime, L.; Davidovich-Rikanati, R.; Sadeh, A.; Abu-Nassar, J.; Marzouk-Kheredin, S.; Yahyaa, Y.; Ibdah, M.; Ghanim, M.; Lewinsohn, E.; Inbar, M.; et al. Phytoecdysteroid and clerodane content in three wild Ajuga species in Israel. ACS Omega 2019, 4, 2369–2376. [Google Scholar] [CrossRef] [Green Version]
- Işıkalan, Ç.; Orcan, P.; Akbaş, F.; Namlı, S.; Kuru, İ.S.; Buluş, Ş. Effect of cytokinins on In vitro propagation of Ajuga xylorrhiza Kit Tan (critically endangered), endemic to Turkey. In Vitro Cell. Dev. Biol. Plant. 2020, 56, 911–914. [Google Scholar] [CrossRef]
- Yu, Y.J.; Do, J.C.; Jung, K.Y.; Kwon, S.Y.; Son, K.H. Studies on the constituents of the herbs of Ajuga multiflora (I). Korean J. Pharmacogn. 1998, 29, 75–78. [Google Scholar]
- Lee, Y.; Kim, H.M.; Kim, J.H.; Lee, J.H.; Zhang, K.X.; Gao, E.M.; Jeon, J.S.; Syed, A.S.; Son, R.H.; Kim, J.Y.; et al. Chemical constituents of the Ajuga multiflora Bunge and their protective effects on dexamethasone-induced muscle atrophy in C2C12 myotubes. Nat. Prod. Res. 2022, 1–8. [Google Scholar] [CrossRef]
- Ryu, M.H.; Aeam, Y.D.; Byun, J.H. Studies on the cytotoxicity and antimicrobial effects of the extract of Ajuga multiflora Bunge. Korean J. Pharmacogn. 2000, 31, 72–76. [Google Scholar]
- Chi, D.F.; Ming-Xue, S.; Wen-Fu, X. Pesticidal character of phytoecdysteroids from Ajuga multiflora Bunge (Labiatae) on larvae of Cryptorrhynchus lapathi L. (Coleoptera: Curculionidae). J. For. Res. 2002, 13, 177–182. [Google Scholar] [CrossRef]
- Rahiminiya, A.; Herizchi, G.H.; Lamardi, S.N.S.; Ebrahimabadi, M.H.; Fazljou, S.M.; Ayati, M.H. Medicinal Importance of Ajuga Species in Iran: Ethnobotanical and traditional applications, phytochemical, and pharmacological studies. Jundishapur J. Nat. Pharm. Prod. 2022, 17, e119209. [Google Scholar] [CrossRef]
- Esposito, T.; Sansone, F.; Auriemma, G.; Franceschelli, S.; Pecoraro, M.; Picerno, P.; Aquino, R.; Mencherini, T. Study on Ajuga reptans extract: A natural antioxidant in microencapsulated powder form as an active ingredient for nutraceutical or pharmaceutical purposes. Pharmaceutics 2020, 12, 671. [Google Scholar] [CrossRef]
- Singh, P.; Grone, N.; Tewes, L.J.; Müller, C. Chemical defense acquired via pharmacophagy can lead to protection from predation for conspecifics in a sawfly. Proc. Biol. Sci. 2022, 13, 20220176. [Google Scholar] [CrossRef]
- Grace, M.H.; Cheng, D.; Raskin, I.; Lila, M.N. Neo-clerodane diterpenes from Ajuga turkestanica. Phytochem. Lett. 2008, 1, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Guibout, L.; Mamadalieva, N.; Balducci, C.; Girault, J.-P.; Lafont, R. The minor ecdysteroids from Ajuga turkestanica. Phytochem. Anal. 2015, 26, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Arthur, S.T.; Zwetsloot, K.A.; Lawrence, M.M.; Nieman, D.C.; Lila, M.A.; Grace, M.H.; Howden, R.; Cooley, I.D.; Tkach, J.F.; Keith, M.D.; et al. Ajuga turkestanica increases Notch and Wnt signaling in aged skeletal muscle. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2584–2592. [Google Scholar] [PubMed]
- Mamadalieva, N.Z.; El-Readi, M.Z.; Ovidi, E.; Ashour, M.L.; Hamoud, R.; Sagdullaev, S.S.; Azimova, S.S.; Tiezzi, A.; Wink, M. Antiproliferative, antimicrobial and antioxidant activities of the chemical constituents of Ajuga turkestanica. Phytopharmacology 2013, 4, 1–18. [Google Scholar]
- Martins, J.P.; Silva, L.C.; Nunes, M.S.; Rübensam, G.; Oliveira, J.R.; Silva, R.B.M.; Campos, M.M. Combined effects of exercise and phytoanabolic extracts in castrated male and female mice. Nutrients 2021, 13, 1177. [Google Scholar] [CrossRef] [PubMed]
- Volodin, V.V.; Chadin, I.F.; Dinan, L.; Lafont, R. Phytoecdysteroids—Plant analogues of insect mold hormones. Rastit. Resur. 2004, 40, 1–18. (In Russian) [Google Scholar]
- Lafont, R.; Dauphin-Villemant, C.; Warren, J.T.; Rees, H.H. Ecdysteroid chemistry and biochemistry. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Ufimstev, K.G.; Shirshova, T.I.; Volodin, V.A. Phytoecdysteroids—Deterrents of Insects-Phytophags; UrORAS: Ekaterinburg, Russia, 2009. (In Russian) [Google Scholar]
- Butenandt, A.; Karlson, P. Über die isolierun geines metamorphose hormons der insektenin kristallisierter. Form. Z. Naturforsch. 1954, 9, 389–391. [Google Scholar] [CrossRef]
- Hampshire, F.; Horn, D.H.S. Structure of crustecdysone, a crustacean moulting hormone. J. Chem. Soc. Chem. Commun. 1966, 2, 37–38. [Google Scholar] [CrossRef]
- Camps, F.; Coll, J. Insect allelochemicals from Ajuga plants. Phytochemistry 1993, 32, 1361–1370. [Google Scholar] [CrossRef]
- Grebenok, R.J.; Adler, J.H. Ecdysteroid biosynthesis during the ontogeny of spinach leaves. Phytochemistry 1993, 33, 341–347. [Google Scholar] [CrossRef]
