A Preterm Rat Model for Immunonutritional Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Caesarean Intervention
2.4. Sample Collection and Processing
2.5. Immunoglobulin Quantification
2.6. Phagocytic Function of Blood Leukocytes
2.7. Intestinal Permeability Assay
2.8. Intestinal Histomorphometric Study
2.9. Statistical Analysis
3. Results
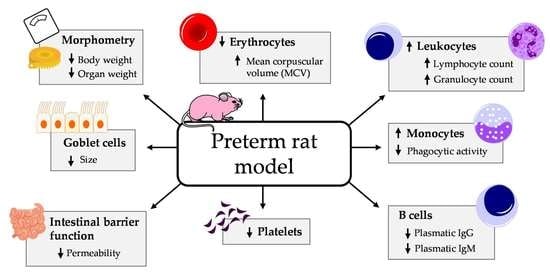
3.1. Effect of Prematurity on Body Weight and Other Morphometric Variables
3.2. Effect of Prematurity on Blood Cell Count
3.3. Effect of Prematurity on Plasma Ig Concentrations
3.4. Effect of Prematurity on Phagocytic Function of Blood Leukocytes
3.5. Effect of Prematurity on Intestinal Barrier Function
3.6. Effect of Prematurity on Histomorphometric Characteristics of the Small Intestine
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rich-Edwards, J.W.; Klungsoyr, K.; Wilcox, A.J.; Skjaerven, R. Duration of pregnancy, even at term, predicts long-term risk of coronary heart disease and stroke mortality in women: A population-based study. Am. J. Obs. Gynecol. 2015, 15, 518.e1–518.e8. [Google Scholar] [CrossRef] [PubMed]
- Melville, J.M.; Moss, T.J. The immune consequences of preterm birth. Front. Neurosci. 2013, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Frey, H.A.; Klebanoff, M.A. The epidemiology, etiology, and costs of preterm birth. Semin. Fetal Neonatal Med. 2016, 21, 68–73. [Google Scholar] [CrossRef]
- Lewis, E.D.; Richard, C.; Larsen, B.M.; Field, C.J. The importance of human milk for immunity in preterm infants. Clin. Perinatol. 2017, 44, 23–47. [Google Scholar] [CrossRef]
- Chang, E. Preterm birth and the role of neuroprotection. BMJ 2015, 350, g6661. [Google Scholar] [CrossRef]
- Rubarth, L.B.; Quinn, J. Respiratory development and respiratory distress syndrome. Neonatal Netw. 2015, 34, 231–238. [Google Scholar] [CrossRef]
- Gritz, E.C.; Bhandari, V. The human neonatal gut microbiome: A brief review. Front. Pediatr. 2015, 3, 17. [Google Scholar]
- Lawn, J.E.; Kerber, K.; Enweronu-Laryea, C.; Cousens, S. 3.6 Million neonatal deaths-what is progressing and what is not? Semin. Perinatol. 2010, 34, 371–386. [Google Scholar] [CrossRef]
- Strunk, T.; Currie, A.; Richmond, P.; Simmer, K.; Burgner, D. Innate immunity in human newborn infants: Prematurity means more than immaturity. J. Matern. Fetal Neonatal Med. 2011, 24, 25–31. [Google Scholar] [CrossRef]
- Adams-Chapman, I. Long-term impact of infection on the preterm neonate. Semin. Perinatol. 2012, 36, 462–470. [Google Scholar] [CrossRef]
- Gasparoni, A.; Ciardelli, L.; Avanzini, A.; Castellazzi, A.M.; Carini, R.; Rondini, G.; Chirico, G. Age-related changes in intracellular Th1/Th2 cytokine production, immunoproliferative T lymphocyte response and natural killer cell activity in newborns, children and adults. Biol. Neonate 2003, 84, 297–303. [Google Scholar] [CrossRef]
- Correa-Rocha, R.; Pérez, A.; Lorente, R.; Ferrando-Martínez, S.; Leal, M.; Gurbindo, D.; Muñoz-Fernández, M.Á. Preterm neonates show marked leukopenia and lymphopenia that are associated with increased regulatory T-cell values and diminished IL-7. Pediatr. Res. 2012, 71, 590–597. [Google Scholar] [CrossRef] [Green Version]
