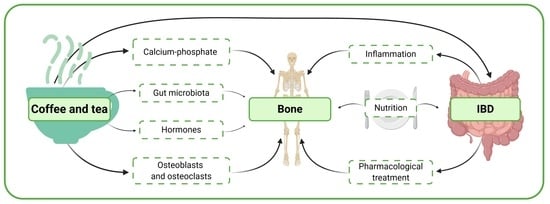
Does Drinking Coffee and Tea Affect Bone Metabolism in Patients with Inflammatory Bowel Diseases?
Abstract
:1. Introduction
2. Caffeine and Tea—Bone Metabolism, Calcium, and Phosphate Management
3. Coffee
3.1. Coffee Consumption
3.2. Coffee Consumption and Risk of IBD
3.3. Coffee Consumption and the Risk of Osteoporosis in IBD Patients
4. Tea
4.1. Tea Consumption
4.2. Tea Consumption and Risk of IBD
4.3. Tea Consumption and Risk of Osteoporosis in IBD Patients
5. Coffee and Tea Consumption and Microbiota in IBD Patients
6. Summary
- ECCO (European Crohn’s and Colitis Organization) notes that there are no clear data regarding the impact of coffee or caffeine on the risk of IBD. However, some patients, particularly individuals suffering from Crohn’s disease report avoidance of coffee, since this product exacerbates the symptoms of the disease.
- AACE/ACE (American Association of Clinical Endocrinologists and American College of Endocrinology) recommend limiting the consumption of beverages with caffeine to 1–2 portions per day in postmenopausal women [90].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, X.; Xiong, D.; Peng, Y.-Q.; Sheng, Z.-F.; Wu, X.-Y.; Wu, X.-P.; Wu, F.; Yuan, L.-Q.; Liao, E.-Y. Epidemiology and Management of Osteoporosis in the People’s Republic of China: Current Perspectives. Clin. Interv. Aging 2015, 10, 1017–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwiatkowska, I.; Lubawy, M.; Formanowicz, D. Nutritional Procedure in Osteoporosis Prevention in Older People. Geriatria 2019, 13, 177–183. [Google Scholar]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s Position Statement on Peak Bone Mass Development and Lifestyle Factors: A Systematic Review and Implementation Recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabor, E.; Kuźniewicz, R.; Zagórski, P.; Martela, K.; Pluskiewicz, W. The Relationship of Knowledge of Osteoporosis and Bone Health in Postmenopausal Women in Silesia Osteo Active Study. J. Clin. Densitom. 2018, 21, 98–104. [Google Scholar] [CrossRef]
- Sairenji, T.; Collins, K.L.; Evans, D.V. An Update on Inflammatory Bowel Disease. Prim. Care 2017, 44, 673–692. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kobayashi, T.; Ueno, F.; Matsui, T.; Hirai, F.; Inoue, N.; Kato, J.; Kobayashi, K.; Kobayashi, K.; Koganei, K.; et al. Evidence-Based Clinical Practice Guidelines for Inflammatory Bowel Disease. J. Gastroenterol. 2018, 53, 305–353. [Google Scholar] [CrossRef] [Green Version]
- Khasawneh, M.; Spence, A.D.; Addley, J.; Allen, P.B. The Role of Smoking and Alcohol Behaviour in the Management of Inflammatory Bowel Disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 553–559. [Google Scholar] [CrossRef]
- Mędrela-Kuder, E.; Szymura, K. Selected Anti-Health Behaviours among Women with Osteoporosis. Rocz. Panstw. Zakl. Hig. 2018, 69, 397–403. [Google Scholar] [CrossRef]