- Hyodo, R.; Fujimoto, Y. Biosynthesis of 20-hydroxyecdysone in Ajuga hairy roots: The possibility of 7-ene introduction at a late stage. Phytochemistry 2000, 53, 733–737. [Google Scholar] [CrossRef]
- Syrov, V.N. Phytoecdysteroids: Their biological effects in the body of higher animals and the outlook for their use in medicine. Eksperimental’naya I Klin. Farmakol. 1994, 57, 61–66. [Google Scholar]
- Bakrim, A.; Ngunjiri, J.; Crouzet, S.; Guibout, L.; Balducci, K.; Girault, J.; Lafont, R. Ecdysteroid profiles of two Ajuga species, A. iva and A. remota. Nat. Prod. Commun. 2014, 9, 1069–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syrov, V.N.; Kurmukov, A.G.; Sakhibov, A.D. Effect of turkesterone and nerobol on the activity of the protein synthesizing system in mice liver. Vopr. Meditsinskoi Khimii 1978, 24, 456–460. [Google Scholar]
- Singh, A.; Duggal, S.; Singh, H.; Singh, J.; Katekhaye, S. Withanolides: Phytoconstituents with significant pharmacological activities. Int. J. Green Pharm. 2010, 4, 229–237. [Google Scholar] [CrossRef]
- Li, R.; Morris-Natschke, S.L.; Lee, K.H. Clerodane diterpenes: Sources, structures, and biological activities. Nat. Prod. Rep. 2016, 33, 1166–1226. [Google Scholar] [CrossRef] [Green Version]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. Terpenoids. In Pharmacognosy. Fundamentals, Applications and Strategies; Badal, S., Delgoda, R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 233–266. [Google Scholar] [CrossRef]
- Frezza, C.; de Vita, D.; Toniolo, C.; Ventrone, A.; Tomassini, L.; Foddai, S.; Nicoletti, M.; Guiso, M.; Bianco, A.; Serafini, M. Harpagide: Occurrence in plants and biological activities. Fitoterapia 2020, 147, 104764. [Google Scholar] [CrossRef]
- Kim, C.W.; Choi, K.C. Potential roles of iridoid glycosides and their underlying mechanisms against diverse cancer growth and metastasis: Do they have an inhibitory effect on cancer progression? Nutrients 2021, 13, 2974. [Google Scholar] [CrossRef]
- Budhiraja, R.D.; Sudhir, S. Review of biological activity of withenolides (antibacterial, antitumor, immunomodulator, antiinflammatory and insect antifeedcent). J. Sci. Ind. Res. 1983, 46, 488–491. [Google Scholar]
- De Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Wang, L.-S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Dinan, L.; Bourne, P.; Whiting, P.; Tsitekli, A.; Saatov, Z.; Dhadialla, T.S.; Hormann, R.E.; Lafont, R.; Coll, J. Synthesis and biological activities of turkesterone 11α-acyl derivatives. J. Insect Sci. 2002, 3, 1–6. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef] [PubMed]
- Titova, M.V.; Popova, E.V.; Konstantinova, S.V.; Kochkin, D.V.; Ivanov, I.M.; Klyushin, A.G.; Titova, E.G.; Nebera, E.A.; Vasilevskaya, E.R.; Tolmacheva, G.S.; et al. Suspension cell culture of Dioscorea deltoidei—A renewable source of biomass and furostanol glycosides for food and pharmaceutical industry. Agronomy 2021, 11, 394. [Google Scholar] [CrossRef]
- Roberts, S.C. Production and engineering of terpenoids in plant cell culture. Nat. Chem. Biol. 2007, 3, 387–395. [Google Scholar] [CrossRef]
- Nosov, A.M.; Popova, E.V.; Kochkin, D.V. Isoprenoid production via plant cell cultures: Biosynthesis, accumulation and scaling-up to bioreactors. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.Y., Murthy, H.N., Zhong, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 563–623. [Google Scholar] [CrossRef]
- Madhavi, D.L.; Smith, M.A.L.; Linas, A.C.; Mitiku, G. Accumulation of ferulic acid in cell cultures of Ajuga pyramidalis Metallica Crispa. J. Agric. Food Chem. 1997, 45, 1506–1508. [Google Scholar] [CrossRef]
- Ali, H.; Khan, M.A.; Ullah, N.; Khan, R.S. Impacts of hormonal elicitors and photoperiod regimes on elicitation of bioactive secondary volatiles in cell cultures of Ajuga bracteosa. J. Photochem. Photobiol. 2018, 183, 242–250. [Google Scholar] [CrossRef]
- Rukh, G.; Ahmad, N.; Rab, A.; Ahmad, N.; Fazal, H.; Akbar, F.; Ullah, I.; Mukhtar, S.; Samad, N. Photo-dependent somatic embryogenesis from non-embryogenic calli and its polyphenolics content in high-valued medicinal plant of Ajuga bracteosa. J. Photochem. Photobiol. B Biol. 2019, 190, 59–65. [Google Scholar] [CrossRef]
- Jan, M.; Singh, S.; Kaloo, Z.A.; Maqbool, F. Callus induction and multiple shoot regeneration in Ajuga bracteosa Wall ex. Benth.—An important medicinal plant growing in Kashmir Himalaya. J. Sci. Innov. Res. 2014, 3, 319–324. [Google Scholar] [CrossRef]
- Din, N.U.; Ali, H.; Shah, S.K.; Israr, S.; Ali, A.; Sher, M.; Khan, R.S.; Khan, M.A. Relationship of light intensity and quality with callus biomass and antioxidant potential in Ajuga bracteosa. Int. J. Biosci. 2019, 15, 506–513. [Google Scholar] [CrossRef]