- Currie, A.J.; Curtis, S.; Strunk, T.; Riley, K.; Liyanage, K.; Prescott, S.; Doherty, D.; Simmer, K.; Richmond, P.; Burgner, D. Preterm infants have deficient monocyte and lymphocyte cytokine responses to group B streptococcus. Infect. Immun. 2011, 79, 1588–1596. [Google Scholar] [CrossRef]
- Neu, J. Gastrointestinal development and meeting the nutritional needs of premature infants. Am. J. Clin. Nutr. 2007, 85, 629S–634S. [Google Scholar] [CrossRef]
- Chin, A.M.; Hill, D.R.; Aurora, M.; Spence, J.R. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin. Cell Dev. Biol. 2017, 66, 81–93. [Google Scholar] [CrossRef]
- Barkhuizen, M.; Van de Berg, W.D.; De Vente, J.; Blanco, C.E.; Gavilanes, A.W.; Steinbusch, H.W. Nitric oxide production in the striatum and cerebellum of a rat model of preterm global perinatal asphyxia. Neurotox. Res. 2017, 31, 400–409. [Google Scholar] [CrossRef]
- Remesal, A.; De Luca, D.; San Feliciano, L.; Isidoro-Garcia, M.; Minucci, A.; Pocino, K.; Casas, J.; Fabrias, G.; Capoluongo, E.D.; de la Cruz, D.L. Effect of prenatal steroidal inhibition of sPLA2 in a rat model of preterm lung. Pulm. Pharmacol. Ther. 2016, 36, 31–36. [Google Scholar] [CrossRef]
- Corsini, I.; Polvani, S.; Tarocchi, M.; Tempesti, S.; Marroncini, G.; Generoso, M.; Bresci, C.; Gozzini, E.; Bianconi, T.; Galli, A.; et al. Peroxisome proliferator-activated receptor-γ agonist pioglitazone reduces the development of necrotizing enterocolitis in a neonatal preterm rat model. Pediatr. Res. 2017, 81, 364–368. [Google Scholar] [CrossRef]
- Satoh, T.; Izumi, H.; Iwabuchi, N.; Odamaki, T.; Namba, K.; Abe, F.; Xiao, J.Z. Bifidobacterium breve prevents necrotising enterocolitis by suppressing inflammatory responses in a preterm rat model. Benef. Microbes 2016, 7, 75–82. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Jiang, P.; Frøkiær, H.; Heegaard, P.M.; Thymann, T.; Sangild, P.T. Delayed development of systemic immunity in preterm pigs as a model for preterm infants. Sci. Rep. 2016, 6, 36816. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Cano, F.J.; Franch, À.; Castellote, C.; Castell, M. The suckling rat as a model for immunonutrition studies in early life. Clin. Dev. Immunol. 2012, 2012, 537310. [Google Scholar] [CrossRef] [PubMed]
- Appleby, C.J.; Towner, R.A. Magnetic resonance imaging of pulmonary damage in the term and premature rat neonate exposed to hyperoxia. Pediatr. Res. 2001, 50, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Grases-Pintó, B.; Abril-Gil, M.; Rodríguez-Lagunas, M.J.; Castell, M.; Pérez-Cano, F.J.; Franch, À. Leptin and adiponectin supplementation modifies mesenteric lymph node lymphocyte composition and functionality in suckling rats. Br. J. Nutr. 2018, 119, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; Van’t Land, B.; Stahl, B.; Garssen, J.; Rodríguez-Lagunas, M.J.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Supplementation with 2′-FL and scGOS/lcFOS ameliorates rotavirus-induced diarrhea in suckling rats. Front. Cell. Infect. Microbiol. 2018, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cano, F.J.; Marín-Gallén, S.; Castell, M.; Rodríguez-Palmero, M.; Rivero, M.; Franch, À.; Castellote, C. Bovine whey protein concentrate supplementation modulates maturation of immune system in suckling rats. Br. J. Nutr. 2007, 98 Suppl 1, S80–S84. [Google Scholar] [CrossRef]
