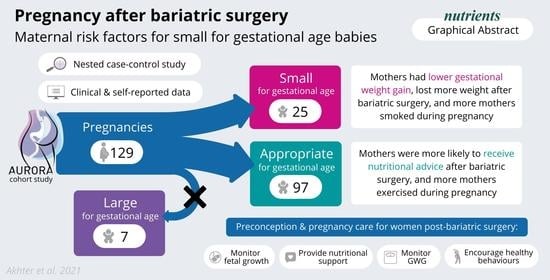
Pregnancy after Bariatric Surgery: A Nested Case-Control Study of Risk Factors for Small for Gestational Age Babies in AURORA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Case and Control Definitions
2.3. Maternal Factors
2.4. Statistical Analysis
3. Results
3.1. Maternal and Infant Characteristics
3.2. Modifiable Factors
3.3. Clinical Indicators
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchi, J.; Berg, M.; Dencker, A.; Olander, E.; Begley, C. Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obes. Rev. 2015, 16, 621–638. [Google Scholar] [CrossRef]
- Slack, E.; Best, K.E.; Rankin, J.; Heslehurst, N. Maternal obesity classes, preterm and post-term birth: A retrospective analysis of 479,864 births in England. BMC Pregnancy Childbirth 2019, 19, 434. [Google Scholar] [CrossRef]
- Stothard, K.J.; Tennant, P.W.G.; Bell, R.; Rankin, J. Maternal Overweight and Obesity and the Risk of Congenital Anomalies: A Systematic Review and Meta-analysis. JAMA 2009, 301, 636–650. [Google Scholar] [CrossRef]
- Edison, E.; Whyte, M.; van Vlymen, J.; Jones, S.; Gatenby, P.; de Lusignan, S.; Shawe, J. Bariatric Surgery in Obese Women of Reproductive Age Improves Conditions That Underlie Fertility and Pregnancy Outcomes: Retrospective Cohort Study of UK National Bariatric Surgery Registry (NBSR). Obes. Surg. 2016, 26, 2837–2842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwong, W.; Tomlinson, G.; Feig, D.S. Maternal and neonatal outcomes after bariatric surgery; a systematic review and meta-analysis: Do the benefits outweigh the risks? Am. J. Obs. Gynecol. 2018, 218, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Maggard, M.A.; Yermilov, I.; Li, Z.; Maglione, M.; Newberry, S.; Suttorp, M.; Hilton, L.; Santry, H.P.; Morton, J.M.; Livingston, E.H.; et al. Pregnancy and Fertility Following Bariatric Surgery: A Systematic Review. JAMA 2008, 300, 2286–2296. [Google Scholar] [CrossRef] [PubMed]
- Gascoin, G.; Gerard, M.; Sallé, A.; Becouarn, G.; Rouleau, S.; Sentilhes, L.; Coutant, R. Risk of low birth weight and micronutrient deficiencies in neonates from mothers after gastric bypass: A case control study. Surg. Obes. Relat. Dis. 2017, 13, 1384–1391. [Google Scholar] [CrossRef] [Green Version]
- Akhter, Z.; Rankin, J.; Ceulemans, D.; Ngongalah, L.; Ackroyd, R.; Devlieger, R.; Vieira, R.; Heslehurst, N. Pregnancy after bariatric surgery and adverse perinatal outcomes: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002866. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Obstetricians and Gynaecologists. The investigation and management of the small-for-gestational age fetus. In RCOG Green-Top Guidelines; Royal College of Obstetricians and Gynaecologists: London, UK, 2013. [Google Scholar]
- Lees, C.C.; Stampalija, T.; Baschat, A.A.; da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef]
