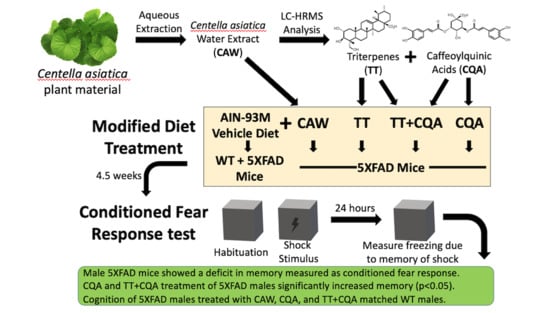
Caffeoylquinic Acids in Centella asiatica Reverse Cognitive Deficits in Male 5XFAD Alzheimer’s Disease Model Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CA Dried Water Extract (CAW)
2.2. CAW Individual Compounds
2.3. LC-HRMS/MS Analysis
2.4. Experimental Mouse Diets
2.5. Animals
2.6. Conditioned Fear Response (CFR)
2.7. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kapoor, L.D. CRC Handbook of Ayurvedic Medicinal Plants; CRC Press: Cleveland, OH, USA, 1990. [Google Scholar]
- Gray, N.E.; Alcazar Magana, A.; Lak, P.; Wright, K.M.; Quinn, J.; Stevens, J.F.; Maier, C.S.; Soumyanath, A. Centella asiatica—Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem. Rev. 2018, 17, 161–194. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.G.; Caruso, M.; Murchison, C.F.; Zhu, J.Y.; Wright, K.M.; Harris, C.J.; Gray, N.E.; Quinn, J.F.; Soumyanath, A. Centella Asiatica Improves Memory and Promotes Antioxidative Signaling in 5XFAD Mice. Antioxidants 2019, 8, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, N.E.; Morre, J.; Kelley, J.; Maier, C.S.; Stevens, J.F.; Quinn, J.F.; Soumyanath, A. Caffeoylquinic acids in Centella asiatica protect against amyloid-beta toxicity. J. Alzheimers Dis. 2014, 40, 359–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, N.E.; Zweig, J.A.; Murchison, C.; Caruso, M.; Matthews, D.G.; Kawamoto, C.; Harris, C.J.; Quinn, J.F.; Soumyanath, A. Centella asiatica attenuates Abeta-induced neurodegenerative spine loss and dendritic simplification. Neurosci. Lett. 2017, 646, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Alcazar Magana, A.; Wright, K.; Vaswani, A.; Caruso, M.; Reed, R.L.; Bailey, C.F.; Nguyen, T.; Gray, N.E.; Soumyanath, A.; Quinn, J.; et al. Integration of mass spectral fingerprinting analysis with precursor ion (MS1) quantification for the characterisation of botanical extracts: Application to extracts of Centella asiatica (L.) Urban. Phytochem. Anal. 2020, 31, 722–738. [Google Scholar] [CrossRef] [Green Version]
- Keiser, A.A.; Turnbull, L.M.; Darian, M.A.; Feldman, D.E.; Song, I.; Tronson, N.C. Sex Differences in context fear generalization and recruitment of hippocampus and amygdala during retrieval. Neuropsychopharmacology 2017, 42, 397–407. [Google Scholar] [CrossRef]
- Han, J.; Miyamae, Y.; Shigemori, H.; Isoda, H. Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience 2010, 169, 1039–1045. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, K.; Davies, J.; Doldan, N.G.; Arao, S.; Ferdousi, F.; Szele, F.G.; Isoda, H. 3,4,5-Tricaffeoylquinic acid induces adult neurogenesis and improves deficit of learning and memory in aging model senescence-accelerated prone 8 mice. Aging 2019, 11, 401–422. [Google Scholar] [CrossRef]
- Ahmad Rather, M.; Justin-Thenmozhi, A.; Manivasagam, T.; Saravanababu, C.; Guillemin, G.J.; Essa, M.M. Asiatic acid attenuated aluminum chloride-induced tau pathology, oxidative stress and apoptosis via AKT/GSK-3beta signaling pathway in wistar rats. Neurotox. Res. 2019, 35, 955–968. [Google Scholar] [CrossRef]
- Lin, X.; Huang, R.; Zhang, S.; Wei, L.; Zhuo, L.; Wu, X.; Tang, A.; Huang, Q. Beneficial effects of asiaticoside on cognitive deficits in senescence-accelerated mice. Fitoterapia 2013, 87, 69–77. [Google Scholar] [CrossRef]
