The Association between Maternal Stress and Glucocorticoid Rhythmicity in Human Milk
Abstract
:1. Introduction
2. Materials and Methods
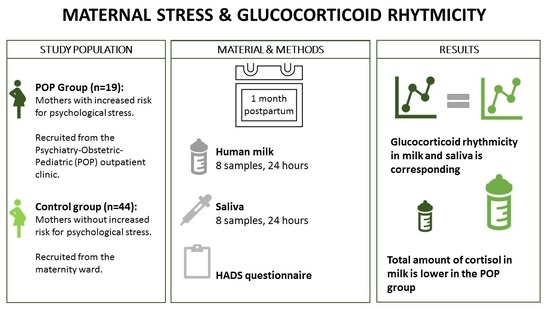
2.1. Study Design and Participants
2.2. Data Collection
2.3. Laboratory Analysis
2.4. Statistical Analysis
2.4.1. Glucocorticoid Rhythmicity Analysis
2.4.2. Other Analyses
3. Results
3.1. Patient Characteristics
3.2. Glucocorticoid Rhythmicity
3.3. Glucocorticoid Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grummer-Strawn, L.M.; Zehner, E.; Stahlhofer, M.; Lutter, C.; Clark, D.; Sterken, E. New World Health Organization guidance helps protect breastfeeding as a human right. Matern. Child Nutr. 2017, 13, e12491. [Google Scholar] [CrossRef] [Green Version]
- Iellamo, A.; Sobel, H.; Engelhardt, K. Working mothers of the World Health Organization Western Pacific offices: Lessons and experiences to protect, promote, and support breastfeeding. J. Hum. Lact 2015, 31, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Garwolinska, D.; Namieśnik, J.; Kot-Wasik, A.; Hewelt-Belka, W. Chemistry of Human Breast Milk-A Comprehensive Review of the Composition and Role of Milk Metabolites in Child Development. J. Agric. Food Chem. 2018, 66, 11881–11896. [Google Scholar] [CrossRef] [PubMed]
- Hollanders, J.J.; Heijboer, A.C.; van der Voorn, B.; Rotteveel, J.; Finken, M.J. Nutritional programming by glucocorticoids in breast milk: Targets, mechanisms and possible implications. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Thau, L.; Gandhi, J.; Sharma, S. Physiology, Cortisol; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Son, G.H.; Cha, H.K.; Chung, S.; Kim, K. Multimodal Regulation of Circadian Glucocorticoid Rhythm by Central and Adrenal Clocks. J. Endocr. Soc. 2018, 2, 444–459. [Google Scholar] [CrossRef]
- Toorop, A.A.; van der Voorn, B.; Hollanders, J.J.; Dijkstra, L.R.; Dolman, K.M.; Heijboer, A.C. Diurnal rhythmicity in breast-milk glucocorticoids, and infant behavior and sleep at age 3 months. Endocrine 2020, 68, 660–668. [Google Scholar] [CrossRef] [PubMed]
- van der Voorn, B.; de Waard, M.; van Goudoever, J.B.; Rotteveel, J.; Heijboer, A.C.; Finken, M.J. Breast-Milk Cortisol and Cortisone Concentrations Follow the Diurnal Rhythm of Maternal Hypothalamus-Pituitary-Adrenal Axis Activity. J. Nutr. 2016, 146, 2174–2179. [Google Scholar] [CrossRef] [Green Version]
- Lindberg, M.; Nolvi, S.; Härkönen, J.; Aatsinki, A.K.; Karlsson, L.; Karlsson, H.; Uusitupa, H.M. Associations between maternal socioeconomic, psychosocial and seasonal factors, infant characteristics and human milk cortisol concentrations. Am. J. Hum. Biol. 2021, e23561. [Google Scholar]
- Hollanders, J.; Dijkstra, L.R.; van der Voorn, B.; Kouwenhoven, S.M.; Toorop, A.A.; van Goudoever, J.B.; Finken, M.J. No Association between Glucocorticoid Diurnal Rhythm in Breastmilk and Infant Body Composition at 3 Months. Nutrients 2019, 11, 2351. [Google Scholar] [CrossRef] [Green Version]