- Belkacemi, L.; Nelson, D.M.; Desai, M.; Ross, M.G. Maternal Undernutrition Influences Placental-Fetal Development. Biol. Reprod. 2010, 83, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Bukowski, R.; Hansen, N.I.; Willinger, M.; Reddy, U.M.; Parker, C.B.; Pinar, H.; Silver, R.M.; Dudley, D.J.; Stoll, B.J.; Saade, G.R.; et al. Fetal Growth and Risk of Stillbirth: A Population-Based Case–Control Study. PLoS Med. 2014, 11, e1001633. [Google Scholar] [CrossRef] [PubMed]
- McIntire, D.D.; Bloom, S.L.; Casey, B.M.; Leveno, K.J. Birth Weight in Relation to Morbidity and Mortality among Newborn Infants. N. Engl. J. Med. 1999, 340, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Savchev, S.; Sanz-Cortes, M.; Cruz-Martinez, R.; Arranz, A.; Botet, F.; Gratacos, E.; Figueras, F. Neurodevelopmental outcome of full-term small-for-gestational-age infants with normal placental function. Ultrasound Obstet. Gynecol. 2013, 42, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, P.G.; Molin, J. Does antenatal identification of small-for-gestational age fetuses significantly improve their outcome? Ultrasound Obstet. Gynecol. 2005, 25, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Jans, G.; Matthys, C.; Bel, S.; Ameye, L.; Lannoo, M.; Van der Schueren, B.; Dillemans, B.; Lemmens, L.; Saey, J.-P.; van Nieuwenhove, Y.; et al. AURORA: Bariatric surgery registration in women of reproductive age—A multicenter prospective cohort study. BMC Pregnancy Childbirth 2016, 16, 195. [Google Scholar] [CrossRef] [Green Version]
- Devlieger, R.; Martens, E.; Martens, G.; Van Mol, C.; Cammu, H. Perinatale Activiteiten in Vlaanderen 2015; SPE: Brussels, Belgium, 2016. [Google Scholar]
- National Research Council. Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef]
- Benhalima, K.; Minschart, C.; Ceulemans, D.; Bogaerts, A.; Van Der Schueren, B.; Mathieu, C.; Devlieger, R. Screening and Management of Gestational Diabetes Mellitus after Bariatric Surgery. Nutrients 2018, 10, 1479. [Google Scholar] [CrossRef] [Green Version]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Strobe Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, S.D.; Han, Z.; Mulla, S.; Murphy, K.E.; Beyene, J.; Ohlsson, A. Preterm birth and low birth weight among in vitro fertilization singletons: A systematic review and meta-analyses. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 146, 138–148. [Google Scholar] [CrossRef]
- Abbassi-Ghanavati, M.; Greer, L.G.; Cunningham, F.G. Pregnancy and Laboratory Studies: A Reference Table for Clinicians. Obstet. Gynecol. 2009, 114, 1326–1331. [Google Scholar] [CrossRef]
- Ceulemans, D.; De Mulder, P.; Lebbe, B.; Coppens, M.; De Becker, B.; Dillemans, B.; Saey, J.-P.; Lemmens, L.; Logghe, H.; Loccufier, A. Gestational weight gain and postpartum weight retention after bariatric surgery: Data from a prospective cohort study. Surg. Obes. Relat. Dis. 2020, 17, 659–666. [Google Scholar] [CrossRef]
- Shawe, J.; Ceulemans, D.; Akhter, Z.; Neff, K.; Hart, K.; Heslehurst, N.; Štotl, I.; Agrawal, S.; Steegers-Theunissen, R.; Taheri, S.; et al. Pregnancy after bariatric surgery: Consensus recommendations for periconception, antenatal and postnatal care. Obes. Rev. 2019, 20, 1507–1522. [Google Scholar] [CrossRef] [Green Version]