- Chan, C.Y.; Subramaniam, S.; Chin, K.-Y.; Ima-Nirwana, S.; Muhammad, N.; Fairus, A.; Mohd Rizal, A.M.; Ng, P.Y.; Nor Aini, J.; Aziz, N.A.; et al. Knowledge, Beliefs, Dietary, and Lifestyle Practices Related to Bone Health among Middle-Aged and Elderly Chinese in Klang Valley, Malaysia. Int. J. Environ. Res. Public Health 2019, 16, 1787. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, L.A.; DiBonaventura, M.; Kawaguchi, I. The Association between Osteoporosis and Patient Outcomes in Japan. J. Med. Econ. 2016, 19, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, H.; Wang, X.; Wei, Z.; Feng, S. Associated Factors for Osteoporosis and Fracture in Chinese Elderly. Med. Sci. Monit. 2019, 25, 5580–5588. [Google Scholar] [CrossRef] [PubMed]
- Thorin, M.H.; Wihlborg, A.; Åkesson, K.; Gerdhem, P. Smoking, Smoking Cessation, and Fracture Risk in Elderly Women Followed for 10 Years. Osteoporos. Int. 2016, 27, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Landin-Wilhelmsen, K.; Wilhelmsen, L.; Bengtsson, B.-Å. Postmenopausal Osteoporosis Is More Related to Hormonal Aberrations than to Lifestyle Factors. Clin. Endocrinol. 1999, 51, 387–394. [Google Scholar] [CrossRef]
- Bijelic, R.; Milicevic, S.; Balaban, J. Risk Factors for Osteoporosis in Postmenopausal Women. Med. Arch. 2017, 71, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Sgambato, D.; Gimigliano, F.; De Musis, C.; Moretti, A.; Toro, G.; Ferrante, E.; Miranda, A.; De Mauro, D.; Romano, L.; Iolascon, G.; et al. Bone Alterations in Inflammatory Bowel Diseases. World J. Clin. Cases 2019, 7, 1908–1925. [Google Scholar] [CrossRef]
- Oh, H.J.; Ryu, K.H.; Park, B.J.; Yoon, B.-H. Osteoporosis and Osteoporotic Fractures in Gastrointestinal Disease. J. Bone Metab. 2018, 25, 213–217. [Google Scholar] [CrossRef]
- Ali, T.; Lam, D.; Bronze, M.S.; Humphrey, M.B. Osteoporosis in Inflammatory Bowel Disease. Am. J. Med. 2009, 122, 599–604. [Google Scholar] [CrossRef] [Green Version]
- Duarte, P.M.; Marques, M.R.; Bezerra, J.P.; Bastos, M.F. The Effects of Caffeine Administration on the Early Stage of Bone Healing and Bone Density A Histometric Study in Rats. Arch. Oral Biol. 2009, 54, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Ide, K.; Cheuk, G.; Cheuk, S.L.; Yazdani, M.; Nakamoto, T.; Thomas, K.A. A Caffeine Diet Can Alter the Mechanical Properties of the Bones of Young Ovariectomized Rats. Ann. Nutr. Metab. 2002, 46, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Avila, M.; Colditz, G.A.; Stampfer, M.J.; Rosner, B.; Speizer, F.E.; Willett, W.C. Caffeine, Moderate Alcohol Intake, and Risk of Fractures of the Hip and Forearm in Middle-Aged Women. Am. J. Clin. Nutr. 1991, 54, 157–163. [Google Scholar] [CrossRef]
- Fernández, M.J.; López, A.; Santa-Maria, A. Apoptosis Induced by Different Doses of Caffeine on Chinese Hamster Ovary Cells. J. Appl. Toxicol. 2003, 23, 221–224. [Google Scholar] [CrossRef]
- Lu, P.-Z.; Lai, C.-Y.; Chan, W.-H. Caffeine Induces Cell Death via Activation of Apoptotic Signal and Inactivation of Survival Signal in Human Osteoblasts. Int. J. Mol. Sci. 2008, 9, 698–718. [Google Scholar] [CrossRef] [Green Version]