- Sahakyan, N.; Petrosyan, M.; Trchounian, A. Comparative analysis of chemical composition and biological activities of Ajuga genevensis L. in In vitro culture and intact plants. Int. J. Biol. Biomol. Agricult. Food Biotechnol. Eng. 2016, 10, 321–326. [Google Scholar]
- Qian, J.J.; Yang, Y.; Li, X.; Chi, D. 20-hydroxyecdysone accumulation and regulation in Ajuga lobata D. Don suspension culture. Biosci. Biotechnol. Biochem. 2016, 80, 591–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.C.; Zhao, J.J.; Chi, D.F. β-Ecdysterone accumulation and regulation in Ajuga multiflora Bunge suspension culture. 3 Biotech 2018, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Filippova, V.N.; Zorinyants, S.E.; Volodina, S.O.; Smolenskaya, I.N. Cell cultures of ecdysteroid-containing Ajuga reptans and Serratula coronata plants. Russ. J. Plant Physiol. 2003, 50, 501–508. [Google Scholar] [CrossRef]
- Nadia, M.; Giancarlo, L.M.; Marzia, I.; FrancoF, V.; Nicoletta, C.-P.; Anacleto, M. Cell cultures of Ajuga reptans L. to bioconvert emodin and aloe–emodin: An HPLC/ESI/MS investigation. Enz. Microb. Technol. 2005, 36, 399–408. [Google Scholar] [CrossRef]
- Eshbakova, K.A.; Zakirova, R.P.; Khasanova, K.I.; Bobakulov, K.M.; Aisa, H.A.; Sagdullaev, S.S.; Nosov, A.M. Phenylpropanoids from callus tissue of Ajuga turkestanica. Chem. Nat. Comp. 2019, 55, 28–31. [Google Scholar] [CrossRef]
- Cheng, D.M.; Yousef, G.G.; Grace, M.H.; Rogers, R.B.; Lila, M.A. In vitro production of metabolism-enhancing phytoecdysteroids from Ajuga turkestanica. Plant Cell Tiss. Organ Cult. 2008, 93, 73–83. [Google Scholar] [CrossRef]
- Lev, S.V.; Zakirova, R.P.; Saatov, Z.; Gorovits, M.V.; Abubakirov, N.K. Ecdysteroids of a culture of tissues and cells of Ajuga turkestanica. Chem. Nat. Comp. 1990, 26, 40–41. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B.H. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia by use of shoot tip culture. Proc. Int. Plant Prop. Soc. 1980, 30, 421–427. [Google Scholar]
- Staba, J.E. Plant tissue culture as a technique for the phytochemist. Recent Adv. Phytochem. 1969, 2, 80. [Google Scholar]
- Rogers, R.B.; Smith, M.A.L. Consequences of In vitro and ex vitro root initiation for miniature rose production. J. Hortic. Sci. 1992, 67, 535–540. [Google Scholar] [CrossRef]
- Callebaut, A.; Hendrickx, G.; Voets, A.; Motte, J.C. Anthocyanins in cell cultures of Ajuga reptans. Phytochemistry 1990, 29, 2153–2158. [Google Scholar] [CrossRef]
- Callebaut, A.; Decleire, M.; Vandermeiren, K. Ajuga reptans (Bugle): In vitro production of anthocyanins. In Biotechnology in Agriculture and Forestry 24. Medicinal and Aromatic Plants V; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1993; pp. 1–22. [Google Scholar]
- Zakirova, R.P.; Putieva, Z.M.; Saatov, Z. Influence of the conditions of cultivation on the growth of callus and cell cultures of Ajuga turkestanica and the biosynthesis of ecdysterone. Chem. Nat. Comp. 1998, 34, 466–468. [Google Scholar] [CrossRef]
- Hikino, H.; Jin, H.; Takemoto, T. Occurrence of insect-moulting substances ecdysterone and inokosterone in callus tissues of Achyranthes radix. Chem. Pharm. Bull. 1971, 19, 438–439. [Google Scholar] [CrossRef] [Green Version]
- Ravishankar, G.A.; Mehta, A.R. Control of ecdysterone biogenesis in tissue cultures of Trianthema portulacastrum. Nat. Prod. 1979, 42, 152–158. [Google Scholar] [CrossRef]
- Karnachuk, R.; Benson, N.; Trofimova, N. Cell culture of Serratula coronate—Prospective ecdysteroid producer. In Biology of Cultivating Cells and Plant Biotechnology; Butenko, R., Ed.; Nauka: Moscow, Russia, 1991; pp. 39–41. (In Russian) [Google Scholar]
- Corio-Costet, M.F.; Chapuis, L.; Delbecque, J.-P. Chenopodium album L. (Fat Hen): In vitro cell culture, and production of secondary metabolites (phytosterols and ecdysteroids). In Biotechnology in Agriculture and Forestry 41. Medicinal and Aromatic Plants X; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 97–112. [Google Scholar] [CrossRef]
- Sinlaparaya, D.; Duanghaklang, P.; Panichajakul, S. Enhancement of 20-hydroxyecdysone production in cell suspension cultures of Vitex glabrata R. Br. by precursors feeding. Afr. J. Biotechnol. 2007, 6, 1639–1642. [Google Scholar] [CrossRef]
- Zakirova, R.P.; Malikova, M.K. Effect of N-nitroso-N-methylurea on the biosynthetic activity of Ajuga turkestanica callus tissue. Chem. Nat. Comp. 2000, 36, 384–386. [Google Scholar] [CrossRef]
- Zakirova, R.P.; Sobolkova, G.I.; Kharitonov, T.D.; Nigmatullaev, A.M.; Sagdullaev, S.S.; Kochkin, D.V.; Nosov, A.M. Obtainment and characteristics of phytoecdysteroid-producing Ajuga turkestanica (Rgl.) plant cell cultures. Appl. Biochem. Microbiol. 2020, 56, 809–814. [Google Scholar] [CrossRef]
- Alekseeva, L. Influence of manganese ions on ecdysteroids biosynthesis in Ajuga reptans (Lamiaceae) plants and cells culture. Rastit. Resur. 2006, 42, 92–100. [Google Scholar]