- Jans, G.; Guelinckx, I.; Voets, W.; Galjaard, S.; Van Haard, P.M.; Vansant, G.M.; Devlieger, R. Vitamin K1 monitoring in pregnancies after bariatric surgery: A prospective cohort study. Surg. Obes. Relat. Dis. 2014, 10, 885–890. [Google Scholar] [CrossRef]
- Conason, A.; Teixeira, J.; Hsu, C.-H.; Puma, L.; Knafo, D.; Geliebter, A. Substance Use Following Bariatric Weight Loss Surgery. Jama Surg. 2013, 148, 145–150. [Google Scholar] [CrossRef]
- Jauniaux, E.; Burton, G.J. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Hum. Dev. 2007, 83, 699–706. [Google Scholar] [CrossRef]
- Son, J.S.; Liu, X.; Tian, Q.; Zhao, L.; Chen, Y.; Hu, Y.; Chae, S.A.; de Avila, J.M.; Zhu, M.-J.; Du, M. Exercise prevents the adverse effects of maternal obesity on placental vascularization and fetal growth. J. Physiol. 2019, 597, 3333–3347. [Google Scholar] [CrossRef] [PubMed]
- Juhl, M.; Olsen, J.; Andersen, P.K.; Nøhr, E.A.; Andersen, A.-M.N. Physical exercise during pregnancy and fetal growth measures: A study within the Danish National Birth Cohort. Am. J. Obstet. Gynecol. 2010, 202, 63.e61–63.e68. [Google Scholar] [CrossRef] [PubMed]
- Clapp, J.F.; Kim, H.; Burciu, B.; Schmidt, S.; Petry, K.; Lopez, B. Continuing regular exercise during pregnancy: Effect of exercise volume on fetoplacental growth. Am. J. Obstet. Gynecol. 2002, 186, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Han, S.; Zhu, J.; Sun, X.; Ji, C.; Guo, X. Pre-Pregnancy Body Mass Index in Relation to Infant Birth Weight and Offspring Overweight/Obesity: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e61627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bramham, K.; Parnell, B.; Nelson-Piercy, C.; Seed, P.T.; Poston, L.; Chappell, L.C. Chronic hypertension and pregnancy outcomes: Systematic review and meta-analysis. BMJ 2014, 348, g2301. [Google Scholar] [CrossRef] [Green Version]
- Stentebjerg, L.L.; Andersen, L.L.T.; Renault, K.; Støving, R.K.; Jensen, D.M. Pregnancy and perinatal outcomes according to surgery to conception interval and gestational weight gain in women with previous gastric bypass. J. Matern. Fetal Neonatal Med. 2017, 30, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Rottenstreich, A.; Elazary, R.; Ezra, Y.; Kleinstern, G.; Beglaibter, N.; Elchalal, U. Hypoglycemia during oral glucose tolerance test among post–bariatric surgery pregnant patients: Incidence and perinatal significance. Surg. Obes. Relat. Dis. 2018, 14, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Roser, M.; Appel, C.; Ritchie, H. Human Height. Available online: https://ourworldindata.org/human-height (accessed on 19 August 2020).



| Pre-Pregnancy BMI | Total Weight Gain Range (kg) |
|---|---|
| Underweight (<18.5 kg/m2) | 12.5–18 |
| Recommended (18.5–25 kg/m2) | 11.5–16 |
| Overweight (25–30 kg/m2) | 7–11.5 |
| Obesity (>30 kg/m2) | 5–9 |
| Infant Characteristics | Total n = 122 | SGA n = 25 (20.5%) | AGA n = 97 (79.5%) | p Value |
|---|---|---|---|---|
| Sex | ||||
| Boys | 69 (56.6%) | 15 (60.0%) | 54 (55.7%) | 0.697 |
| Girls | 53 (43.4%) | 10 (40.0%) | 43 (44.3%) | |
| Birth weight | ||||
| Mean ± SD | 3064.1 ± 535.5 | 2594.4 ± 328.0 | 3185.2 ± 512.1 | 0.000 |
| <2500 g | 16 (13.0%) | 9 (36.0%) | 7 (7.2%) | 0.002 |