- Pundir, S.; Finken, M.J.; Mäkelä, J.; Nuora, A.; Junttila, N.; Wall, C.R.; Linderborg, K.; Lagström, H. Maternal influences on the glucocorticoid concentrations of human milk: The STEPS study. Clin. Nutr. 2019, 38, 1913–1920. [Google Scholar] [CrossRef]
- Aparicio, M.; Browne, P.D.; Hechler, C.; Beijers, R.; Rodríguez, J.M.; de Weerth, C.; Fernández, L. Human milk cortisol and immune factors over the first three postnatal months: Relations to maternal psychosocial distress. PLoS ONE 2020, 15, e0233554. [Google Scholar] [CrossRef]
- Vreeburg, S.A.; Hoogendijk, W.J.; DeRijk, R.H.; van Dyck, R.; Smit, J.H.; Zitman, F.G.; Penninx, B.W. Salivary cortisol levels and the 2-year course of depressive and anxiety disorders. Psychoneuroendocrinology 2013, 38, 1494–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bremmer, M.A.; Deeg, D.J.; Beekman, A.T.; Penninx, B.W.; Lips, P.; Hoogendijk, W.J. Major depression in late life is associated with both hypo- and hypercortisolemia. Biol. Psychiatry 2007, 62, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W.; Beekman, A.T.; Corsi, A.M.; Bremmer, M.; Hoogendijk, W.J.; Guralnik, J.M.; Bandinelli, S. Late-life depressive symptoms are associated with both hyperactivity and hypoactivity of the hypothalamo-pituitary-adrenal axis. Am. J. Geriatr. Psychiatry 2007, 15, 522–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarcho, M.R.; Slavich, G.M.; Tylova-Stein, H.; Wolkowitz, O.M.; Burke, H.M. Dysregulated diurnal cortisol pattern is associated with glucocorticoid resistance in women with major depressive disorder. Biol. Psychol. 2013, 93, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Zorn, J.V.; Schür, R.R.; Boks, M.P.; Kahn, R.S.; Joëls, M.; Vinkers, C.H. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 77, 25–36. [Google Scholar] [CrossRef]
- Knorr, U.; Vinberg, M.; Kessing, L.V.; Wetterslev, J. Salivary cortisol in depressed patients versus control persons: A systematic review and meta-analysis. Psychoneuroendocrinology 2010, 35, 1275–1286. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- van der Voorn, B.; Martens, F.; Peppelman, N.S.; Rotteveel, J.; Blankenstein, M.A.; Finken, M.J.; Heijboer, A.C. Determination of cortisol and cortisone in human mother’s milk. Clin. Chim. Acta 2015, 444, 154–155. [Google Scholar] [CrossRef]
- Pruessner, J.C.; Kirschbaum, C.; Meinlschmid, G.; Hellhammer, D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003, 28, 916–931. [Google Scholar] [CrossRef]
- Seth, S.; Lewis, A.J.; Galbally, M. Perinatal maternal depression and cortisol function in pregnancy and the postpartum period: A systematic literature review. BMC Pregnancy Childbirth 2016, 16, 124. [Google Scholar] [CrossRef] [Green Version]
- Groer, M.W.; Morgan, K. Immune, health and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrinology 2007, 32, 133–139. [Google Scholar] [CrossRef]
- Tsubouchi, H.; Nakai, Y.; Toda, M.; Morimoto, K.; Chang, Y.S.; Ushioda, N.; Shimoya, K. Change of salivary stress marker concentrations during pregnancy: Maternal depressive status suppress changes of those levels. J. Obstet. Gynaecol. Res. 2011, 37, 1004–1009. [Google Scholar] [CrossRef]