- Sahakyan, N.Z.; Petrosyan, M.T.; Volodin, V.V.; Volodina, S.O.; Aghajanyan, J.A.; Popov, Y.G. Isolated culture of Ajuga genevensis L. as a potential source of biological active substances. New Armen. Med. J. 2008, 2, 65–73. [Google Scholar]
- Yany, Y.Y.; Chi, D.F.; Yu, J. Changing of antioxidant enzyme activities and ß-ecdysone synthesis after adding ABA into Ajuga lobata D. Don suspension cell culture system. J. Nat. Sci. Hunan Normal Univ. 2015, 38, 16–21. [Google Scholar]
- Wang, X.M.; Chi, D.F. The effect of jasmonic acid methylester on cell growth and ß-ecdysteron accumulation in Ajuga lobata. Acta Pratacult. Sin. 2018, 27, 95–109. [Google Scholar]
- Wang, Y.C.; Yang, Y.Y.; Chi, D. Transcriptome analysis of abscisic acid induced 20E regulation in suspension Ajuga lobata cells. 3 Biotech 2018, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Zakirova, R.P.; Malikova, M.K. Accumulation dynamics of ecdysterone and carbohydrates in callus tissue of Ajuga turkestanica. Chem. Nat. Comp. 2001, 37, 266–268. [Google Scholar] [CrossRef]
- Cheng, D.M.; Yousef, G.; Rogers, R.B.; Lila, M.A. In vitro production of phytoecdysteroids from Ajuga turkestanica. FASEB J. 2007, 21, A1079–A1080. [Google Scholar]
- Zakirova, R.P.; Yakubova, M.R. Productivity of Ajuga turkestanica callus tissue during multiyear cultivation. Chem. Nat. Comp. 2002, 38, 356–357. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nishida, K.; Noguchi, M.; Tamaki, E. Some factors affecting the anthocyanin formation by Populus cells in suspension culture. Agric. Bio. Chem. 1973, 37, 561–567. [Google Scholar] [CrossRef]
- Dougall, D.K.; Vagelien, D.L. The stability of accumulated anthocyanin in suspension cultures of the parental line and high and low accumulating subclones of wild carrot. Plant Cell Tiss. Organ Cult. 1987, 8, 113–123. [Google Scholar] [CrossRef]
- Mizukami, H.; Tomita, K.; Ohashi, H.; Hiraoka, N. Anthocyanin production in callus cultures of roselle (Hibiscus sabdariffa L.). Plant. Cell Rep. 1988, 7, 553–556. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kinoshila, Y.; Watanabe, S.; Yamada, Y. Anthocyanin production in suspension cultures of high-producing cells. Agric. Biol. Chem. 1989, 53, 417–423. [Google Scholar] [CrossRef]
- Do, C.B.; Cormier, F. Accumulation of anthocyanins enhanced by a high osmotic potential in grape (Vitis vinifera L.) cell suspensions. Plant Cell Rep. 1990, 9, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Akita, M.; Sakamoto, K.; Liu, J.; Shigeoka, T.; Koyano, T.; Kawamura, M.; Furuya, T. Large-scale production of anthocyanin by Aralia cordata cell suspension cultures. Appl. Microbiol. Biotechnol. 1993, 40, 215–218. [Google Scholar] [CrossRef]
- Akita, T.; Hina, Y.; Nishi, T. Production of betacyanins by a cell suspension culture of table beet (Beta vulgaris L.). Biosci. Biotechnol. Biochem. 2000, 64, 1807–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamakawa, T.; Kato, S.; Ishida, K.; Kodama, T.; Minoda, Y. Production of anthocyanins by Vitis cells in suspension cultures. Agric. Biol. Chem. 1983, 47, 2185–2191. [Google Scholar] [CrossRef]
- Madhavi, D.L.; Juthangkoon, S.; Lewen, K.; Berber-Jimenez, M.D.; Smith, M.A.L. Characterization of anthocyanins from Ajuga pyramidalis Metallica Crispa cell cultures. J. Agric. Food Chem. 1996, 44, 1170–1176. [Google Scholar] [CrossRef]
- Callebaut, A.; Voets, A.M.; Motte, J.C. Anthocyanin production by plant cell cultures on media based on milk whey. Biotechnol. Lett. 1990, 12, 215–218. [Google Scholar] [CrossRef]
- Terahara, N.; Callebaut, A.; Ohba, R.; Nagata, T.; Ohnishi-Kameyama, M.; Suzuki, M. Triacylated anthocyanins from Ajuga reptans flowers and cell cultures. Phytochemistry 1996, 42, 199–203. [Google Scholar] [CrossRef]
- Norihiko, T.; Callebaut, A.; Ohba, R.; Nagata, T.; Ohnishi-Kameyama, M.; Suzuki, M. Acylated anthocyanidin 3-sophoroside-5-glucosides from Ajuga reptans flowers and the corresponding cell cultures. Phytochemistry 2001, 58, 493–500. [Google Scholar] [CrossRef]
- Callebaut, A.; Terahara, N.; Decleire, M. Anthocyanin acyltransferases in cell cultures of Ajuga reptans. Plant Sci. 1996, 118, 109–118. [Google Scholar] [CrossRef]
- Callebaut, A.; Terahara, N.; Haan, M.; Decleire, M. Stability of anthocyanin composition in Ajuga reptans callus and cell suspension cultures. Plant Cell Tiss. Organ Cult. 1997, 50, 195–201. [Google Scholar] [CrossRef]
- Nagarajan, R.P.; Keshavarz, E.; Gerson, D.F. Optimization of anthocyanin yield in a mutated carrot cell line (Daucus carota) and its implications in large-scale production. J. Ferment. Bioeng. 1989, 68, 102–106. [Google Scholar] [CrossRef]