- Rigo-Adrover, M.M.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Preclinical immunomodulation by the probiotic Bifidobacterium breve M-16V in early life. PLoS One 2016, 11, e0166082. [Google Scholar] [CrossRef]
- Grases-Pintó, B.; Abril-Gil, M.; Castell, M.; Pérez-Cano, F.J.; Franch, À. Enhancement of immune maturation in suckling rats by leptin and adiponectin supplementation. Sci. Rep. 2019, 9, 1786. [Google Scholar] [CrossRef]
- Torres-Castro, P.; Abril-Gil, M.; Rodríguez-Lagunas, M.J.; Castell, M.; Pérez-Cano, F.J.; Franch, À. TGF-β2, EGF, and FGF21 growth factors present in breast milk promote mesenteric lymph node lymphocytes maturation in suckling rats. Nutrients 2018, 10, 1171. [Google Scholar] [CrossRef]
- Ramírez-Santana, C.; Pérez-Cano, F.J.; Castellote, C.; Castell, M.; Rivero, M.; Rodríguez-Palmero, M.; Franch, À. Higher immunoglobulin production in conjugated linoleic acid-supplemented rats during gestation and suckling. Br. J. Nutr. 2009, 102, 858–868. [Google Scholar] [CrossRef]
- Rigo-Adrover, M.M.; van Limpt, K.; Knipping, K.; Garssen, J.; Knol, J.; Costabile, A.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Preventive effect of a synbiotic combination of galacto- and fructooligosaccharides mixture with Bifidobacterium breve M-16V in a model of multiple rotavirus infections. Front. Immunol. 2018, 9, 1318. [Google Scholar] [CrossRef]
- Puiman, P.; Stoll, B. Animal models to study neonatal nutrition in humans. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Underwood, M.A. Human milk for the premature infant. Pediatr. Clin. North Am. 2013, 60, 189–207. [Google Scholar] [CrossRef]
- Migraine, A.; Nicklaus, S.; Parnet, P.; Lange, C.; Monnery-Patris, S.; Des Robert, C.; Darmaun, D.; Flamant, C.; Amarger, V.; Rozé, J.C. Effect of preterm birth and birth weight on eating behavior at 2 years of age. Am. J. Clin. Nutr. 2013, 97, 1270–1277. [Google Scholar] [CrossRef]
- Andres, O.; Schulze, H.; Speer, C.P. Platelets in neonates: Central mediators in haemostasis, antimicrobial defence and inflammation. Thromb. Haemost. 2015, 113, 3–12. [Google Scholar]
- Cowman, J.; Quinn, N.; Geoghegan, S.; Müllers, S.; Oglesby, I.; Byrne, B.; Somers, M.; Ralph, A.; Voisin, B.; Ricco, A.J.; et al. Dynamic platelet function on von Willebrand factor is different in preterm neonates and full-term neonates: Changes in neonatal platelet function. J. Thromb. Haemost. 2016, 14, 2027–2035. [Google Scholar] [CrossRef]
- Hoffbrand, A.V. Folate deficiency in premature infants. Arch. Dis. Child. 1970, 45, 441–444. [Google Scholar] [CrossRef]
- Alkan Ozdemir, S.; Ozer, E.A.; Kose, S.; Ilhan, O.; Ozturk, C.; Sutcuoglu, S. Reference values of serum IgG and IgM levels in preterm and term newborns. J. Matern. Fetal Neonatal Med. 2016, 29, 972–976. [Google Scholar] [CrossRef]