- van der Voorn, B.; Hollanders, J.J.; Kieviet, N.; Dolman, K.M.; de Rijke, Y.B.; van Rossum, E.F.; Finken, M.J. Maternal Stress During Pregnancy Is Associated with Decreased Cortisol and Cortisone Levels in Neonatal Hair. Horm. Res. Paediatr. 2018, 90, 299–307. [Google Scholar] [CrossRef]
- Sullivan, E.C.; Hinde, K.; Mendoza, S.P.; Capitanio, J.P. Cortisol concentrations in the milk of rhesus monkey mothers are associated with confident temperament in sons, but not daughters. Dev. Psychobiol. 2011, 53, 96–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poscic, N.; Gabai, G.; Stefanon, B.; Da Dalt, L.; Sgorlon, S. Milk cortisol response to group relocation in lactating cows. J. Dairy Res. 2017, 84, 36–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stalder, T.; Kirschbaum, C.; Kudielka, B.M.; Adam, E.K.; Pruessner, J.C.; Wüst, S.; Clow, A. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology 2016, 63, 414–432. [Google Scholar] [CrossRef]
- Salacz, P.; Csukly, G.; Haller, J.; Valent, S. Association between subjective feelings of distress, plasma cortisol, anxiety, and depression in pregnant women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 165, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Glover, V.; Marks, M.; Kammerer, M. Diurnal pattern of cortisol output in postnatal depression. Psychoneuroendocrinology 2009, 34, 1184–1188. [Google Scholar] [CrossRef]
- Dedovic, K.; Engert, V.; Duchesne, A.; Lue, S.D.; Andrews, J.; Efanov, S.I.; Pruessner, J.C. Cortisol awakening response and hippocampal volume: Vulnerability for major depressive disorder? Biol. Psychiatry 2010, 68, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Dedovic, K.; Ngiam, J. The cortisol awakening response and major depression: Examining the evidence. Neuropsychiatr. Dis. Treat. 2015, 11, 1181–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangold, D.; Marino, E.; Javors, M. The cortisol awakening response predicts subclinical depressive symptomatology in Mexican American adults. J. Psychiatr. Res. 2011, 45, 902–909. [Google Scholar] [CrossRef] [Green Version]
- Ronaldson, A.; Carvalho, L.A.; Kostich, K.; Lazzarino, A.I.; Urbanova, L.; Steptoe, A. The effects of six-day SSRI administration on diurnal cortisol secretion in healthy volunteers. Psychopharmacology 2018, 235, 3415–3422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manthey, L.; Leeds, C.; Giltay, E.J.; van Veen, T.; Vreeburg, S.A.; Penninx, B.W.; Zitman, F.G. Antidepressant use and salivary cortisol in depressive and anxiety disorders. Eur. Neuropsychopharmacol. 2011, 21, 691–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Total N = 63 | POP Group N = 19 (30%) | Control Group N = 44 (70%) | |
|---|---|---|---|
| Mothers’ age, years—mean (±SD) | 33.7 (±4.6) | 32.5 (±4.6) | 34.6 (±4.6) |
| Gestational age, weeks—mean (±SD) | 39.7 (±1.3) | 39.5 (±1.4) | 39.8 (±1.4) |
| Parity—median [IQR] | 2 [1,2] | 2 [1,2] | 1 [1,2] |
| BMI—mean (±SD) | 22.46 (±2.6) | 22.69 (±2.2) | 22.36 (±2.8) |
| Birth weight, grams—mean (±SD) | 3509 (±479) | 3434 (±436) | 3541 (±497) |