- Tsuang, Y.-H.; Sun, J.-S.; Chen, L.-T.; Sun, S.C.-K.; Chen, S.-C. Direct Effects of Caffeine on Osteoblastic Cells Metabolism: The Possible Causal Effect of Caffeine on the Formation of Osteoporosis. J. Orthop. Surg. 2006, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Guan, X.X.; Zhu, Z.L.; Guo, J.; Huang, Y.C.; Hou, W.W.; Yu, H.Y. Caffeine Inhibits the Viability and Osteogenic Differentiation of Rat Bone Marrow-Derived Mesenchymal Stromal Cells. Br. J. Pharmacol. 2010, 161, 1542–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heaney, R.P. Effects of Caffeine on Bone and the Calcium Economy. Food Chem. Toxicol. 2002, 40, 1263–1270. [Google Scholar] [CrossRef]
- Massey, L.K.; Whiting, S.J. Caffeine, Urinary Calcium, Calcium Metabolism and Bone. J. Nutr. 1993, 123, 1611–1614. [Google Scholar] [CrossRef]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of Caffeine on Human Health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef]
- Samoggia, A.; Riedel, B. Consumers’ Perceptions of Coffee Health Benefits and Motives for Coffee Consumption and Purchasing. Nutrients 2019, 11, 653. [Google Scholar] [CrossRef] [Green Version]
- Massey, L.K.; Wise, K.J. Impact of Gender and Age on Urinary Water and Mineral Excretion Responses to Acute Caffeine Doses. Nutr. Res. 1992, 12, 605–612. [Google Scholar] [CrossRef]
- Heaney, R.P.; Rafferty, K. Carbonated Beverages and Urinary Calcium Excretion. Am. J. Clin. Nutr. 2001, 74, 343–347. [Google Scholar] [CrossRef]
- Whiting, S.J.; Whitney, H.L. Effect of Dietary Caffeine and Theophylline on Urinary Calcium Excretion in the Adult Rat. J. Nutr. 1987, 117, 1224–1228. [Google Scholar] [CrossRef]
- Folwarczna, J.; Zych, M.; Nowińska, B.; Pytlik, M.; Janas, A. Unfavorable Effect of Trigonelline, an Alkaloid Present in Coffee and Fenugreek, on Bone Mechanical Properties in Estrogen-Deficient Rats. Mol. Nutr. Food Res. 2014, 58, 1457–1464. [Google Scholar] [CrossRef]
- Kiyama, R. Estrogenic Activity of Coffee Constituents. Nutrients 2019, 11, 1401. [Google Scholar] [CrossRef] [Green Version]
- Higdon, J.V.; Frei, B. Coffee and Health: A Review of Recent Human Research. Crit. Rev. Food Sci. Nutr. 2006, 46, 101–123. [Google Scholar] [CrossRef]
- Lire Wachamo, H. Review on Health Benefit and Risk of Coffee Consumption. Med. Aromat. Plants 2017, 6, 1–12. [Google Scholar] [CrossRef]
- Nieber, K. The Impact of Coffee on Health. Planta Med. 2017, 83, 1256–1263. [Google Scholar] [CrossRef] [Green Version]
- Reyes, C.M.; Cornelis, M.C. Caffeine in the Diet: Country-Level Consumption and Guidelines. Nutrients 2018, 10, 1772. [Google Scholar] [CrossRef] [Green Version]
- Verster, J.C.; Koenig, J. Caffeine Intake and Its Sources: A Review of National Representative Studies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1250–1259. [Google Scholar] [CrossRef]
- Andrews, K.W.; Schweitzer, A.; Zhao, C.; Holden, J.M.; Roseland, J.M.; Brandt, M.; Dwyer, J.T.; Picciano, M.F.; Saldanha, L.G.; Fisher, K.D.; et al. The Caffeine Contents of Dietary Supplements Commonly Purchased in the US: Analysis of 53 Products with Caffeine-Containing Ingredients. Anal. Bioanal. Chem. 2007, 389, 231–239. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Herrick, K. Caffeine Intake from Food and Beverage Sources and Trends among Children and Adolescents in the United States: Review of National Quantitative Studies from 1999 to 2011. Adv. Nutr. 2015, 6, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.P. Caffeine. In Nutritional Neuroscience; Liebermann, H.R., Kanarek, R.B., Prasad, C., Eds.; Taylor & Francis: Philadelphia, PA, USA, 2005; pp. 335–354. ISBN 0-415-31599-9. [Google Scholar]