- Ozeki, Y.; Komamine, A. Changes in activities of enzymes involved in general phenylpropanoid metabolism during the induction and reduction of anthocyanin synthesis in a carrot suspension culture as regulated by 2,4-D. Plant Cell Physiol. 1985, 26, 903–911. [Google Scholar]
- Sahakyan, N.Z.; Petrosyan, M.T.; Popov, Y.G.; Volodin, V.V.; Matistov, N.V.; Gruzdev, I.V.; Shirshova, T.I. Content of neutral lipids and fatty acids in callus cultures and leaves of intact plants of Ajuga genevensis and Ajuga chia. Biotechnol. Biotechnol. Equip. 2010, 24, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Di Paola, R.; Esposito, E.; Mazzon, E.; Riccardi, L.; Caminiti, R.; Dal Toso, R.; Pressi, G.; Cuzzocrea, S. Teupolioside, a phenylpropanoid glycosides of Ajuga reptans, biotechnologically produced by IRBN22 plant cell line, exerts beneficial effects on a rodent model of colitis. Biochem. Pharmacol. 2009, 77, 845–857. [Google Scholar] [CrossRef] [Green Version]
- Rakhimov, D.A.; Shadmanova, N.A. Polysaccharides of plant tissue cultures. iii. polysaccharides of a callus culture of Ajuga turkestanica. Chem. Nat. Comp. 1994, 30, 3404–3405. [Google Scholar] [CrossRef]
- Korkina, L.G.; Mikhal'chik, E.; Suprun, M.V.; Pastore, S.; Dal Toso, R. Molecular mechanisms underlying wound healing and anti-inflammatory properties of naturally occurring biotechnologically produced phenylpropanoid glycosides. Cell. Mol. Biol. 2007, 53, 84–91. [Google Scholar] [CrossRef]
- Muraca, L.; Scuteri, A.; Burdino, E.; Marcianò, G.; Rania, V.; Catarisano, L.; Casarella, A.; Cione, E.; Palleria, C.; Colosimo, M.; et al. Effectiveness and safety of a new nutrient fixed combination containing pollen extract plus teupolioside, in the management of LUTS in patients with benign prostatic hypertrophy: A pilot study. Life 2022, 12, 965. [Google Scholar] [CrossRef]
- Smith, M.A.L.; Reid, J.F.; Hansen, A.C.; Li, Z.; Madhavi, D.L. Non-destructive machine vision analysis of pigment-producing cell cultures. J. Biotechnol. 1995, 40, 1–11. [Google Scholar] [CrossRef]
- Smith, M.A.L.; Rogers, R.B. Pigment production in Ajuga cell cultures. In Plant Tissue Culture Concepts and Laboratory Exercises, 2nd ed.; Trigiano, R.N., Ed.; Routledge: London, UK, 2000; pp. 361–372. [Google Scholar]
- Sivanesan, I.; Saini, R.K.; Noorzai, R. In vitro propagation, carotenoid, fatty acid and tocopherol content of Ajuga multiflora Bunge. 3 Biotech 2016, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Jeong, B.R.; Sivanesan, I. Impact of light quality and sucrose on adventitious shoot regeneration and bioactive compound accumulation in Ajuga multiflora Bunge. Sci. Hort. 2018, 236, 222–228. [Google Scholar] [CrossRef]
- Ali, H.; Khan, M.A.; Kayani, W.K.; Khan, T.; Mashwani, Z.; Ullah, N.; Raham Sher Khan, R.S. Thidiazuron regulated growth, secondary metabolism and essential oil profiles in shoot cultures of Ajuga bracteosa. Ind. Crop. Prod. 2018, 121, 418–427. [Google Scholar] [CrossRef]
- Ullah, M.A.; Gul, F.Z.; Khan, T.; Bajwa, M.N.; Drouet, S.; Tungmunnithum, D.; Giglioli-Guivarc'h, N.; Liu, C.; Hano, C.; Abbasi, B.H. Differential induction of antioxidant and anti-inflammatory phytochemicals in agitated micro-shoot cultures of Ajuga integrifolia Buch. Ham. ex D. Don with biotic elicitors. AMB Express 2021, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Nagakari, M.; Kushiro, T.; Matsumoto, T.; Tanaka, N.; Kakinuma, K.; Fujimoto, Y. Incorporation of acetate and cholesterol into 20-hydroxyecdysone by hairy root clone of Ajuga reptans var. atropurpurea. Phytochemistry 1994, 36, 907–910. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Ohyama, K.; Nomura, K.; Hyodo, R.; Takahashi, K.; Yamada, J.; Morisaki, M. Biosynthesis of sterols and ecdysteroids in Ajuga hairy roots. Lipids 2000, 35, 279–288. [Google Scholar] [CrossRef]
- Hussain, M.J.; Abbas, Y.; Nazli, N.; Fatima, S.; Drouet, S.; Hano, C.; Abbasi, B.H. Root cultures, a boon for the production of valuable compounds: A comparative review. Plants 2022, 11, 439. [Google Scholar] [CrossRef]
- Tanaka, N.; Matsumoto, T. Regenerants from Ajuga hairy roots with high productivity of 20-hydroxyecdysone. Plant Cell Rep. 1993, 13, 87–90. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tanaka, N. Production of phytoecdysteroids by hairy root cultures of Ajuga reptans var. atropurpurea. Agric. Biol. Chem. 1991, 55, 1019–1025. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, N.; Matsumoto, T. Characterization of Ajuga plants regenerated from hairy roots. Plant Tiss. Cult. Lett. 1993, 10, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Uozumi, N.; Kohketsu, K.; Kobayashi, T. Growth and kinetic parameters of Ajuga hairy root in fed–batch culture on monosaccharide medium. J. Chem. Technol. Biotechnol. 1993, 57, 155–161. [Google Scholar] [CrossRef]