| Cesarean section—number (%) | 26 (42%) | 4 (21%) | 22 (50%) * |
| Antidepressant medication—number (%) | 14 (22%) | 11 (58%) | 2 (5%) |
| HADS score 1 month postpartum HADS-A Score—mean (±SD) HADS-D Score—mean (±SD) | 4.10 (±3.10) 2.66 (±3.10) | 5.58 (±3.69) 4.11 (±4.80) | 3.44 (±2.59) * 2.02 (±1.67) * |
| Socioeconomic status—mean (±SD) | 0.49 (±1.21) | 0.41 (±1.24) | (±1.21) |
| Outcome | POP Group | Control Group | p-Value |
|---|---|---|---|
| Milk Cortisol | Milk Cortisol | ||
| Peak height (nmol/L) | 10.95 (±0.97) | 11.34 (±0.69) | 0.75 |
| Peak width (h) | 1.66 (±0.20) | 2.06 (±0.17) | 0.17 |
| Peak timing (in 2 h) | 3.34 (±0.20) | 3.80 (±0.17) | 0.12 |
| Milk Cortisone | Milk Cortisone | ||
| Peak height (nmol/L) | 27.95 (±1.40) | 28.49 (±0.92) | 0.75 |
| Peak width (h) | 3.41 (±0.29) | 3.74 (±0.21) | 0.37 |
| Peak timing (2 h) | 4.26 (±0.28) | 4.40 (±0.19) | 0.69 |
| Saliva Cortisol | Saliva Cortisol | ||
| Peak height (nmol/L) | 6.65 (±0.83) | 6.11 (±0.50) | 0.57 |
| Peak width (h) | 2.18 (±0.36) | 2.87 (±0.36) | 0.26 |
| Peak timing (2 h) | 3.72 (±0.38) | 4.21 (±0.33) | 0.39 |
| Saliva Cortisone | Saliva Cortisone | ||
| Peak height (nmol/L) | 25.31 (±1.95) | 24.65 (±1.05) | 0.75 |
| Peak width (h) | 3.02 (±0.35) | 3.40 (±0.24) | 0.380 |
| Peak timing (2 h) | 4.52 (±0.34 | 4.62 (±0.21) | 0.80 |
| Variable | POP | Control |
|---|---|---|
| AUCg Cortisol Milk, median [IQR] | 3.42 [2.63–4.59] | 4.73 [3.59–6.10] * |
| AUCg Cortisone Milk, median [IQR] | 16.12 [13.19–19.85] | 17.86 [15.72–21.63] |
| AUCg Cortisol Saliva, median [IQR] | 2.53 [1.78–2.92] | 3.07 [2.32–3.92] |
| AUCg Cortisone Saliva, median [IQR] | 13.49 [10.40–19.14] | 15.34 [12.54–18.80] |
| Glucocorticoid | Study Group | Correlation between Milk and Saliva (r) | 95% CI |
|---|---|---|---|
| Cortisol | POP | 0.841 * | 0.683–1.000 |
| Control | 0.829 * | 0.702–0.956 | |
| POP versus control | 0.070 | −0.140–0.279 | |
| Cortisone | POP | 0.861 * | 0.746–0.975 |
| Control | 0.896 * | 0.819–0.974 | |
| POP versus control | −0.023 | −0.162–0.107 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romijn, M.; van Tilburg, L.J.L.; Hollanders, J.J.; van der Voorn, B.; de Goede, P.; Dolman, K.M.; Heijboer, A.C.; Broekman, B.F.P.; Rotteveel, J.; Finken, M.J.J. The Association between Maternal Stress and Glucocorticoid Rhythmicity in Human Milk. Nutrients 2021, 13, 1608. https://doi.org/10.3390/nu13051608
Romijn M, van Tilburg LJL, Hollanders JJ, van der Voorn B, de Goede P, Dolman KM, Heijboer AC, Broekman BFP, Rotteveel J, Finken MJJ. The Association between Maternal Stress and Glucocorticoid Rhythmicity in Human Milk. Nutrients. 2021; 13(5):1608. https://doi.org/10.3390/nu13051608
Chicago/Turabian StyleRomijn, Michelle, Luca J. L. van Tilburg, Jonneke J. Hollanders, Bibian van der Voorn, Paul de Goede, Koert M. Dolman, Annemieke C. Heijboer, Birit F. P. Broekman, Joost Rotteveel, and Martijn J. J. Finken. 2021. "The Association between Maternal Stress and Glucocorticoid Rhythmicity in Human Milk" Nutrients 13, no. 5: 1608. https://doi.org/10.3390/nu13051608