- Jawhar, S.; Trawicka, A.; Jenneckens, C.; Bayer, T.A.; Wirths, O. Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Abeta aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 2012, 33, 196 e129–e140. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, T.P.; Robertson, A.; Chipman, P.H.; Rafuse, V.F.; Brown, R.E. Motor function deficits in the 12 month-old female 5xFAD mouse model of Alzheimer’s disease. Behav. Brain Res. 2018, 337, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Bradwejn, J.; Zhou, Y.; Koszycki, D.; Shlik, J. A double-blind, placebo-controlled study on the effects of Gotu Kola (Centella asiatica) on acoustic startle response in healthy subjects. J. Clin. Psychopharmacol. 2000, 20, 680–684. [Google Scholar] [CrossRef]
- Gohil, K.J.; Patel, J.A.; Gajjar, A.K. Pharmacological review on Centella asiatica: A potential herbal cure-all. Indian J. Pharm. Sci. 2010, 72, 546–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijeweera, P.; Arnason, J.T.; Koszycki, D.; Merali, Z. Evaluation of anxiolytic properties of gotukola—(Centella asiatica) extracts and asiaticoside in rat behavioral models. Phytomedicine 2006, 13, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Bobeck, E.N.; Gomes, I.; Pena, D.; Cummings, K.A.; Clem, R.L.; Mezei, M.; Devi, L.A. The bigLEN-GPR171 peptide receptor system within the basolateral amygdala regulates anxiety-like behavior and contextual fear conditioning. Neuropsychopharmacology 2017, 42, 2527–2536. [Google Scholar] [CrossRef] [Green Version]
- Lissek, S.; Powers, A.S.; McClure, E.B.; Phelps, E.A.; Woldehawariat, G.; Grillon, C.; Pine, D.S. Classical fear conditioning in the anxiety disorders: A meta-analysis. Behav. Res. Ther. 2005, 43, 1391–1424. [Google Scholar] [CrossRef]
- Donley, M.P.; Rosen, J.B. Novelty and fear conditioning induced gene expression in high and low states of anxiety. Learn. Mem. 2017, 24, 449–461. [Google Scholar] [CrossRef] [Green Version]
- Soumyanath, A.; Zhong, Y.P.; Henson, E.; Wadsworth, T.; Bishop, J.; Gold, B.G.; Quinn, J.F. Centella asiatica extract improves behavioral deficits in a mouse model of Alzheimer’s disease: Investigation of a possible mechanism of action. Int. J. Alzheimer’s Dis. 2012, 2012, 381974. [Google Scholar] [CrossRef] [Green Version]
- Gray, N.E.; Harris, C.J.; Quinn, J.F.; Soumyanath, A. Centella asiatica modulates antioxidant and mitochondrial pathways and improves cognitive function in mice. J. Ethnopharmacol. 2016, 180, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Gray, N.E.; Zweig, J.A.; Caruso, M.; Martin, M.D.; Zhu, J.Y.; Quinn, J.F.; Soumyanath, A. Centella asiatica increases hippocampal synaptic density and improves memory and executive function in aged mice. Brain Behav. 2018, 8, e01024. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.E.; Zweig, J.A.; Caruso, M.; Zhu, J.Y.; Wright, K.M.; Quinn, J.F.; Soumyanath, A. Centella asiatica attenuates hippocampal mitochondrial dysfunction and improves memory and executive function in beta-amyloid overexpressing mice. Mol. Cell. Neurosci. 2018, 93, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.A.; Wong, A.A.; Fertan, E.; Brown, R.E. Whisker exploration behaviours in the 5xFAD mouse are affected by sex and retinal degeneration. Genes Brain Behav. 2020, 19, e12532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Leary, T.P.; Mantolino, H.M.; Stover, K.R.; Brown, R.E. Age-related deterioration of motor function in male and female 5xFAD mice from 3 to 16 months of age. Genes Brain Behav. 2020, 19, e12538. [Google Scholar] [CrossRef] [PubMed]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef] [PubMed]