- Ali, H.; Khan, M.A.; Kayani, W.K.; Dilshad, E.; Rani, R.; Khan, R.S. Production of biomass and medicinal metabolites through adventitious roots in Ajuga bracteosa under different spectral lights. J. Photochem. Photobiol. B 2019, 193, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Ali, H.; Khan, T.; Kayani, W.; Khan, M.A. Impacts of methyl jasmonate and phenyl acetic acid on biomass accumulation and antioxidant potential in adventitious roots of Ajuga bracteosa Wall ex Benth., a high valued endangered medicinal plant. Physiol. Mol. Biol. Plants 2017, 23, 229–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakirova, R.P.; Abdukadyrov, I.T.; Yakubova, M.R. A possibility of increasing in ecdysteroid content in callus and suspension cultures of Ajuga turkestanica by induced morphogenesis. Biotechnol. Russ. 2012, 3, 44–47. [Google Scholar]

| Species | Biological Activities Reported ** | Phytoecdysteroids | Other Compounds | References |
|---|---|---|---|---|
| A. bracteosa | Antimicrobial (antiviral, antiplasmodial (antimalarial), against hepatitis C, antibacterial), anti-inflammatory (including against arthritis and osteoporosis), cytotoxic, antidiabetic, hepatoprotective, antioxidant, analgesic antidepressant, anticoagulant, anti-cancer, immunoregulatory, insecticidal, cardiotonic, anti-Alzheimer, antihypertensive | 20-hydroxyecdysone makisterone A ajugalactone cyasterone 3-epicyasterone 3-epi-22-acetylcyasterone | Withanolides: bracteosin A and B, ajugin 1, C, D, E, withaferin A Iridoids: reptoside, 6-deoxyharpagide Other sterols: ß-sitosterol, stigmasterol Neo-clerodane diterpenoids: clerodin, 14,15-dihydroclerodin, ivain II, 14,15-dihydroajugapitin, 14,15-dihydro-15-hydroxyajugapitin, ajugarin I, lupulin A, 15-epi-lupulin B, ajubractins A-E, bracteonin-A, 3-epi-caryoptin, 3-epi-14,15-dihydrocaryoptin, 15-hydroxyajubractin C, 14-hydro-15-hydroxyajugachin A Other compounds: pyrocatechol, resorcinol, catechin, gallic acid, chlorogenic acid, caffeic acid, syringic acid, p-coumaric acid, ferulic acid, vanillic acid, coumarin, sinapinic acid, trans-cinnamic acid, rutin, quercetin, kaempferol, 3,4′-dihydroxy-3,6,7-trimethoxyflavone, 7-hydroxy-3,6,3′,4′-tetramethoxyflavone, ajuganane, bis(2S-methylheptyl) phthalate, heptacos-3-en-25-one, bractic acid, bractin A and B Essential oils | [2,4,6,26,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] |
| A. genevensis (including synonym A. pyramidalis) | Antioxidant, antimicrobial (including antifungal), anti-inflammatory, antiproliferative, antitumor | No information | Neo-clerodane diterpenoids: ajugavensins A–C, ajugapyrin A (in A. pyramidalis) Iridoids: harpagide, aucubin, catalpol, harpagoside, 8-O-acetyl-harpagide Other compounds: coumaroyl glucoside and its isomer, caffeic acid, p-coumaric acid, ferulic acid, rosmarinic acid, oleanolic acid, maslinic acid, hyperoside, quercitrin, quercetin glucuronide, apigenin, apigenin-C-hexoside-C-pentoside, forsythoside A, luteolin and its derivatives, campesterol | [43,44,45,46,47,48,49] |
| A. chamaepitys | Anti-inflammatory, antioxidant, antimicrobial, antitumor, anti-arthritic, antimalarial, wound-healing, activity against ulcerative colitis | 20-hydroxyecdysone makisterone A cyasterone | Iridoids: 8-O-acetylharpagide, harpagide, aucubin, catalpol, harpagoside, ajugoside, reptoside, 5-O-β- D -glucopyranosyl-harpagide, 5-O-β-D-glucopyranosyl-8-O-acetylharpagide, asperulosidic acid, deacetylasperulosidic acid Other compounds: ferulic acid, isoquercitrin, rutin, quercitrin, luteolin, 53 volatile compounds (major–β-pinene, ethyl linoleate, germacrene D, kaurene, (E)-phytol, γ-terpinene, limonene), acteoside, chrysoeriol 7-O-glucopyranoside, pigenin 7-O-rhamnopyranoside | [43,50,51,52,53,54,55] |
| A. multiflora | Muscle-protective, cytotoxic against murine leukemia tumor, antibacterial, pesticidal | 20-hydroxyecdysone 29-hydroxyprecyasterone cyasterone makisterone A | Iridoids: 8-O-acetylharpagide, harpagide Other compounds: apigenin, apigenin 7-glucuronide, bis(2-ethylhexyl) phthalate, carotenoids (including E-lutein, all-E-β-carotene, 9′-Z-neoxanthin, all-E-violaxanthin, all-E-zeaxanthin, all-E-β-cryptoxanthin), fatty acids (including linoleic, linolenic, and palmitic) | [2,56,57,58,59,60] |