- Hansen, T.S.; Jess, T.; Vind, I.; Elkjaer, M.; Nielsen, M.F.; Gamborg, M.; Munkholm, P. Environmental Factors in Inflammatory Bowel Disease: A Case-Control Study Based on a Danish Inception Cohort. J. Crohn’s Colitis 2011, 5, 577–584. [Google Scholar] [CrossRef]
- Ng, S.C.; Tang, W.; Leong, R.W.; Chen, M.; Ko, Y.; Studd, C.; Niewiadomski, O.; Bell, S.; Kamm, M.A.; de Silva, H.J.; et al. Environmental Risk Factors in Inflammatory Bowel Disease: A Population-Based Case-Control Study in Asia-Pacific. Gut 2015, 64, 1063–1071. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.-Y.; Zhao, Q. Beverage Consumption and Risk of Ulcerative Colitis. Medicine 2017, 96, e9070. [Google Scholar] [CrossRef]
- Yang, Y.; Xiang, L.; He, J. Beverage Intake and Risk of Crohn Disease. Medicine 2019, 98, e15795. [Google Scholar] [CrossRef]
- Barthel, C.; Wiegand, S.; Scharl, S.; Scharl, M.; Frei, P.; Vavricka, S.R.; Fried, M.; Sulz, M.C.; Wiegand, N.; Rogler, G.; et al. Patients’ Perceptions on the Impact of Coffee Consumption in Inflammatory Bowel Disease: Friend or Foe?—A Patient Survey. Nutr. J. 2015, 14, 78. [Google Scholar] [CrossRef] [Green Version]
- Głąbska, D.; Guzek, D.; Lech, G. Analysis of the Nutrients and Food Products Intake of Polish Males with Ulcerative Colitis in Remission. Nutrients 2019, 11, 2333. [Google Scholar] [CrossRef] [Green Version]
- Gacek, L.; Bączyk, G.; Skokowska, B.; Bielawska, A.; Brzezińska, R. The Level of Patients’ Knowladge about the Inflammatory Bowel Disease and Healthy Lifestyle. Pielęgniarstwo Polskie 2017, 63, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Liu, T.; Hu, L.; Li, J.; Gan, C.; Xu, J.; Chen, F.; Xiang, Z.; Wang, X.; Sheng, J. Effect of Caffeine on Ovariectomy-Induced Osteoporosis in Rats. Biomed. Pharmacother. 2019, 112, 108650. [Google Scholar] [CrossRef]
- Chau, Y.-P.; Au, P.C.M.; Li, G.H.Y.; Sing, C.-W.; Cheng, V.K.F.; Tan, K.C.B.; Kung, A.W.C.; Cheung, C.-L. Serum Metabolome of Coffee Consumption and Its Association with Bone Mineral Density: The Hong Kong Osteoporosis Study. J. Clin. Endocrinol. Metab. 2019, 105, e619–e627. [Google Scholar] [CrossRef]
- Hasling, C.; Søndergaard, K.; Charles, P.; Mosekilde, L. Calcium Metabolism in Postmenopausal Osteoporotic Women Is Determined by Dietary Calcium and Coffee Intake. J. Nutr. 1992, 122, 1119–1126. [Google Scholar] [CrossRef]
- Chang, H.-C.; Hsieh, C.-F.; Lin, Y.-C.; Tantoh, D.M.; Ko, P.-C.; Kung, Y.-Y.; Wang, M.-C.; Hsu, S.-Y.; Liaw, Y.-C.; Liaw, Y.-P. Does Coffee Drinking Have Beneficial Effects on Bone Health of Taiwanese Adults? A Longitudinal Study. BMC Public Health 2018, 18, 1273. [Google Scholar] [CrossRef]
- Hallström, H.; Byberg, L.; Glynn, A.; Lemming, E.W.; Wolk, A.; Michaëlsson, K. Long-Term Coffee Consumption in Relation to Fracture Risk and Bone Mineral Density in Women. Am. J. Epidemiol. 2013, 178, 898–909. [Google Scholar] [CrossRef]
