Aflatoxin B1 DNA-Adducts in Hepatocellular Carcinoma from a Low Exposure Area
Abstract
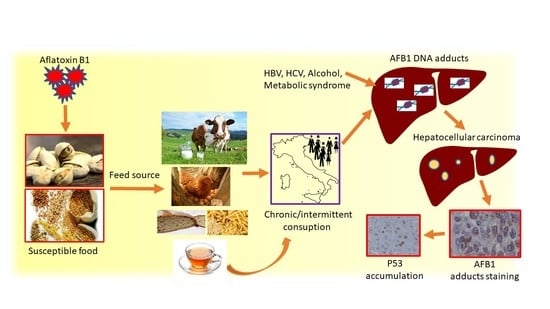
:1. Introduction
2. Patients and Methods
2.1. Patients
- Cohort 1: 131 surgically resected HCCs were studied by immunohistochemistry (IHC) to determine the presence of AFB1-adducts, mutated TP53 aberrant accumulation, and CD68, to characterize AFB1-adducts positive cells among the tumor-infiltrating cell populations. Restriction fragment length polymorphism (RFLP) analysis of TP53 R249S transversion was performed in a subgroup of 77 patients, while 27 matched HCC and cirrhotic tissues were previously sequenced to detect TP53 gene mutations [13].
- Cohort 2: 45 surgically-resected CC were studied by IHC to determine the presence of AFB1-adducts.
- Cohort 3: 20 explanted end-stage cirrhotic livers were studied by IHC to determine the presence of AFB1 adducts, mutated TP53 aberrant accumulation, CD68 IHC to characterize AFB1 adducts positive cells.
2.2. Rat Model of HCC Exposed to AFB1
2.3. Immunohistochemical Assay of AFB1 Adducts
2.4. Statistical Analysis
3. Results
3.1. AFB1 Adducts Are Present in HCC but Not in CC Tissues
3.2. DNA Adducts Are Present in AFB1-Exposed Rats HCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Statement of Ethics
References
- Liu, Y.; Wu, F. Global burden of aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wild, C.P.; Gong, Y.Y. Mycotoxins and human disease: A largely ignored global health issue. Carcinogenesis 2010, 31, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Front. Microbiol. 2016, 7, 2170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viegas, S.; Viegas, C.; Martins, C.; Assuncao, R. Occupational Exposure to Mycotoxins-Different Sampling Strategies Telling a Common Story Regarding Occupational Studies Performed in Portugal (2012–2020). Toxins 2020, 12, 513. [Google Scholar] [CrossRef] [PubMed]
- Wogan, G.N. Aflatoxins as risk factors for hepatocellular carcinoma in humans. Cancer Res. 1992, 1, 2114s–2118s. [Google Scholar]
- Sotomayor, R.E.; Washington, M.; Nguyen, L.; Nyang’anyi, R.; Hinton, D.M.; Chou, M. Effects of intermittent exposure to aflatoxin B1 on DNA and RNA adduct formation in rat liver: Dose-response and temporal patterns. Toxicol. Sci. 2003, 73, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Lunn, R.M.; Zhang, Y.J.; Wang, L.Y.; Chen, C.J.; Lee, P.H.; Lee, C.S.; Tsai, W.Y.; Santella, R.M. p53 mutations, chronic hepatitis B virus infection, and aflatoxin exposure in hepatocellular carcinoma in Taiwan. Cancer Res. 1997, 57, 3471–3477. [Google Scholar] [PubMed]
- Chu, Y.J.; Yang, H.I.; Wu, H.C.; Lee, M.H.; Liu, J.; Wang, L.Y.; Lu, S.N.; Jen, C.L.; You, S.L.; Santella, R.M.; et al. Aflatoxin B1 exposure increases the risk of hepatocellular carcinoma associated with hepatitis C virus infection or alcohol consumption. Eur. J. Cancer 2018, 94, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Mycotoxin Control in Low- and Middle-Income Countries. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Working-Group-Reports/Mycotoxin-Control-In-Low-And-Middle-income-Countries-2015 (accessed on 21 December 2021).
- Giorni, P.; Magan, N.; Pietri, A.; Bertuzzi, T.; Battilani, P. Studies on Aspergillus section Flavi isolated from maize in northern Italy. Int. J. Food. Microbiol. 2007, 113, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Risk Assessment of Aflatoxins in Food. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2020.6040 (accessed on 21 December 2021).