- Palmeira, P.; Quinello, C.; Silveira-Lessa, A.L.; Zago, C.A.; Carneiro-Sampaio, M. IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012, 2012, 985646. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, X.; He, J.; Diraviyam, T.; Zhang, X. Quantitative investigation on correlation between IgG and FcRn during gestation and lactating periods in rat. Am. J. Reprod. Immunol. 2016, 75, 81–85. [Google Scholar] [CrossRef]
- Desesso, J.M.; Williams, A.L.; Ahuja, A.; Bowman, C.J.; Hurtt, M.E. The placenta, transfer of immunoglobulins, and safety assessment of biopharmaceuticals in pregnancy. Crit. Rev. Toxicol. 2012, 42, 185–210. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, J.; Mario, L.C.; Rodrigues, M.N.; Favaron, P.O.; Miglino, M.A. Immunoglobulin transport during gestation in domestic animals and humans — A Review. Open J. Anim. Sci. 2014, 4, 323–336. [Google Scholar] [CrossRef]
- Collins, A.; Weitkamp, J.H.; Wynn, J.L. Why are preterm newborns at increased risk of infection? Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F391–F394. [Google Scholar] [CrossRef]
- Mirpuri, J.; Raetz, M.; Sturge, C.R.; Wilhelm, C.L.; Benson, A.; Savani, R.C.; Hooper, L.V.; Yarovinsky, F. Proteobacteria-specific IgA regulates maturation of the intestinal microbiota. Gut Microbes. 2014, 5, 28–39. [Google Scholar] [CrossRef]
- Gustafson, C.E.; Higbee, D.; Yeckes, A.R.; Wilson, C.C.; De Zoeten, E.F.; Jedlicka, P.; Janoff, E.N. Limited expression of APRIL and its receptors prior to intestinal IgA plasma cell development during human infancy. Mucosal Immunol. 2014, 7, 467–477. [Google Scholar] [CrossRef]
- Prosser, A.; Hibbert, J.; Strunk, T.; Kok, C.H.; Simmer, K.; Richmond, P.; Burgner, D.; Currie, A. Phagocytosis of neonatal pathogens by peripheral blood neutrophils and monocytes from newborn preterm and term infants. Pediatr. Res. 2013, 74, 503–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Jong, E.; Strunk, T.; Burgner, D.; Lavoie, P.M.; Currie, A. The phenotype and function of preterm infant monocytes: Implications for susceptibility to infection. J. Leukoc. Biol. 2017, 102, 645–656. [Google Scholar] [CrossRef]
- Walthall, K.; Cappon, G.D.; Hurtt, M.E.; Zoetis, T. Postnatal development of the gastrointestinal system: A species comparison. Birth Defects Res. B Dev. Reprod. Toxicol. 2005, 74, 132–156. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.M.; Myers, L.S.; Kurundkar, A.R.; Maheshwari, A.; Nusrat, A.; Lin, P.W. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am. J. Pathol. 2012, 180, 626–635. [Google Scholar] [CrossRef] [PubMed]
- van Elburg, R.M.; Fetter, W.P.; Bunkers, C.M.; Heymans, H.S. Intestinal permeability in relation to birth weight and gestational and postnatal age. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, F52–F55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, L.T.; Laker, M.F.; Nelson, R. Intestinal permeability in the newborn. Arch. Dis. Child. 1984, 59, 236–241. [Google Scholar] [CrossRef]