| A. reptans | Antioxidant antimicrobial (antibacterial, antifungal), anti-inflammatory, androgenic, neuroprotective, antipredatory | 20-hydroxyecdysone ajugalactone ajugasterone A, B cyasterone 29-norcyasterone 29-norsengosterone 2-acetyl-29-norcyasterone 3-acetyl-29-norcyasterone sengosterone 24,25-dehydroprecyasterone 20-hydroxyecdysone 22-acetate 20-hydroxyecdysone 25-acetate (viticosterone E) breviflorasterone reptanslactone A and B sendreisterone polypodine B reptansterone viticosterone E 28-epi-sengosterone 5,29-dihydroxycapitasterone, 2-dehydroajugalactone, 3-dehydroajugalactone | Iridoids: reposide, ajugoside, ajugol, 8-O-acetylharpagide, harpagide, ajureptoside, aucubin, catalpol, harpagoside Sterols: clerosterol, 22,23-didehydroclerosterol, campesterol, ß-Sitosterol Neo-clerodane diterpenoids: ajugareptansin, ajugareptansone A, ajugareptansone B, ajugachin A, ajugavensin A, ajugorientin (3β-hydroxyajugavensin B), 14,15-dehydroajugareptansin, 3α-hydroxyajugamarin F4, areptin A, areptin B, ajugatansin A1, ajugatansin B1, ajugatansin D1, ajugareptone Other compounds: chlorogenic acid, caffeic acid, luteolin, luteolin-7-O-glucoside, apigenin, p-Coumaric acid, ferulic acid, leucoseptoside A, verbascoside, cistanoside A, forsythoside A, echinocoside, caffeoyl glucose, isoquercitrin, rutin, quercitrin | [2,7,8,38,44,61,62,63] |
| A. turkestanica | Antiproliferative, antimicrobial (antibacterial), antioxidant, hypoglycemic, hypolipidemic, anabolic, hepatoprotective, increase in protein synthesis in skeletal muscle and in liver, increase signaling in aged skeletal muscles, muscle strength improvement, stimulation of aquaporins–human skin hydration, erythropoesis-stimulating | 20-hydroxyecdysone cyasterone 11-hydroxy-cyasterone 11-hydroxy-sidisterone 11-hydroxy-Δ24-capitasterone ajugalactone 22-acetylcyasterone turkesterone ajugasterone B atrotosterone C abutasterone ajugasterone C 25-hydroxy-atrotosterone A 25-hydroxy-dacryhainansterone turkesterone 22-acetate 22-oxo-turkesterone turkesterone 22-acetonide | Neo-clerodane diterpenes: ajugapitin (clerodendrin D), chamaepitin, ajugachin B, lupulin A, 14,15-dihydroajugachin B, 14-hydro-15-methoxyajugachin B Iridoids: harpagide, 8-O-acetylharpagide | [2,7,17,58,64,65,66,67,68] |
| Species | Explant Source | Culture Type | Medium Composition | Reference |
|---|---|---|---|---|
| A. bracteosa | Leaves Petioles Internodes | Callus Callus Callus | I: MS + 5.0 mg/L BA I: MS + 2.0 mg/L BA + 3.0 mg/L IAA I: MS + 2.0 mg/L BA + 5.0 mg/L NAA | [96] |
| A. bracteosa | Leaves of in vitro plantlets | Callus | I: MS + 2.0 mg/L BA + 1.0 mg/L 2,4-D | [95] |
| A. bracteosa | Sterile hypocotyls | Callus, suspension | I, M: MS + 1.0 mg/L BA | [94] |
| A. bracteosa | Sterile hypocotyls | Callus | I: MS + 1.0 mg/L TDZ + 0.5 mg/L NAA | [97] |
| A. genevensis | Leaves and roots | Callus | M: MS + 2.0 mg/L glycine + 2.0 mg/L IAA + 0.2 mg/L kinetin | [98] |
| A. lobata | Roots | Callus, suspension | I: MS + 1 mg/L BA + 0.4 mg/L 2,4-D M: MS + 0.4 mg/L 2,4-D for callus and 0.4 mg/L 2, 4-D + 0.5 mg/L BA for suspension | [99] |
| A. multiflora | Leaves | Callus, suspension | I: MS + 0.2 mg/L BA + 0.2 mg/L kinetin + 0.4 mg/L 2,4-D) M: MS + 0.4 mg/L 2,4-D for callus and 0.6 mg/L 2,4-D for cell suspension | [100] |
| A. pyramidalis | In vitro developed leaves | Callus | WPM + 100 μM Fe as FeNa2EDTA + rose vitamins + 0.1 g/L myoinositol, + 0.15 g/L PVP + 0.05 g/L L-ascorbic acid + 2.26 μM 2,4-D + 3.49 μM kinetin | [93] |
| A. reptans | Roots | Callus, suspension | M (callus): MS + Staba vitamins + 100 mg/L inositol. M (suspension): MS + Staba vitamins + 100 mg/L mesoinositol + 1 mg/L NAA + 2 g/L PVP | [101] |
| A. reptans | Not specified | Callus, suspension | M: MS + Staba vitamins + 1 mg/L 2,4-D + 0.2 mg/L BA + 100 mg/L mesoinositol + 1 mg/L NAA + 2 g/L PVP. | [18] |
| A. reptans | Seeds | Callus, suspension | B5 (Gamborg) + 1 mg/L NAA + 1 mg/L kinetin + 0.2 mg/L 2,4-D | [102] |
| A. turkestanica | Leaves | Callus | M: MS + 100 mg/L meso-inositol + 0.4 mg/L thiamine HCl | [103] |
| A. turkestanica | Leave of in vitro plants | Callus, suspension | I, M: B5 (Gamborg) + 2.31 M 2,4-D | [104] |
| A. turkestanica | Ovary tissues | Callus, suspension | I, M (callus): MS + 1 mg/L NAA + 0.002 mg/L TDZ I, M (suspension): 1/2MS + 1 mg/L NAA + 0.0002 mg/L TDZ | [105] |
| Species | Culture Type | Extract Analyzed | Compounds Identified | Content and Optimum Elicitation Treatment | Reference |
|---|---|---|---|---|---|
| A. genevensis | Leaf-originated callus | Water, methanol, or ethanol extracts | Non-identified ecdysteroids | - | [122] |
| A. lobata | Cell suspension | Not available | 20-Hydroxyecdysone | Elicitation: 0.1 mg/L ABA * | [123] |
| A. lobata | Root-originated cell suspension | Methanol extracts | 20-Hydroxyecdysone | Up to 12.75 mg/gDW Elicitation: 10 mg/L MVA (day 1) + 50 μL/L α-Pinene (day 1) + 80 mmol/L SNP (day 7) | [99] |
| A. lobata | Cell suspension | Not available | 20-Hydroxyecdysone | Up to 3.53 mg/gDW Elicitation: 100 μmol/L MeJ | [124] |
| A. lobata | Cell suspension | Methanol extracts | 20-Hydroxyecdysone | Up to 7 mg/gDW Elicitation: 0.15 mg/L ABA | [125] |
| A. multiflora | Leaf-originated cell suspension | Methanol extracts | 20-Hydroxyecdysone | Up to 6 mg/gDW Elicitation: 10 mg/L MVA | [100] |
| A. reptans | Callus | Methanol extracts | 20-Hydroxyecdysone | 0.121% | [18] |