- Guyton, K.Z.; Rusyn, I.; Chiu, W.A.; Corpet, D.E.; van den Berg, M.; Ross, M.K.; Christiani, D.C.; Beland, F.A.; Smith, M.T. Application of the key characteristics of carcinogens in cancer hazard identification. Carcinogenesis 2018, 39, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Fornari, F.; Milazzo, M.; Chieco, P.; Negrini, M.; Marasco, E.; Capranico, G.; Mantovani, V.; Marinello, J.; Sabbioni, S.; Callegari, E.; et al. In hepatocellular carcinoma miR-519d is up-regulated by p53 and DNA hypomethylation and targets CDKN1A/p21, PTEN, AKT3 and TIMP2. J. Pathol. 2012, 227, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, W.; Deng, M.; Liu, D.; Ma, Q.; Feng, X. Immunohistochemical Determination of p53 Protein Overexpression for Predicting p53 Gene Mutations in Hepatocellular Carcinoma: A Meta-Analysis. PLoS ONE 2016, 11, e0159636. [Google Scholar] [CrossRef] [PubMed]
- Shirabe, K.; Toshima, T.; Taketomi, A.; Taguchi, K.; Yoshizumi, T.; Uchiyama, H.; Harimoto, N.; Kajiyama, K.; Egashira, A.; Maehara, Y. Hepatic aflatoxin B1-DNA adducts and TP53 mutations in patients with hepatocellular carcinoma despite low exposure to aflatoxin B1 in southern Japan. Liver Int. 2011, 31, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Gallo, P.; Imbimbo, S.; Alvino, S.; Castellano, V.; Arace, O.; Soprano, V.; Esposito, M.; Serpe, F.; Sansone, D. Contamination by Aflatoxins B/G in Food and Commodities Imported in Southern Italy from 2017 to 2020: A Risk-Based Evaluation. Toxins 2021, 13, 368. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, B.G.; Bhat, N.K.; Avadhani, N.G. Preferential attack of mitochondrial DNA by aflatoxin B1 during hepatocarcinogenesis. Science 1982, 215, 73–75. [Google Scholar] [CrossRef]
- Shamsuddin, A.M.; Harris, C.C.; Hinzman, M.J. Localization of aflatoxin B1--nucleic acid adducts in mitochondria and nuclei. Carcinogenesis 1987, 8, 109–114. [Google Scholar] [CrossRef]
- Aguilar, F.; Hussain, S.P.; Cerutti, P. Aflatoxin B1 induces the transversion of G-T in codon 249 of the p53 tumor suppressor gene in human hepatocytes. Proc. Natl. Acad. Sci. USA 1993, 90, 8586–8590. [Google Scholar] [CrossRef] [Green Version]
- Smela, M.E.; Currier, S.S.; Bailey, E.A.; Essigmann, J.M. The chemistry and biology of aflatoxin B1: From mutational spectrometry to carcinogenesis. Carcinogenesis 2001, 22, 535–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulla, J.E.; Chen, Z.Y.; Eaton, D.L. Aflatoxin B1-induced Rat Hepatic Hyperplastic Nodules Do Not Exhibit a Site-specific Mutation within the p53 gene. Cancer Res. 1993, 53, 9–11. [Google Scholar] [PubMed]



| DNA-Adducts Positive | DNA-Adducts Negative | Significance | |
|---|---|---|---|
| Hepatocarcinoma (N = 131) | 25/131 (19.1%) | 106/131 (80.9%) | Chi-square, p = 0.048 |
| male | 16 (64%) | 89 (84%) | Chi-square, p = 0.07 |
| female | 9 (36%) | 17 (16%) | Chi-square, n.s. |
| TP53 accumulation | 12 (48%) | 29 (27.4%) | |
| Viral infection | 21 (84%) | 79 (74.5%) | |
| Non viral | 4 (16%) | 27 (25.5%) | |
| HCV | 14 (56%) | 49 (46.2%) | |
| HBV | 5 (20%) | 17 (16%) | |
| HBV + HCV | 1 (4%) | 6 (5.7%) | |
| HBV + HDV | 1 (4%) | 0 | |
| metabolic | 3 (12%) | 18 (17%) | |
| alcohol | 1 (4%) | 9 (8.5%) | |
| HCV + Previous HBV | 0 (0%) | 7 (6.6%) | |
| Smoking history | 1/18 * (5.5%) | 12/71 * (16.9%) | Chi-square, n.s. |
| Cholangiocarcinoma (N = 45) | 0 | 45 | |
| male | 21 | ||
| female | 24 | ||
| TP53 accumulation | 14 (31%) | ||
| Non viral | 40 (88.9%) | ||
| HCV | 5 (11.1%) | ||
| HBV | 0 | ||
| Smoking history | 8/29 * | ||
| Liver transplantation (N = 20) | 1/20 | 19/20 | |
| male | 1 | 13 | |
| female | 0 | 6 | |
| TP53 accumulation | 0 | 0 | |
| HCV | 1 | 11 | |
| HBV | 0 | 3 | |
| HBV + HCV | 0 | 1 | |
| metabolic | 0 | 3 | |
| alcohol | 0 | 1 | |
| Smoking history | 0/1 | 3/19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gramantieri, L.; Gnudi, F.; Vasuri, F.; Mandrioli, D.; Fornari, F.; Tovoli, F.; Suzzi, F.; Vornoli, A.; D’Errico, A.; Piscaglia, F.; et al. Aflatoxin B1 DNA-Adducts in Hepatocellular Carcinoma from a Low Exposure Area. Nutrients 2022, 14, 1652. https://doi.org/10.3390/nu14081652
Gramantieri L, Gnudi F, Vasuri F, Mandrioli D, Fornari F, Tovoli F, Suzzi F, Vornoli A, D’Errico A, Piscaglia F, et al. Aflatoxin B1 DNA-Adducts in Hepatocellular Carcinoma from a Low Exposure Area. Nutrients. 2022; 14(8):1652. https://doi.org/10.3390/nu14081652
Chicago/Turabian StyleGramantieri, Laura, Federica Gnudi, Francesco Vasuri, Daniele Mandrioli, Francesca Fornari, Francesco Tovoli, Fabrizia Suzzi, Andrea Vornoli, Antonia D’Errico, Fabio Piscaglia, and et al. 2022. "Aflatoxin B1 DNA-Adducts in Hepatocellular Carcinoma from a Low Exposure Area" Nutrients 14, no. 8: 1652. https://doi.org/10.3390/nu14081652