- Barrett-Connor, E.; Chang, J.C.; Edelstein, S.L. Coffee-Associated Osteoporosis Offset by Daily Milk Consumption. The Rancho Bernardo Study. JAMA 1994, 271, 280–283. [Google Scholar] [CrossRef]
- Choi, E.; Choi, K.-H.; Park, S.M.; Shin, D.; Joh, H.-K.; Cho, E. The Benefit of Bone Health by Drinking Coffee among Korean Postmenopausal Women: A Cross-Sectional Analysis of the Fourth & Fifth Korea National Health and Nutrition Examination Surveys. PLoS ONE 2016, 11, e0147762. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, Z.-H.; Lei, T.; Tang, Z. Subjective Evaluation of the Frequency of Coffee Intake and Relationship to Osteoporosis in Chinese Men. J. Health Popul. Nutr. 2016, 35, 24. [Google Scholar] [CrossRef] [Green Version]
- Al-Othman, A.; Al-Musharaf, S.; Al-Daghri, N.M.; Yakout, S.; Alkharfy, K.M.; Al-Saleh, Y.; Al-Attas, O.S.; Alokail, M.S.; Moharram, O.; Sabico, S.; et al. Tea and Coffee Consumption in Relation to Vitamin D and Calcium Levels in Saudi Adolescents. Nutr. J. 2012, 11, 56. [Google Scholar] [CrossRef] [Green Version]
- Barbalho, S.M.; Bosso, H.; Salzedas-Pescinini, L.M.; de Alvares Goulart, R. Green Tea: A Possibility in the Therapeutic Approach of Inflammatory Bowel Diseases? Green Tea and Inflammatory Bowel Diseases. Complement. Ther. Med. 2019, 43, 148–153. [Google Scholar] [CrossRef]
- Zhang, Z.-F.; Yang, J.-L.; Jiang, H.-C.; Lai, Z.; Wu, F.; Liu, Z.-X. Updated Association of Tea Consumption and Bone Mineral Density: A Meta-Analysis. Medicine 2017, 96, e6437. [Google Scholar] [CrossRef]
- Weerawatanakorn, M.; Hung, W.-L.; Pan, M.-H.; Li, S.; Li, D.; Wan, X.; Ho, C.-T. Chemistry and Health Beneficial Effects of Oolong Tea and Theasinensins. Food Sci. Hum. Wellness 2015, 4, 133–146. [Google Scholar] [CrossRef] [Green Version]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-Analyses. Gastroenterology 2019, 157, 647–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Ding, H.; Vanarsa, K.; Soomro, S.; Baig, S.; Hicks, J.; Mohan, C. Low Dose Epigallocatechin Gallate Alleviates Experimental Colitis by Subduing Inflammatory Cells and Cytokines, and Improving Intestinal Permeability. Nutrients 2019, 11, 1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitzer, Z.T.; Elias, R.J.; Vijay-Kumar, M.; Lambert, J.D. (−)-Epigallocatechin-3-Gallate Decreases Colonic Inflammation and Permeability in a Mouse Model of Colitis, but Reduces Macronutrient Digestion and Exacerbates Weight Loss. Mol. Nutr. Food Res. 2016, 60, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S. Chronic Inflammatory Diseases and Green Tea Polyphenols. Nutrients 2017, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.U.; Li, Y.; Huang, Y.; Zhu, L.; Feng, S.; Wu, J.; Wang, X. Treatment of Inflammatory Bowel Disease via Green Tea Polyphenols: Possible Application and Protective Approaches. Inflammopharmacology 2018, 26, 319–330. [Google Scholar] [CrossRef]
- Liu, T.; Xiang, Z.; Chen, F.; Yin, D.; Huang, Y.; Xu, J.; Hu, L.; Xu, H.; Wang, X.; Sheng, J. Theabrownin Suppresses in Vitro Osteoclastogenesis and Prevents Bone Loss in Ovariectomized Rats. Biomed. Pharmacother. 2018, 106, 1339–1347. [Google Scholar] [CrossRef]