- Sadleir, K.R.; Eimer, W.A.; Cole, S.L.; Vassar, R. Abeta reduction in BACE1 heterozygous null 5XFAD mice is associated with transgenic APP level. Mol. Neurodegener. 2015, 10, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sbrini, G.; Brivio, P.; Fumagalli, M.; Giavarini, F.; Caruso, D.; Racagni, G.; Dell’agli, M.; Sangiovanni, E.; Calabrese, F. Centella asiatica l. Phytosome improves cognitive performance by promoting bdnf expression in rat prefrontal cortex. Nutrients 2020, 12, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boondam, Y.; Songvut, P.; Tantisira, M.H.; Tapechum, S.; Tilokskulchai, K.; Pakaprot, N. Inverted U-shaped response of a standardized extract of Centella asiatica (ECa 233) on memory enhancement. Sci. Rep. 2019, 9, 8404. [Google Scholar] [CrossRef] [Green Version]

| Compound | Compounds in Extract (mg/g ± SEM) | Compounds in Diet (µg/g ± SEM) | ||
|---|---|---|---|---|
| CAW Extract | Standard Diet | AIN-93M Diet | AIN-93M Diet + 1% CAW Extract Calculated | |
| Caffeoylquinic acids | ||||
| 1,3-dicaffeoylquinic acid | 0.67 ± 0.01 | <LOQ | <LOQ | 6.7 |
| 1,5-dicaffeoylquinic acid | 0.64 ± 0.01 | <LOQ | <LOQ | 6.4 |
| Chlorogenic Acid | 5.25 ± 0.02 | 9.3 ± 0.1 | <LOQ | 52.5 |
| Isochlorogenic Acid A | 2.29 ± 0.01 | 9.5 ± 2 | <LOQ | 22.9 |
| Isochlorogenic Acid B | 3.60 ± 0.02 | 5.5 ± 2 | <LOQ | 36.0 |
| Isochlorogenic Acid C | 2.64 ± 0.01 | 9.7 ± 2 | <LOQ | 26.4 |
| Neochlorogenic Acid | 1.49 ± 0.01 | 2.2 ± 0.1 | <LOQ | 14.9 |
| Total Caffeoylquinic Acids | 16.57 ± 0.04 | 36.2 ± 3 | <LOQ | 165.7 |
| Triterpenes | ||||
| Asiatic acid | 0.57 ± 0.01 | <LOQ | <LOQ | 5.7 |
| Asiaticoside | 23.87 ± 1.72 | <LOQ | <LOQ | 238.7 |
| Madecassic Acid | 0.94 ± 0.02 | <LOQ | <LOQ | 9.4 |
| Madecassoside | 18.64 ± 1.84 | <LOQ | <LOQ | 186.4 |
| Total Triterpenes | 44.01 ± 2.52 | <LOQ | <LOQ | 440.1 |
| Food Consumption Rate (g/kg body weight/d) | Sex Comparison | ||
|---|---|---|---|
| Females | Males | ||
| Treatment | Avg ± SE | Avg ± SE | p-Value |
| Vehicle † | 128 ± 2 bc | 130 ± 3 | 0.541 |
| 1% CAW | 137 ± 4 c | 122 ± 4 | 0.014 |
| CQA | 111 ± 4 a | 123 ± 4 | 0.032 |
| TT | 112 ± 4 a | 118 ± 2 | 0.203 |
| TT + CQA | 120 ± 4 ab | 125 ± 6 | 0.504 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matthews, D.G.; Caruso, M.; Alcazar Magana, A.; Wright, K.M.; Maier, C.S.; Stevens, J.F.; Gray, N.E.; Quinn, J.F.; Soumyanath, A. Caffeoylquinic Acids in Centella asiatica Reverse Cognitive Deficits in Male 5XFAD Alzheimer’s Disease Model Mice. Nutrients 2020, 12, 3488. https://doi.org/10.3390/nu12113488
Matthews DG, Caruso M, Alcazar Magana A, Wright KM, Maier CS, Stevens JF, Gray NE, Quinn JF, Soumyanath A. Caffeoylquinic Acids in Centella asiatica Reverse Cognitive Deficits in Male 5XFAD Alzheimer’s Disease Model Mice. Nutrients. 2020; 12(11):3488. https://doi.org/10.3390/nu12113488
Chicago/Turabian StyleMatthews, Donald G., Maya Caruso, Armando Alcazar Magana, Kirsten M. Wright, Claudia S. Maier, Jan F. Stevens, Nora E. Gray, Joseph F. Quinn, and Amala Soumyanath. 2020. "Caffeoylquinic Acids in Centella asiatica Reverse Cognitive Deficits in Male 5XFAD Alzheimer’s Disease Model Mice" Nutrients 12, no. 11: 3488. https://doi.org/10.3390/nu12113488