| 2500–4000 g | 103 (84.4%) | 16 (64%) | 87 (89.7%) | |
| ≥4000 g | 3 (2.5%) | 0 | 3 (3.1%) | |
| Gestational age | ||||
| Mean ± SD | 38.4 ± 1.9 | 38.6 ± 1.6 | 38.3 ± 2.0 | 0.696 |
| <37 weeks | 19 (15.6%) | 4 (16.0%) | 15 (15.5%) | 1.000 |
| 37–42 weeks | 103 (84.4%) | 21 (84.0%) | 82 (84.5%) | |
| ≥42 weeks | 0 | 0 | 0 |
| Maternal Characteristics | Total n = 122 | SGA n = 25 | AGA n = 97 | p Value |
|---|---|---|---|---|
| Age at delivery, years Mean ± SD | 30.1 ± 4.6 | 30.4 ± 4.7 | 30 ± 4.5 | 0.727 |
| Height, cm Mean ± SD | 165.5 ± 6.9 | 164.1 ± 5.7 | 165.8 ± 7.2 | 0.267 |
| Parity | ||||
| Nulliparous | 38 (31.2%) | 8 (32%) | 30 (30.9%) | 0.586 |
| Multiparous | 77 (63.1%) | 13 (52%) | 64 (66%) | |
| Preconception BMI, kg/m2 | ||||
| Underweight <18.5 | 2 (1.6%) | 2 (8%) | 0 | 0.193 |
| Recommended 18.5–24.9 | 34 (27.9%) | 7 (28%) | 27 (27.8%) | |
| Overweight 25.0–29.9 | 46 (37.7%) | 9 (36%) | 37 (38.1%) | |
| Obesity class I 30–34.9 | 24 (19.7%) | 6 (24%) | 18 (18.6%) | |
| Obesity class II 35–39.9 | 14 (11.5%) | 1 (0.4%) | 13 (13.4%) | |
| Obesity class III ≥ 40 | 1 (0.8%) | 0 | 1 (1%) | |
| Pre-surgery BMI, kg/m2 | ||||
| Overweight 25.0–29.9 | 1 (0.9%) | 0 | 1 (1%) | 0.477 |
| Obesity class I 30–434.9 | 7 (5.7%) | 0 | 7 (7.1%) | |
| Obesity class II 35–39.9 | 29 (23.8%) | 8 (32%) | 21 (21.6%) | |
| Obesity class III ≥ 40 | 77 (63.1%) | 16 (64%) | 61 (62.9%) | |
| Ethnicity | ||||
| White European | 63 (51.6%) | 13 (52%) | 50 (51.5%) | 1.000 |
| Other | 8 (6.6%) | 1 (4%) | 7 (7.2%) | |
| Marital status | ||||
| Married | 38 (31.2%) | 3 (12%) | 35 (36.1%) | 0.046 |
| Single | 29 (23.8%) | 8 (32%) | 21 (21.6%) | |
| Education level | ||||
| Higher education | 20 (16.4%) | 1 (4%) | 19 (19.6%) | 0.153 |
| Secondary or below | 47 (38.5%) | 10 (40%) | 37 (38.1%) | |
| Employment status | ||||
| Full or part-time work | 38 (31.2%) | 3 (12%) | 35 (36.1%) | 0.046 |
| Unemployed | 29 (23.8%) | 8 (32%) | 21 (21.6%) | |
| Method of conception | ||||
| Spontaneous | 105 (86.1%) | 25 (100%) | 80 (82.5%) | 0.039 |
| Fertility treatment | 14 (11.5%) | 0 | 14 (14.4%) | |
| Type of bariatric surgery | ||||
| RYGB | 89 (73.0%) | 20 (80%) | 69 (71.1%) | 0.962 |
| LAGB | 19 (15.6%) | 3 (12%) | 16 (16.5%) | |
| SG | 9 (7.4%) | 2 (8%) | 7 (7.2%) | |
| BPD | 3 (2.5%) | 0 | 3 (3.1%) |
| Modifiable Factors | Total n = 122 | SGA n = 25 | AGA n = 97 | Sig. Test p Value | OR (95% CI) | p Value |
|---|---|---|---|---|---|---|
| GWG, kg Mean ± SD | 12.3 ± 6.5 | 9.8 ± 7.1 | 13.0 ± 6.2 | 0.023 | 0.92 (0.85–0.99) | 0.029 |
| IOM GWG guidelines | ||||||
| Inadequate | 27 (22.1%) | 11 (44.0%) | 16 (16.5%) | 0.012 | 2.55 (0.82–7.93) | 0.105 |
| Recommended | 33 (27.0%) | 7 (28.0%) | 26 (26.8%) | Reference group | - | |
| Excessive | 57 (46.7%) | 7 (28.0%) | 50 (51.5%) | 0.52 (0.16–1.64) | 0.265 | |
| Nutritional advice | ||||||
| Yes | 58 (47.5%) | 8 (32.0%) | 50 (51.5%) | 0.004 | 0.14 (0.04–0.51) | 0.003 |
| No | 13 (10.7%) | 7 (28.0%) | 6 (6.2%) | Reference group | - | |
| Smoking in pregnancy | ||||||
| Yes | 10 (8.2%) | 4 (16.0%) | 6 (6.2%) | 0.082 | 3.83 (0.88–16.69) | 0.073 |