- Shen, C.-L.; Chyu, M.-C.; Wang, J.-S. Tea and Bone Health: Steps Forward in Translational Nutrition12345. Am. J. Clin. Nutr. 2013, 98, 1694S–1699S. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.-L.; Chyu, M.-C.; Yeh, J.K.; Zhang, Y.; Pence, B.C.; Felton, C.K.; Brismée, J.-M.; Arjmandi, B.H.; Doctolero, S.; Wang, J.-S. Effect of Green Tea and Tai Chi on Bone Health in Postmenopausal Osteopenic Women: A 6-Month Randomized Placebo-Controlled Trial. Osteoporos. Int. 2012, 23, 1541–1552. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Wang, L.; Ma, Q.; Cui, Q.; Lv, Q.; Zhang, W.; Li, X. Association between Tea Consumption and Osteoporosis: A Meta-Analysis. Medicine 2017, 96, e9034. [Google Scholar] [CrossRef]
- Li, X.; Qiao, Y.; Yu, C.; Guo, Y.; Bian, Z.; Yang, L.; Chen, Y.; Yan, S.; Xie, X.; Huang, D.; et al. Tea Consumption and Bone Health in Chinese Adults: A Population-Based Study. Osteoporos. Int. 2019, 30, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Han, G.-Y.; Jing, L.-P.; Chen, Z.-Y.; Chen, Y.-M.; Xiao, S.-M. Tea Consumption Is Associated with Increased Bone Strength in Middle-Aged and Elderly Chinese Women. J. Nutr. Health Aging 2018, 22, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Qu, H.; Xu, L.; Shi, D.-Z. Tea Consumption May Decrease the Risk of Osteoporosis: An Updated Meta-Analysis of Observational Studies. Nutr. Res. 2017, 42, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Prosberg, M.; Bendtsen, F.; Vind, I.; Petersen, A.M.; Gluud, L.L. The Association between the Gut Microbiota and the Inflammatory Bowel Disease Activity: A Systematic Review and Meta-Analysis. Scand. J. Gastroenterol. 2016, 51, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Kruis, W.; Frič, P.; Pokrotnieks, J.; Lukáš, M.; Fixa, B.; Kaščák, M.; Kamm, M.A.; Weismueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining Remission of Ulcerative Colitis with the Probiotic Escherichia Coli Nissle 1917 Is as Effective as with Standard Mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Nishitsuji, K.; Watanabe, S.; Xiao, J.; Nagatomo, R.; Ogawa, H.; Tsunematsu, T.; Umemoto, H.; Morimoto, Y.; Akatsu, H.; Inoue, K.; et al. Effect of Coffee or Coffee Components on Gut Microbiome and Short-Chain Fatty Acids in a Mouse Model of Metabolic Syndrome. Sci. Rep. 2018, 8, 16173. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Ogata, K.; Takeshita, T.; Shibata, Y.; Matsumi, R.; Kageyama, S.; Asakawa, M.; Yamashita, Y. Effect of Coffee on the Compositional Shift of Oral Indigenous Microbiota Cultured in Vitro. J. Oral Sci. 2019, 61, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Kleber Silveira, A.; Moresco, K.S.; Mautone Gomes, H.; da Silva Morrone, M.; Kich Grun, L.; Pens Gelain, D.; de Mattos Pereira, L.; Giongo, A.; Rodrigues De Oliveira, R.; Fonseca Moreira, J.C. Guarana (Paullinia Cupana Mart.) Alters Gut Microbiota and Modulates Redox Status, Partially via Caffeine in Wistar Rats. Phytother. Res. 2018, 32, 2466–2474. [Google Scholar] [CrossRef]