- Sangild, P.T.; Thymann, T.; Schmidt, M.; Stoll, B.; Burrin, D.G.; Buddington, R.K. Invited review: The preterm pig as a model in pediatric gastroenterology. J. Anim. Sci. 2013, 91, 4713–4729. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.J.; Leaphart, C.L.; Mollen, K.P.; Hackam, D.J. The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock 2007, 27, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, K.R.; Liu, S.X.; Tian, R.; Kushnir, A.; Turner, J.R.; Li, H.L.; Chou, P.M.; Weber, C.R.; De Plaen, I.G. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am. J. Pathol. 2013, 182, 1596–1606. [Google Scholar] [CrossRef]
- Le Huërou-Luron, I.; Blat, S.; Boudry, G. Breast- v. formula-feeding: Impacts on the digestive tract and immediate and long-term health effects. Nutr. Res. Rev. 2010, 23, 23–36. [Google Scholar] [CrossRef]
- Taylor, S.N.; Basile, L.A.; Ebeling, M.; Wagner, C.L. Intestinal permeability in preterm infants by feeding type: mother’s milk versus formula. Breastfeed Med. 2009, 4, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Saleem, B.; Okogbule-Wonodi, A.C.; Fasano, A.; Magder, L.S.; Ravel, J.; Kapoor, S.; Viscardi, R.M. Intestinal barrier maturation in very low birthweight infants: Relationship to feeding and antibiotic exposure. J. Pediatr. 2017, 183, 31.e1–36.e1. [Google Scholar] [CrossRef] [PubMed]
- Colaizy, T.T.; Bartick, M.C.; Jegier, B.J.; Green, B.D.; Reinhold, A.G.; Schaefer, A.J.; Bogen, D.L.; Schwarz, E.B.; Stuebe, A.M.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Impact of optimized breastfeeding on the costs of necrotizing enterocolitis in extremely low birthweight infants. J. Pediatr. 2016, 175, 100–105.e2. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.; Schanler, R.J.; Kim, J.H.; Patel, A.L.; Trawöger, R.; Kiechl-Kohlendorfer, U.; Chan, G.M.; Blanco, C.L.; Abrams, S.; Cotten, C.M.; et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J. Pediatr. 2010, 156, 562–567. [Google Scholar] [CrossRef]
- Clark, J.A.; Doelle, S.M.; Halpern, M.D.; Saunders, T.A.; Holubec, H.; Dvorak, K.; Boitano, S.A.; Dvorak, B. Intestinal barrier failure during experimental necrotizing enterocolitis: Protective effect of EGF treatment. Am. J. Physiol. Liver Physiol. 2006, 291, G938–G949. [Google Scholar] [CrossRef]
- Hackam, D.J.; Upperman, J.S.; Grishin, A.; Ford, H.R. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin. Pediatr. Surg. 2005, 14, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Stelloh, C.; Allen, K.P.; Mattson, D.L.; Lerch-Gaggl, A.; Reddy, S.; El-Meanawy, A. Prematurity in mice leads to reduction in nephron number, hypertension, and proteinuria. Transl. Res. 2012, 159, 80–89. [Google Scholar] [CrossRef]
- Castellote, C.; Casillas, R.; Ramírez-Santana, C.; Pérez-Cano, F.J.; Castell, M.; Moretones, M.G.; López-Sabater, M.C.; Franch, A. Premature delivery influences the immunological composition of colostrum and transitional and mature human milk. J. Nutr. 2011, 1181–1187. [Google Scholar] [CrossRef]
- Amaral, M.A.; Guedes, G.H.B.F.; Epifanio, M.; Wagner, M.B.; Jones, M.H.; Mattiello, R. Network meta-analysis of probiotics to prevent respiratory infections in children and adolescents. Pediatr. Pulmonol. 2017, 52, 833–843. [Google Scholar] [CrossRef]