| Polypodine B | 0.001% | ||||
| 29-Norcyasterone | 0.002% | ||||
| 29-Norsengosterone | 0.001% | ||||
| A. reptans | Root-originated callus and cell suspension | Ethanol extracts | 20-Hydroxyecdysone | 0.68% in callus, 0.43–0.50% in suspension | [101] |
| A. reptans | Callus | Methanol extracts | 20-Hydroxyecdysone, Polypodine B, 29-Norcyasterone, Ajugalactone, Ajugasterone | Elicitation: 2.5 mM MnSO4 * | [121] |
| A. turkestanica | Leaf-originated callus and cell suspension | Methanol followed by petroleum ether and ethyl acetate extraction | 20-Hydroxyedysone, | 23.6 µg/mg extract (20E) Elicitation: 125 μM MeJ | [104] |
| Turkesterone Cyasterone Cyasterone 22-acetate | Trace amounts Trace amounts Trace amounts | ||||
| A. turkestanica | Ovary-originated callus | Methanol extracts | 20-hydroxyedysone | 0.1–0.12% | [105] |
| Turkesterone | 0.032–0.036% | ||||
| A. turkestanica | Not specified | Methanol followed by butanol extracts | 20-Hydroxyedysone | 0.029% | [113] |
| A. turkestanica | Ovary-originated callus | Methanol extract | 20-Hydroxyedysone | 0.035% | [126] |
| A. turkestanica | Mutant callus (N-NMU treated) | Methanol extract | 20-Hydroxyedysone Turkesterone | 0.2% Not given | [119] |
| A. turkestanica | Leaf-originated callus and cell suspension | Water-ethanol extracts | 20-Hydroxyecdysone | Up to 2.5 mg/gDW | [120] |
| Turkesterone | 0.04–0.05 mg/gDW |
| Species | Culture Type | Extract Analyzed | Compounds Identified | Content and Optimum Elicitation Treatment | Reference |
|---|---|---|---|---|---|
| A. bracteosa | Hypocotyle-originated callus and cell suspension | Acetone-water extracts for total phenolics and flavonoids, hydro-distillation through a Clevenger-type apparatus for volatiles | Total phenolics, total flavonoids 29 volatile compounds including: β-Pinene | 7.0 mg GAE/g DW 3.8 mg QE/g DW Elicitation: 0.5 mg/L MeJ 2.1–9.5% | [94] |
| β-Ocimene | 1.4–8.3% | ||||
| 1-Terpinene-4-ol | 5.8–9.6% | ||||
| Caryophyllene | 1.3–6.2% | ||||
| β-Farnesene | 0.82–7.8% | ||||
| Myrtenal | 2.2–8.4% | ||||
| Citronellyl acetate | 2.1–7.3% | ||||
| Caryophyllene oxide | 1.5–5.5% | ||||
| β-Elemene | 2.2–8.8% | ||||
| A. chia (accepted name Ajuga chamaepitys subsp. chia) | Leaf-originated callus | Hexane extracts | Neutral lipids, palmitic, stearic, oleic, linolenic, linoleic, arachic fatty acids | 0.89–1.48% | [145] |
| A. genevensis | Leaf-originated callus | Water, methanol, or ethanol extracts | Alanine Serine | 0.217 mg/gDW 0.98 mg/gDW | [122] |
| A. genevensis | Leaf- and root-originated callus | Water, ethanol, or methanol extracts | 10-Methylnonadecane | 57.0% | [98] |
| Methoxyacetic acid 2-tetradecyl ester | 17.75% | ||||
| 1-Bromopentadecane | 14.55% | ||||
| A. genevensis | Leaf- and root-originated callus | Hexane extracts | Neutral lipids, palmitic, stearic, oleic, linolenic, linoleic, arachic fatty acids | 0.65–0.96% | [145] |
| A. pyramidalis | Leaf-originated callus and cell suspension | Methanol-HCl extracts | Ferulic acid | 138 mg/100 gFW | [93,137] |
| Anthocyanins | up to 17–42 mg/100 gFW | ||||
| A. reptans | Leaf-originated cell suspension | Ethanol-water extract | Teupolioside | 4 g/L | [146] |
| A. reptans | Flower-originated callus and cell suspensions | Methanol-acetic acid-water extracts | Cyanidin- and delphinidin-based anthocyanins | 1–2.5% DW | [111,112,139,140,142] |
| A. turkestanica | Leaf-originated callus | Methanol extracts | Leonoside A, lavandulifolioside | Not given | [103] |
| A. turkestanica | Ovary-originated callus | Water, oxalate buffer, alkali, precipitation with alcohol | Polysaccharides: WSPS PS HMC A and B | 7.2% 7.0% 13.1% | [147] |
| A. turkestanica | Mutant callus (N-NMU treated) | Methanol extract | Harpagide, 8-O-Ac-harpagide | Not given | [119] |
| A. turkestanica | Ovary-originated callus | Ethanol, water, oxalate extracts | Polysaccharides: SWPS PS | Up to 13.7% Up to 5.7% | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, E.; Titova, M.; Tynykulov, M.; Zakirova, R.P.; Kulichenko, I.; Prudnikova, O.; Nosov, A. Sustainable Production of Ajuga Bioactive Metabolites Using Cell Culture Technologies: A Review. Nutrients 2023, 15, 1246. https://doi.org/10.3390/nu15051246
Popova E, Titova M, Tynykulov M, Zakirova RP, Kulichenko I, Prudnikova O, Nosov A. Sustainable Production of Ajuga Bioactive Metabolites Using Cell Culture Technologies: A Review. Nutrients. 2023; 15(5):1246. https://doi.org/10.3390/nu15051246
Chicago/Turabian StylePopova, Elena, Maria Titova, Marat Tynykulov, Rano P. Zakirova, Irina Kulichenko, Olga Prudnikova, and Alexander Nosov. 2023. "Sustainable Production of Ajuga Bioactive Metabolites Using Cell Culture Technologies: A Review" Nutrients 15, no. 5: 1246. https://doi.org/10.3390/nu15051246