| No | 54 (44.3%) | 8 (32.0%) | 46 (47%) | Reference group | - | |
| Sports and exercise | ||||||
| More than once a month | 24 (19.7%) | 2 (8.0%) | 22 (22.7%) | 0.123 | 0.29 (0.06–1.49) | 0.14 |
| Once a month or less | 38 (31.1%) | 9 (26.0%) | 29 (30.0%) | Reference group | - |
| Serum Levels | Reference Range * | AURORA Range (N) | SGA Mean ± SD | AGA Mean ± SD | p Value |
|---|---|---|---|---|---|
| Haemoglobin (g/dL) | 9.7–14.7 | 8.7–13.9 (110) | 11.3 ± 1.3 | 11.4 ± 1.1 | 0.851 |
| RBC count (106/μL) | 2.81–4.49 | 3.12–4.94 (100) | 3.87 ± 0.35 | 3.90 ± 0.34 | 0.777 |
| Iron (μg/dL) | 44–178 | 24–169 (49) | 81.1 ± 41.0 | 90.1 ± 38.4 | 0.517 |
| Folate (ng/mL) | 0.8–24 | 4.4–19.7 (77) | 15.9 ± 5.0 | 14.3 ± 5.1 | 0.245 |
| Vitamin B12 (ng/L) | 130–656 | 77–760 (103) | 207.8 ± 95.2 | 236.9 ± 110.6 | 0.232 |
| Calcium (mmol/L) | 2.05–2.25 | 2.08–2.39 (34) | 2.22 ± 0.08 | 2.26 ± 0.08 | 0.276 |
| Vitamin D 25-OH (ng/mL) | 10–22 | 4.5–68 (87) | 33.2 ± 15.3 | 29.1 ± 13.3 | 0.373 |
| Albumin (g/L) | 25–45 | 29.8–41.4 (76) | 36.8 ± 2.6 | 37.1 ± 2.3 | 0.951 |
| Clinical Indicators | Total n = 122 | SGA n = 25 | AGA n = 97 | Sig. Test p Value | OR (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Preconception BMI, kg/m2 | ||||||
| Mean ± SD | 28.3 ± 5.1 | 26.4 ± 5.0 | 28.8 ± 5.1 | 0.066 | ||
| per 1 kg/m2 increase | 0.90 (0.81–0.99) | 0.038 | ||||
| per 5 kg/m2 increase | 0.58 (0.35–0.97) | 0.038 | ||||
| Gestational diabetes | ||||||
| Yes | 28 (23%) | 4 (16%) | 24 (24.7%) | 0.541 | 0.69 (0.21–2.29) | 0.543 |
| No | 77 (63.1%) | 15 (60%) | 62 (63.9%) | Reference group | - | |
| Hypertension | ||||||
| Yes | 7 (5.7%) | 0 | 7 (7.2%) | 0.342 | * 0.24 (0.01–4.37) | 0.336 |
| No | 112 (91.8%) | 24 (96%) | 88 (90.7%) | Reference group | - | |
| Type of surgery | ||||||
| RYGB/BPD | 92 (76.7%) | 20 (80%) | 72 (74.2%) | 0.658 | 1.28 (0.43–3.79) | 0.658 |
| LAGB/SG | 28 (23.3%) | 5 (20%) | 23 (23.7%) | Reference group | - | |
| Surgery to conception interval, months Mean ± SD | 49.8 ± 37.5 | 48.9 ± 34.0 | 50.0 ± 38.5 | 0.856 | 1.00 (0.99–1.01) | 0.890 |
| Weight loss from surgery to conception, kg Mean ± SD | 40.4 ± 17.4 | 45.6 ± 14.4 | 39.0 ± 17.9 | 0.057 | 1.02 (1.00–1.05) | 0.100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhter, Z.; Heslehurst, N.; Ceulemans, D.; Rankin, J.; Ackroyd, R.; Devlieger, R. Pregnancy after Bariatric Surgery: A Nested Case-Control Study of Risk Factors for Small for Gestational Age Babies in AURORA. Nutrients 2021, 13, 1699. https://doi.org/10.3390/nu13051699
Akhter Z, Heslehurst N, Ceulemans D, Rankin J, Ackroyd R, Devlieger R. Pregnancy after Bariatric Surgery: A Nested Case-Control Study of Risk Factors for Small for Gestational Age Babies in AURORA. Nutrients. 2021; 13(5):1699. https://doi.org/10.3390/nu13051699
Chicago/Turabian StyleAkhter, Zainab, Nicola Heslehurst, Dries Ceulemans, Judith Rankin, Roger Ackroyd, and Roland Devlieger. 2021. "Pregnancy after Bariatric Surgery: A Nested Case-Control Study of Risk Factors for Small for Gestational Age Babies in AURORA" Nutrients 13, no. 5: 1699. https://doi.org/10.3390/nu13051699