- Wang, H.T.; Anvari, S.; Anagnostou, K. The role of probiotics in preventing allergic disease. Children 2019, 6, 24. [Google Scholar] [CrossRef]
- Nauta, A.J.; Ben Amor, K.; Knol, J.; Garssen, J.; van der Beek, E.M. Relevance of pre- and postnatal nutrition to development and interplay between the microbiota and metabolic and immune systems. Am. J. Clin. Nutr. 2013, 98, 586S–593S. [Google Scholar] [CrossRef]
- Hagen, P.C.; Skelley, J.W. Efficacy of Bifidobacterium species in prevention of necrotizing enterocolitis in very-low birth weight infants. A systematic review. J. Pediatr. Pharmacol. Ther. 2019, 24, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.P.; Miller, M.M.; Sherman, J.; Niklas, V. Lactoferrin and necrotizing enterocolitis. Curr. Opin. Pediatr. 2014, 26, 146–150. [Google Scholar] [CrossRef] [Green Version]






| Term | Preterm | |||
|---|---|---|---|---|
| Weight (g) | Relative Weight (%) | Weight (g) | Relative Weight (%) | |
| Spleen | 0.14 ± 0.00 | 0.55 ± 0.01 | 0.12 ± 0.01 * | 0.58 ± 0.03 |
| Thymus | 0.09 ± 0.00 | 0.34 ± 0.02 | 0.07 ± 0.00 * | 0.33 ± 0.02 |
| Liver | 0.73 ± 0.03 | 2.82 ± 0.08 | 0.67 ± 0.03 * | 3.08 ± 0.09 |
| Small intestine | 0.84 ± 0.03 | 3.29 ± 0.08 | 0.77 ± 0.03 * | 3.51 ± 0.08 |
| Large intestine | 0.16 ± 0.01 | 0.63 ± 0.03 | 0.12 ± 0.01 * | 0.57 ± 0.03 |
| Length (cm) | Relative Length (%) | Length (cm) | Relative Length (%) | |
| Small intestine | 41.04 ± 0.87 | 1.62 ± 0.03 | 37.20 ± 0.91 * | 1.72 ± 0.05 |
| Large intestine | 6.88 ± 0.22 | 0.27 ± 0.01 | 6.19 ± 0.16 * | 0.29 ± 0.01 |
| Term | Preterm | |
|---|---|---|
| Leukocytes (×109/L) | 2.18 ± 0.20 | 4.57 ± 0.79 * |
| Erythrocytes (×1012/L) | 3.23 ± 0.08 | 2.83 ± 0.12 * |
| Hb (g/L) | 77.58 ± 1.84 | 72.56 ± 1.89 |
| HCT (%) | 27.16 ± 0.79 | 25.01 ± 1.16 |
| MCV (fL) | 84.28 ± 1.30 | 88.42 ± 0.60 * |
| MCH (pg) | 24.03 ± 0.42 | 25.82 ± 0.78 |
| Platelets (×109/L) | 529.18 ± 33.41 | 408.38 ± 56.36 * |
| Term | Preterm | |
|---|---|---|
| Villi width (μm) | 128.21 ± 15.91 | 173.54 ± 22.26 |
| Villi height (μm) | 602.03 ± 92.70 | 765.77 ± 136.41 |
| Villi area (μm2) | 75319.14 ± 22354.29 | 131378.51 ± 36991.03 |
| Villi perimeter (μm) | 1328.82 ± 191.19 | 1679.68 ± 278.57 |
| Goblet cells/villi | 6.79 ± 1.33 | 4.99 ± 0.44 |
| Goblet cell area (μm2) | 379.96 ± 26.72 | 239.08 ± 22.13 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grases-Pintó, B.; Torres-Castro, P.; Abril-Gil, M.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J.; Franch, À. A Preterm Rat Model for Immunonutritional Studies. Nutrients 2019, 11, 999. https://doi.org/10.3390/nu11050999
Grases-Pintó B, Torres-Castro P, Abril-Gil M, Castell M, Rodríguez-Lagunas MJ, Pérez-Cano FJ, Franch À. A Preterm Rat Model for Immunonutritional Studies. Nutrients. 2019; 11(5):999. https://doi.org/10.3390/nu11050999
Chicago/Turabian StyleGrases-Pintó, Blanca, Paulina Torres-Castro, Mar Abril-Gil, Margarida Castell, María J. Rodríguez-Lagunas, Francisco J. Pérez-Cano, and Àngels Franch. 2019. "A Preterm Rat Model for Immunonutritional Studies" Nutrients 11, no. 5: 999. https://doi.org/10.3390/nu11050999