- Lee, I.-A.; Low, D.; Kamba, A.; Llado, V.; Mizoguchi, E. Oral Caffeine Administration Ameliorates Acute Colitis by Suppressing Chitinase 3-like 1 Expression in Intestinal Epithelial Cells. J. Gastroenterol. 2014, 49, 1206–1216. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Xie, Q.; Kong, P.; Liu, L.; Sun, S.; Xiong, B.; Huang, B.; Yan, L.; Sheng, J.; Xiang, H. Polyphenol- and Caffeine-Rich Postfermented Pu-Erh Tea Improves Diet-Induced Metabolic Syndrome by Remodeling Intestinal Homeostasis in Mice. Infect. Immun. 2017, 86, e00601-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Mocanu, V.; Cai, C.; Dang, J.; Slater, L.; Deehan, E.C.; Walter, J.; Madsen, K.L. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome-A Systematic Review. Nutrients 2019, 11, 2291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bancirova, M. Comparison of the Antioxidant Capacity and the Antimicrobial Activity of Black and Green Tea. Food Res. Int. 2010, 43, 1379–1382. [Google Scholar] [CrossRef]
- Hänninen, A.; Toivonen, R.; Pöysti, S.; Belzer, C.; Plovier, H.; Ouwerkerk, J.P.; Emani, R.; Cani, P.D.; De Vos, W.M. Akkermansia Muciniphila Induces Gut Microbiota Remodelling and Controls Islet Autoimmunity in NOD Mice. Gut 2018, 67, 1445–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human Intestinal Lumen and Mucosa-Associated Microbiota in Patients with Colorectal Cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Dominianni, C.; Shapiro, J.A.; Church, T.R.; Wu, J.; Miller, G.; Yuen, E.; Freiman, H.; Lustbader, I.; Salik, J.; et al. The Gut Microbiota in Conventional and Serrated Precursors of Colorectal Cancer. Microbiome 2016, 4, 69. [Google Scholar] [CrossRef] [Green Version]
- Flemer, B.; Warren, R.D.; Barrett, M.P.; Cisek, K.; Das, A.; Jeffery, I.B.; Hurley, E.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. The Oral Microbiota in Colorectal Cancer Is Distinctive and Predictive. Gut 2018, 67, 1454–1463. [Google Scholar] [CrossRef] [Green Version]
- Mazzon, E.; Muià, C.; Paola, R.D.; Genovese, T.; Menegazzi, M.; De Sarro, A.; Suzuki, H.; Cuzzocrea, S. Green Tea Polyphenol Extract Attenuates Colon Injury Induced by Experimental Colitis. Free Radic. Res. 2005, 39, 1017–1025. [Google Scholar] [CrossRef]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Clarke, B.L.; Harris, S.T.; Hurley, D.L.; Kleerekoper, M.; Lewiecki, E.M.; Miller, P.D.; Narula, H.S.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis—2016—Executive Summary. Endocr. Pract. 2016, 22, 1111–1118. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratajczak, A.E.; Szymczak-Tomczak, A.; Zawada, A.; Rychter, A.M.; Dobrowolska, A.; Krela-Kaźmierczak, I. Does Drinking Coffee and Tea Affect Bone Metabolism in Patients with Inflammatory Bowel Diseases? Nutrients 2021, 13, 216. https://doi.org/10.3390/nu13010216
Ratajczak AE, Szymczak-Tomczak A, Zawada A, Rychter AM, Dobrowolska A, Krela-Kaźmierczak I. Does Drinking Coffee and Tea Affect Bone Metabolism in Patients with Inflammatory Bowel Diseases? Nutrients. 2021; 13(1):216. https://doi.org/10.3390/nu13010216
Chicago/Turabian StyleRatajczak, Alicja Ewa, Aleksandra Szymczak-Tomczak, Agnieszka Zawada, Anna Maria Rychter, Agnieszka Dobrowolska, and Iwona Krela-Kaźmierczak. 2021. "Does Drinking Coffee and Tea Affect Bone Metabolism in Patients with Inflammatory Bowel Diseases?" Nutrients 13, no. 1: 216. https://doi.org/10.3390/nu13010216